-
摘要: 二氧化碳捕获和利用 (CCU)是目前降低大气中二氧化碳浓度的最好方法之一,而且具有很好的发展前景。在此基础上人们探究了以CO2为碳源合成有机化合物的方法。噁唑烷酮分子通常被应用于合成一些药物,并且在有机合成中也具有重要的意义,其合成方法近年来层出不穷,以二氧化碳为碳源的方法更是吸引了诸多研究工作者。早期人们利用二氧化碳和氮丙啶进行环加成反应,使用碱金属、Cr、Al等金属催化剂来提高反应的收率。考虑到成本和绿色合成的原则,选用廉价易得的离子液体或者不使用催化剂的方法更适合大规模的生产。除此之外,二氧化碳还可以和2-氨基醇等化合物在不同反应条件下得到优良甚至极好的产率。因此,本工作对近几年通过二氧化碳分别和氮丙啶、2-氨基醇、不饱和胺以及1, 2-二卤代烃反应合成噁唑烷酮的方法进行了总结和概述。Abstract: Carbon dioxide capture and utilization (CCU) is the best way to solve the problem of reducing concentration of carbon dioxide in the atmosphere, and it has a good prospect for development. On this basis, chemical researchers have explored the methods of synthesizing valuable organic compounds with CO2 as carbon source. The oxazolidinones are commonly used to synthesize drugs, and they are significant in organic synthesis as chiral molecules and intermediates. The synthetic methods of oxazolidinones have emerged in recent years. Furthermore, the methods of using carbon dioxide as a carbon source have attracted many researchers. In earlier years, people explored cycloaddition reactions of carbon dioxide and aziridines to synthesize oxazolidinones, and they took alkali metals, Cr, Al or other metals as catalysts to improve the efficiency of the reactions. Because of the cost and the principle of green synthesis, it is more suitable for large-scale reaction to select cheap and easily available ionic liquids or no catalysts. In addition, carbon dioxide and compounds such as β-amino alcohols, unsaturated amines and 1, 2-dihalohydrogenated compounds can obtain moderate or even excellent yields under different reaction conditions. In this paper, we summarized the synthetic methods of oxazolidinones using CO2 with different raw materials in recent years.
-
Key words:
- carbon dioxide /
- oxazolidinones /
- aziridines /
- epoxides /
- β-amino alcohols
-
Figure 4 Plausible catalytic cycle using aluminum complexes[51]
(with permission from Wiley-VCH)
Figure 5 A possible mechanism for the PEG6000(NBu3Br)2-catalyzed cycloaddition of CO2 with aziridine [60]
(with permission from ACS Publications)
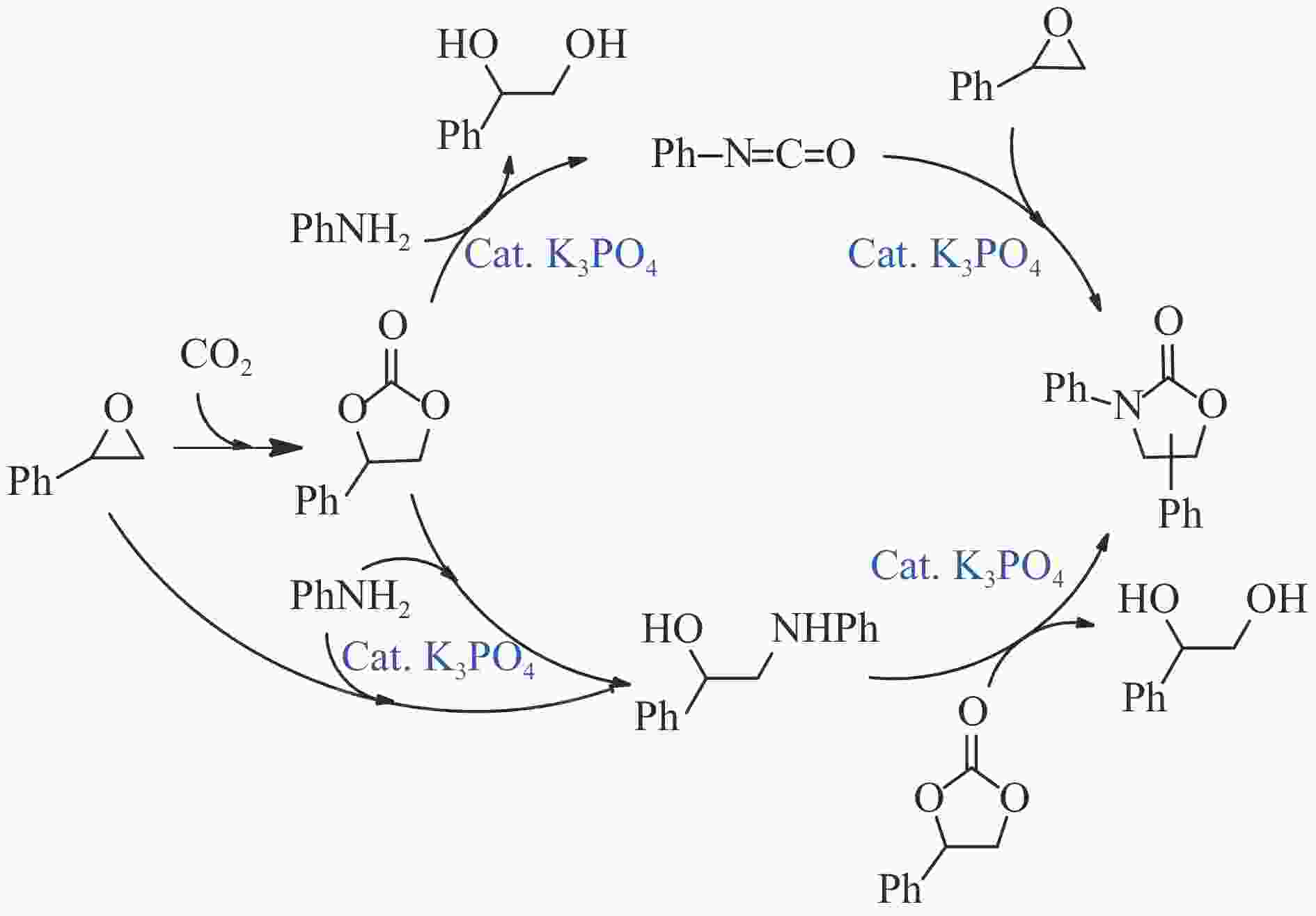
Figure 8 Plausible mechanism for potassium phosphate-catalyzed synthesis of oxazolidinones from amines, aryl epoxides and CO2[101]
(with permission from Royal Society of Chemistry)
Figure 11 A route to synthesize oxazolidinones from dihalogenated compounds[128]
(with permission from Wiley-VCH)
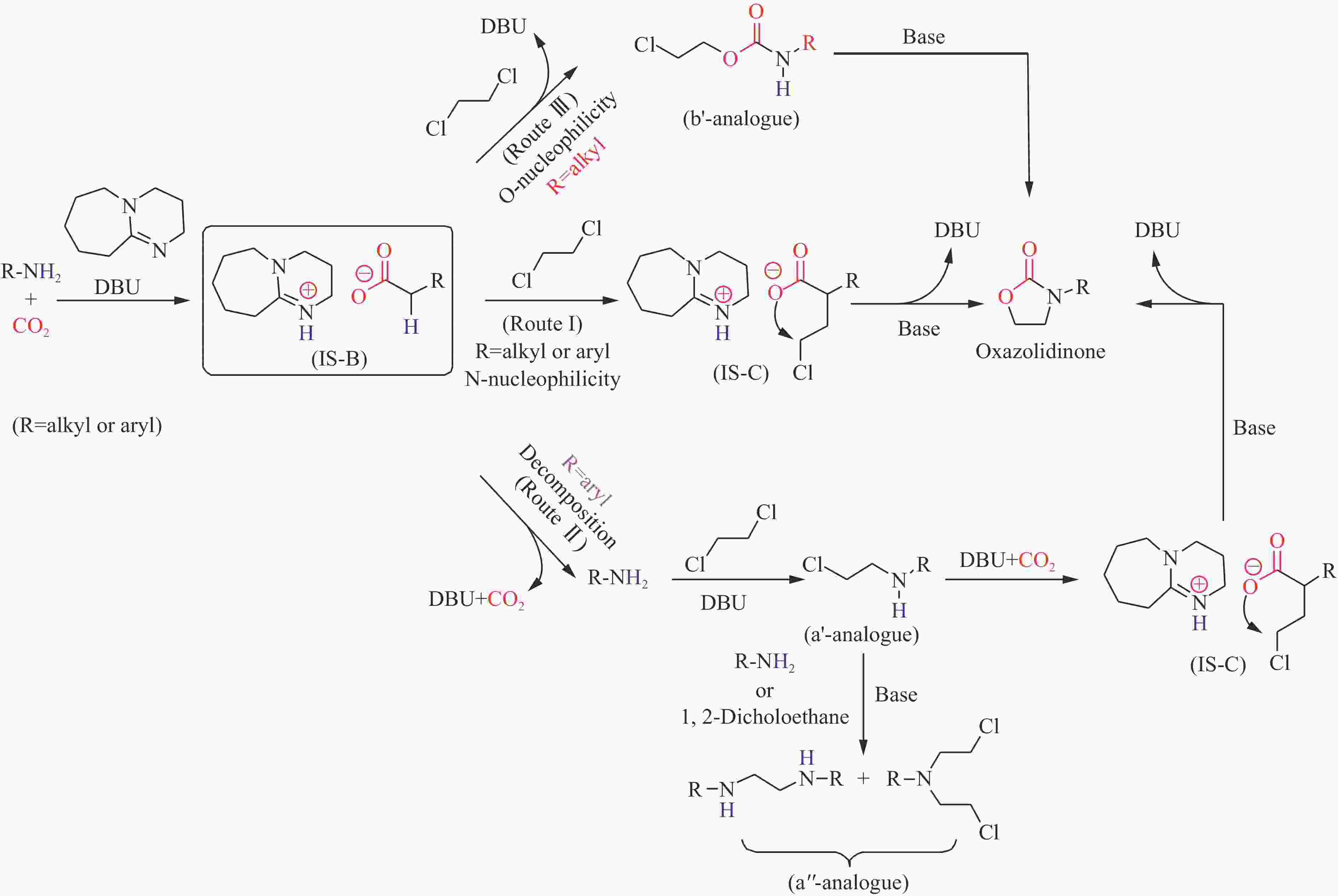
Figure 12 Plausible mechanism for DBU catalyzed synthesis of oxazolidinones from CO2, amines and 1,2-dichloroethane[130]
(with permission from Royal Society of Chemistry)
-
[1] OTTO A, GRUBE T, SCHIEBAHN S, STOLTEN D. Closing the loop: Captured CO2 as a feedstock in the chemical industry[J]. Energy Environ Sci,2015,8:3283−3297. doi: 10.1039/C5EE02591E [2] OEHLMANN N N, REBELEIN J G. The conversion of carbon monoxide and carbon dioxide by nitrogenases[J]. ChemBioChem,2022,23:e202100453. [3] DIBENEDETTO A, NOCITO F. The future of carbon dioxide chemistry[J]. ChemSusChem,2020,13:6219−6228. [4] ZHANG Z, WANG T, BLUNT M J, ANTHONY E J, PARK A-H A, HUGHES R W, WEBLEY P A, YAN J. Advances in carbon capture, utilization and storage[J]. Appl Energy,2020,278:115627. doi: 10.1016/j.apenergy.2020.115627 [5] AL-MAMOORI A, KRISHNAMURTHY A, ROWNAGHI A A, REZAEI F. Carbon capture and utilization update, energy technol[J]. 2017, 5: 834–849. [6] PSARRAS P C, COMELLO S, BAINS P, CHAROENSAWADPONG P, REICHELSTEIN S, WILCOX J. Carbon capture and utilization in the industrial sector[J]. Environ Sci Technol,2017,51:11440−11449. doi: 10.1021/acs.est.7b01723 [7] FU H-C, YOU F, LI H-R, HE L-N. CO2 capture and in situ catalytic transformation[J]. Front Chem,2019,7:525. doi: 10.3389/fchem.2019.00525 [8] CHENG B-B, YU B, HU C-W. Indirect conversion of ambient pressure CO2 into oxazolidin-2-ones by a copper-based magnetic nanocatalyst[J]. RSC Adv,2016,6:87179−87187. doi: 10.1039/C6RA15857A [9] WANG W, WANG S, MA X, GONG J. Recent advances in catalytic hydrogenation of carbon dioxide[J]. Chem Soc Rev,2011,40:3703−3727. doi: 10.1039/c1cs15008a [10] MANJOLINHO F, ARNDT M, GOOSSEN K, GOOSSEN L J. Catalytic C–H carboxylation of terminal alkynes with carbon dioxide[J]. ACS Catal,2012,2:2014−2021. doi: 10.1021/cs300448v [11] MA R, HE L-N, ZHOU Y-B. An efficient and recyclable tetraoxo-coordinated zinc catalyst for the cycloaddition of epoxides with carbon dioxide at atmospheric pressure[J]. Green Chem,2016,18:226−231. doi: 10.1039/C5GC01826A [12] SONG Q W, CHEN W Q, MA R, YU A, LI Q Y, CHANG Y, HE L N. Bifunctional silver(I) complex-catalyzed CO2 conversion at ambient conditions: Synthesis of alpha-methylene cyclic carbonates and derivatives[J]. ChemSusChem,2015,8:821−827. doi: 10.1002/cssc.201402921 [13] LUO R, LIN X, CHEN Y, ZHANG W, ZHOU X, JI H. Cooperative catalytic activation of Si-H bonds: CO2-based synthesis of formamides from amines and hydrosilanes under mild conditions[J]. ChemSusChem,2017,10:1224−1232. doi: 10.1002/cssc.201601490 [14] PANDIT N, SINGLA R K, SHRIVASTAVA B. Current updates on oxazolidinone and its significance[J]. J Med Chem,2012,2012:159285. [15] ZAPPIA G, MENENDEZ P, DELLE MONACHE, MISITI G D, NEVOLA L, BOTTA B. The contribution of oxazolidinone frame to the biological activity of pharmaceutical drugs and natural products[J]. Mini Rev Med Chem,2007,7:389−409. doi: 10.2174/138955707780363783 [16] LEMAIRE S, TULKENS PAUL M, VAN BAMBEKE F. Cellular Pharmacokinetics of the novel biaryloxazolidinone radezolid in phagocytic cells: Studies with macrophages and polymorphonuclear neutrophils[J]. ACC,2010,54:2540−2548. [17] SHINABARGER D L, MAROTTI K R, MURRAY R W, LIN A H, MELCHIOR E P, SWANEY S M, DUNYAK D S, DEMYAN W F, BUYSSE J M. Mechanism of action of oxazolidinones: Effects of linezolid and eperezolid on translation reactions[J]. ACC,1997,41:2132−2136. [18] CHELLAT M F, RAGUŽ L, RIEDL R. Targeting antibiotic resistance[J]. Angew Chem Int Ed,2016,55:6600−6626. doi: 10.1002/anie.201506818 [19] LEACH K L, BRICKNER S J, NOE M C, MILLER P F. Linezolid, the first oxazolidinone antibacterial agent[J]. Ann N Y Acad Sci,2011,1222:49−54. doi: 10.1111/j.1749-6632.2011.05962.x [20] MORAN G J, FANG E, COREY G R, DAS A F, ANDA C DE, PROKOCIMER P. Tedizolid for 6 days versus linezolid for 10 days for acute bacterial skin and skin-structure infections (ESTABLISH-2): A randomised, double-blind, Phase 3. Non-inferiority trial[J]. Lancet Infect Dis,2014,14:696−705. doi: 10.1016/S1473-3099(14)70737-6 [21] EVANS J B D A, SHIH T L. Enantioselective aldol condensations. 2. erythro-selective chiral aldol condensations via noronenolates[J]. J Am Chem Soc,1981,103:2127−2129. doi: 10.1021/ja00398a058 [22] HERAVI M M, ZADSIRJAN V, FARAJPOUR B. Applications of oxazolidinones as chiral auxiliaries in the asymmetric alkylation reaction applied to total synthesis[J]. RSC Adv,2016,6:30498−30551. doi: 10.1039/C6RA00653A [23] SHYMANSKA N V, AN I H, PIERCE J G. A rapid synthesis of 4-oxazolidinones: total synthesis of synoxazolidinones A and B[J]. Angew Chem Int Ed,2014,53:5401−5404. doi: 10.1002/anie.201402310 [24] ASHIDA N, IDA K, KOIDE Y, VAVRICKA C J, IZUMI M, KIYOTA H. Synthesis of the oxazolidinone fragment of thelepamide[J]. Nat Prod Res,2011,36:1686−1692. [25] MANDAL P S, VIJAY KUMAR A. Metal-free one-pot domino synthesis of oxazolidinone derivatives[J]. Asian J Org Chem,2022,11:e202100735. [26] TOHRU Y, NOBUAKI K, SHINJI M, NOBORU S. A new synthesis of cyclic ureas, cyclic urethanes, and a quinazolinedione selenium-assisted carbonylation of aromatic amines with carbon monoxide[J]. Bull Chem Soc JPn,1987,60:1793−1799. doi: 10.1246/bcsj.60.1793 [27] LIU J-M, PENG X-G, LIU J-H, ZHENG S-Z, SUN W, XIA C-G. Synthesis of 2-oxazolidinones by Salen-Co-complexes catalyzed oxidative carbonylation of β-amino alcohols[J]. Tetrahedron Lett,2007,48:929−932. doi: 10.1016/j.tetlet.2006.12.028 [28] GU Z-Y, XIA J-B. [3 + 1 + 1] Cyclization of vinyl oxiranes with azides and CO by tandem palladium catalysis: efficient synthesis of oxazolidinones[J]. Org Chem Fron,2021,8:4112−4117. doi: 10.1039/D1QO00591J [29] CLOSE B W J. Anticonvulsant drugs. IV. Some 2-oxazolidones[J]. Jan,1951,73:95−98. [30] CASTRO-OSMA J A, EARLAM A, LARA-SÁNCHEZ A, OTERO A, NORTH M. Synthesis of oxazolidinones from epoxides and isocyanates catalysed by aluminium heteroscorpionate complexes[J]. ChemCatChem,2016,8:2100−2108. doi: 10.1002/cctc.201600407 [31] TODA Y, GOMYOU S, TANAKA S, KOMIYAMA Y, KIKUCHI A, SUGA H. Tetraarylphosphonium salt-catalyzed synthesis of oxazolidinones from isocyanates and epoxides[J]. Org Lett,2017,19:5786−5789. doi: 10.1021/acs.orglett.7b02722 [32] TODA Y, TANAKA S, GOMYOU S, KIKUCHI A, SUGA H. 4-Hydroxymethyl-substituted oxazolidinone synthesis by tetraarylphosphonium salt-catalyzed reactions of glycidols with isocyanates[J]. ChemComm,2019,55:5761−5764. [33] ZHU X, QI Y, YANG Y, GUO D, HUANG Z, ZHANG L, WEI Y, ZHOU S, WANG S. Rare-earth-metal-complex-catalyzed hydroalkoxylation and tandem hydroalkoxylation/cyclohydroamination of isocyanates: Synthesis of carbamates and oxazolidinones[J]. Inorg Chem,2022,61:3202−3211. doi: 10.1021/acs.inorgchem.1c03673 [34] MCGHEE D R W. Replacement of phosgene with carbon dioxide: Synthesis of alkyl carbonates[J]. J Org Chem,1995,60:6205−6207. doi: 10.1021/jo00124a044 [35] HOBSON S T, RICHIERI R A, PARSEGHIAN M H. Phosgene: Toxicology, animal models, and medical countermeasures[J]. Toxicol Mech Methods,2021,31:293−307. doi: 10.1080/15376516.2021.1885544 [36] KONG D-L, HE L-N, WANG J-Q. Facile synthesis of oxazolidinones catalyzed by n-Bu4NBr3/n-Bu4NBr directly from olefins, chloramine-T and carbon dioxide[J]. Catal Commun,2010,11:992−995. doi: 10.1016/j.catcom.2010.04.003 [37] GAO X-T, GAN C-C, LIU S-Y, ZHOU F, WU H-H, ZHOU J. Utilization of CO2 as a C1 building block in a tandem asymmetric A3 coupling-carboxylative cyclization sequence to 2-oxazolidinones[J]. ACS Catal,2017,7:8588−8593. doi: 10.1021/acscatal.7b03370 [38] KHATUN R, BISWAS S, BISWAS I H, RIYAJUDDIN S, HAQUE N, GHOSH K, ISLAM S M. Cu-NPs@COF: A potential heterogeneous catalyst for CO2 fixation to produce 2-oxazolidinones as well as benzimidazoles under moderate reaction conditions[J]. J CO2 Util,2020,40:101180. doi: 10.1016/j.jcou.2020.101180 [39] GANSÄUER A. Aziridines and epoxides in organic synthesis. herausgegeben von Andrei K. Yudin[J]. Angew Chem,2006,118:5863−5863. [40] SCHAEFER F C. Homologs of triethylenemelamine[J]. J Am Chem Soc,7195,5,7:5928−5930. [41] SHEN Y-M, DUAN W-L, SHI M. Chemical fixation of carbon dioxide Co-catalyzed by a combination of schiff bases or phenols and organic bases[J]. Eur J Org Chem,2004,2004:3080−3089. doi: 10.1002/ejoc.200400083 [42] PHUNG C, PINHAS A R. The high yield and regioselective conversion of an unactivated aziridine to an oxazolidinone using carbon dioxide with ammonium iodide as the catalyst[J]. Tetrahedron Lett,2010,51:4552−4554. doi: 10.1016/j.tetlet.2010.06.110 [43] NOMURA R, NAKANO T, NISHIO Y, OGAWA S, NINAGAWA A, MATSUDA H. Regioselective cycloaddition of 1, 2-disubstituted aziridines to heterocumulenes catalyzed by organoantimony halides[J]. Chem Ber,1989,122:2407−2409. doi: 10.1002/cber.19891221232 [44] TASCEDDA P, DUÑACH E. Electrosynthesis of cyclic carbamates from aziridines and carbon dioxide[J]. ChemComm,2000,2000:449−450. [45] WU Y, HE L-N, DU Y, WANG J-Q, MIAO C-X, LI W. Zirconyl chloride: An efficient recyclable catalyst for synthesis of 5-aryl-2-oxazolidinones from aziridines and CO2 under solvent-free conditions[J]. Tetrahedron Lett,2009,65:6204−6210. doi: 10.1016/j.tet.2009.05.034 [46] MILLER A W, NGUYEN S T. (Salen)chromium(III)/DMAP: An efficient catalyst system for the selective synthesis of 5-Substituted sxazolidinones from carbon dioxide and aziridines[J]. Org Lett,2004,6:2301−2304. doi: 10.1021/ol049689t [47] ADHIKARI D, MILLER A W, BAIK M H, NGUYEN S T. Intramolecular ring-opening from a CO2-derived nucleophile as the origin of selectivity for 5-substituted oxazolidinone from the (salen)Cr-catalyzed [aziridine + CO2] coupling[J]. Chem Sci,2015,6:1293−1300. doi: 10.1039/C4SC02785J [48] SUDO A, MORIOKA Y, KOIZUMI E, SANDA F, ENDO T. Highly efficient chemical fixations of carbon dioxide and carbon disulfide by cycloaddition to aziridine under atmospheric pressure[J]. Tetrahedron Lett,2003,44:7889−7891. doi: 10.1016/j.tetlet.2003.09.011 [49] HANCOCK M T, PINHAS A R. A convenient and inexpensive conversion of an aziridine to an oxazolidinone[J]. Tetrahedron Lett,2003,44:5457−5460. doi: 10.1016/S0040-4039(03)01325-X [50] CARMINATI D, GALLO E, DAMIANO C, CASELLI A, INTRIERI D. Ruthenium porphyrin catalyzed synthesis of oxazolidin-2-ones by cycloaddition of CO2 to aziridines[J]. Eur J Inorg Chem,2018,2018:5258−5262. doi: 10.1002/ejic.201801208 [51] SENGODEN M, NORTH M, WHITWOOD A C. Synthesis of oxazolidinones by using carbon dioxide as a C1 building block and an aluminium‐based catalyst[J]. ChemSusChem,2019,12:3296−3303. doi: 10.1002/cssc.201901171 [52] BRESCIANI G, ZACCHINI S, MARCHETTI F, PAMPALONI G. Non-precious metal carbamates as catalysts for the aziridine/CO2 coupling reaction under mild conditions[J]. Dalton Trans,2021,50:5351−5359. doi: 10.1039/D1DT00525A [53] KIM J H, LEE S H, KIM N H, KANG E J. Sustainable synthesis of five-membered heterocycles using carbon dioxide and Fe-iminopyridine catalysts[J]. J CO2 Util,2021,50:101595. doi: 10.1016/j.jcou.2021.101595 [54] WANG Y-L, LI B, LAAKSONEN A. Coarse-grained simulations of ionic liquid materials: From monomeric ionic liquids to ionic liquid crystals and polymeric ionic liquids[J]. Phys Chem Chem Phys,2021,23:19435−19456. doi: 10.1039/D1CP02662C [55] KAWANAMI H, MATSUMOTO H, IKUSHIMA Y. Effective scCO2-ionic liquid reaction system based on symmetric aliphatic ammonium salts for the rapid CO2 fixation with aziridine to 2-oxazolidinone[J]. Chem Lett,2005,34:60−61. doi: 10.1246/cl.2005.60 [56] YANG Z-Z, HE L-N, PENG S-Y, LIU A-H. Lewis basic ionic liquids-catalyzed synthesis of 5-aryl-2-oxazolidinones from aziridines and CO2 under solvent-free conditions[J]. Green Chem,2010,12:1850−1854. doi: 10.1039/c0gc00286k [57] ZHOU Y, ZHANG W, MA L, ZHOU Y, WANG J. Amino acid anion paired mesoporous poly(ionic liquids) as metal-/halogen-free heterogeneous catalysts for carbon dioxide fixation[J]. ACS Sustainable Chem Eng,2019,7:9387−9398. doi: 10.1021/acssuschemeng.9b00591 [58] SONZINI P, DAMIANO C, INTRIERI D, MANCA G, GALLO E. A metal-free synthesis of N-aryl oxazolidin-2-ones by the one‐pot reaction of carbon dioxide with N-aryl aziridines[J]. Adv Synth Catal,2020,362:2961−2969. doi: 10.1002/adsc.202000175 [59] BRESCIANI G, BORTOLUZZI M, PAMPALONI G, MARCHETTI F. Diethylammonium iodide as catalyst for the metal-free synthesis of 5-aryl-2-oxazolidinones from aziridines and carbon dioxide[J]. Org Bio Chem,2021,19:4152−4161. doi: 10.1039/D1OB00458A [60] LIU A-H, HE L-N. Quaternary ammonium bromide functionalized polyethylene glycol: A highly efficient and recyclable catalyst for selective synthesis of 5-Aryl-2-oxazolidinones from carbon dioxide and aziridines under solvent-free conditions[J]. J Org Chem,2008,73:4709−4712. doi: 10.1021/jo800269v [61] KUMAR S, JAIN S L. Polyethylene glycol wrapped potassium bromide assisted chemical fixation of carbon dioxide[J]. Ind Eng Chem Res,2014,53:541−546. doi: 10.1021/ie4033439 [62] NALE D B, RANA S, PARIDA K, BHANAGE B M. Amine functionalized MCM-41 as a green, efficient, and heterogeneous catalyst for the regioselective synthesis of 5-aryl-2-oxazolidinones, from CO2 and aziridines[J]. Appl Catal A: Gen,2014,469:340−349. doi: 10.1016/j.apcata.2013.10.011 [63] KATHALIKKATTIL A C, ROSHAN R, THARUN J, BABU R, JEONG G-S, KIM D-W, CHO S J, PARK D-W. A sustainable protocol for the facile synthesis of zinc-glutamate MOF: An efficient catalyst for room temperature CO2 fixation reactions under wet conditions[J]. ChemComm,2016,52:280−283. [64] SAPTAL V, SHINDE D B, BANERJEE R, BHANAGE B M. State-of-the-art catechol porphyrin COF catalyst for chemical fixation of carbon dioxide via cyclic carbonates and oxazolidinones[J]. Catal Sci Tech,2016,6:6152−6158. doi: 10.1039/C6CY00362A [65] SADEGHZADEH S M, ZHIANI R, MORADI M. CO2 transformation under mild conditions using tripolyphosphate-grafted KCC-1-NH2[J]. Phosphorus Sulfur,2018,193:535−544. doi: 10.1080/10426507.2018.1455197 [66] LIU Y, CHEN C, HU Y L. Efficient and convenient catalytic regioselective synthesis of 2-oxazolidinones from CO2 and aziridines over reusable SBA-15 supported hydroxyacetate-functionalized ionic liquid[J]. J Porous Mater,2022,29:131−142. doi: 10.1007/s10934-021-01153-6 [67] DOU X-Y, HE L-N, YANG Z-Z, WANG J-L. Catalyst-free process for the synthesis of 5-aryl-2-oxazolidinones via cycloaddition reaction of aziridies and carbon dioxide[J]. Synlett,2010,2010:2159−2163. doi: 10.1055/s-0030-1258510 [68] PHUNG C, ULRICH R M, IBRAHIM M, TIGHE N T G, LIEBERMAN D L, PINHAS A R. The solvent-free and catalyst-free conversion of an aziridine to an oxazolidinone using only carbon dioxide[J]. Green Chem,2011,13:3224−3229. doi: 10.1039/c1gc15850c [69] O'BRIEN P. Sharpless asymmetric aminohydroxylation: Scope, limitations, and use in synthesis[J]. Angew Chem Int Ed,1999,38:326−329. doi: 10.1002/(SICI)1521-3773(19990201)38:3<326::AID-ANIE326>3.0.CO;2-T [70] YANG D, XIE C-X, WU X-T, FEI L-R, FENG L, MA C. Metal-free β-amino alcohol synthesis: A two-step smiles rearrangement[J]. J Org Chem,2020,85:14905−14915. doi: 10.1021/acs.joc.0c01543 [71] SCHIROK H. Microwave-assisted flexible synthesis of 7-azaindoles[J]. J Org Chem,2006,71:5538−5545. doi: 10.1021/jo060512h [72] LIEBSCHER J, JIN S, OTTO A, WOYDOWSKI K. Synthetic application of chiral pool derived heterocycles[J]. J Heterocyclic Chem,2000,37:509−518. doi: 10.1002/jhet.5570370308 [73] AGER D J, PRAKASH I, SCHAAD D R. 1, 2-Amino alcohols and their heterocyclic derivatives as chiral auxiliaries in asymmetric synthesis[J]. Chem Rev,1996,96:835−876. doi: 10.1021/cr9500038 [74] RUEDIGER E, MARTEL A, MEANWELL N, SOLOMON C, TURMEL B. Novel 3'- deoxy analogs of the anti-HBV agent entecavir: Synthesis of enantiomers from a single chiral epoxide[J]. Tetrahedron Lett,2004,45:739−742. doi: 10.1016/j.tetlet.2003.11.052 [75] STÅHLBERG J, HENRIKSSON H, DIVNE C, ISAKSSON R, PETTERSSON G, JOHANSSON G, JONES T A. Structural basis for enantiomer binding and separation of a common β-blocker: Crystal structure of cellobiohydrolase Cel7A with bound (S)-propranolol at 1.9 Å resolution1 1Edited by R. Huber[J]. J Mol Biol,2001,305:79−93. doi: 10.1006/jmbi.2000.4237 [76] YOUNG R R, GROWDON J H, SHAHANI B T. Beta-adrenergic mators (R)-terbutaline and (R)-Ssalbutamol from (R)-cyanohydrins1[J]. J Org Chem,1997,62:3867−3873. doi: 10.1021/jo970032d [77] FACHE F, SCHULZ E, TOMMASINO M L, LEMAIRE M. Nitrogen-containing ligands for asymmetric homogeneous and heterogeneous catalysis[J]. Chem Rev,2000,100:2159−2232. doi: 10.1021/cr9902897 [78] ZHU S, MENG L, ZHANG Q, WEI L. Synthesis and evaluation of febrifugine analogues as potential antimalarial agents[J]. Bioorg Med Chem Lett,2006,16:1854−1858. doi: 10.1016/j.bmcl.2006.01.009 [79] CHUNG S-K, KANG D-H. Stereoselective synthesis of β-amino alcohols: Diastereoselective reduction of chiral α′-amino enones derived from amino acids[J]. Tetrahedron: Asymmetry,1997,8:3027−3030. doi: 10.1016/S0957-4166(97)00374-1 [80] JUÁREZ R, CONCEPCIÓN P, CORMA A, GARCÍA H. Ceria nanoparticles as heterogeneous catalyst for CO2 fixation by ω-aminoalcohols[J]. ChemComm,2010,46:4181−4183. [81] MATSUDA H., BABA A., NOMURA R., KORI M., OGAWA S. Improvement of the process in the synthesis of 2-oxazolidinones from 2-amino alcohols and carbon dioxide by use of triphenylstibine oxide as catalyst[J]. Ind Eng Chem Res,1985,24:239−242. doi: 10.1021/i300018a013 [82] RYOKI NOMURA M Y, MATSUDA H. Preparation of cyclic ureas from carbon dioxide and diamines catalyzed by triphenylstibine pxide[J]. Ind Eng Chem Res,1987,26:1056−1059. doi: 10.1021/ie00066a002 [83] JUÁREZ R, CONCEPCIÓN P, CORMA A, GARCÍA H. Ceria nanoparticles as heterogeneous catalyst for CO2 fixation by ω-aminoalcohols[J]. Chem Commun,2010,46:4181−4183. [84] PULLA S, FELTON C M, GARTIA Y, RAMIDI P, GHOSH A. Synthesis of 2-oxazolidinones by direct condensation of 2-aminoalcohols with carbon dioxide using chlorostannoxanes[J]. ACS Sustainable Chem Eng,2013,1:309−312. doi: 10.1021/sc300077m [85] TAMURA M, HONDA M, NORO K, NAKAGAWA Y, TOMISHIGE K. Heterogeneous CeO2-catalyzed selective synthesis of cyclic carbamates from CO2 and aminoalcohols in acetonitrile solvent[J]. J Catal,2013,305:191−203. doi: 10.1016/j.jcat.2013.05.013 [86] FOO S W, TAKADA Y, YAMAZAKI Y, SAITO S. Dehydrative synthesis of chiral oxazolidinones catalyzed by alkali metal carbonates under low pressure of CO2[J]. Tetrahedron Lett,2013,54:4717−4720. doi: 10.1016/j.tetlet.2013.06.100 [87] KAWANAMI H, IKUSHIMA Y. Synthesis of 2-oxazolidinone from β-aminoalcohol using supercritical carbon dioxide[J]. J Jpn Petrol Inst,2002,45:321−324. doi: 10.1627/jpi.45.321 [88] DINSMORE C J, MERCER S P. Carboxylation and mitsunobu reaction of amines to give carbamates: Retention vs inversion of configuration is substituent-dependent[J]. Org Lett,2004,6:2885−2888. doi: 10.1021/ol0491080 [89] FU J-T, SENBOKU H, ARAI M. Preparation of cyclic urethanes from amino alcohols and carbon dioxide using ionic liquid catalysts with alkali metal promoters[J]. Int J Mol Sci,2006,7:438−450. doi: 10.3390/i7100438 [90] FEROCI M, INESI A, MUCCIANTE V AND ROSSI L. New synthesis of oxazolidin-2-ones[J]. Tetrahedron Lett,1999,40:6059−6060. [91] PAZ J, PÉREZ-BALADO C, IGLESIAS B, MUÑOZ L. Carbon dioxide as a carbonylating agent in the synthesis of 2-oxazolidinones, 2-oxazinones, and cyclic ureas: Scope and limitations[J]. J Org Chem,2010,75:3037−3046. doi: 10.1021/jo100268n [92] MAITI S K, RAMANATHAN A, SUBRAMANIAM B. 110th Anniversary: Near-total epoxidation selectivity and hydrogen peroxide utilization with Nb-EISA catalysts for propylene epoxidation[J]. Ind Eng Chem Res,2019,58:17727−17735. doi: 10.1021/acs.iecr.9b03461 [93] NIJHUIS T A, MAKKEE M, MOULIJN J A, WECKHUYSEN B M. The production of propene oxide: Catalytic processes and recent developments[J]. Ind Eng Chem Res,2006,45:3447−3459. doi: 10.1021/ie0513090 [94] CLIMENT M J, CORMA A, IBORRA S. Heterogeneous catalysts for the one-pot synthesis of chemicals and fine chemicals[J]. Chem Rev,2011,111:1072−1133. doi: 10.1021/cr1002084 [95] HAYASHI T, TANAKA K, HARUTA M. Selective vapor-phase epoxidation of propylene over Au/TiO2 catalysts in the presence of oxygen and hydrogen[J]. J Catal,1998,178:566−575. doi: 10.1006/jcat.1998.2157 [96] GAO X, YU B, YANG Z, ZHAO Y, ZHANG H, HAO L, HAN B, LIU Z. Ionic liquid-catalyzed C–S bond construction using CO2 as a C1 building block under mild conditions: A metal-free route to synthesis of benzothiazoles[J]. ACS Catal,2015,5:6648−6652. doi: 10.1021/acscatal.5b01874 [97] HAO L, ZHAO Y, YU B, YANG Z, ZHANG H, HAN B, GAO X, LIU. Z. Imidazolium-based ionic liquids catalyzed formylation of amines using carbon dioxide and phenylsilane at room temperature[J]. ACS Catal,2015,5:4989−4993. doi: 10.1021/acscatal.5b01274 [98] WANG B, ELAGEED E H M, ZHANG D, YANG S, WU S, ZHANG G, GAO G. One-pot conversion of carbon dioxide, ethylene oxide, and amines to 3-aryl-2-oxazolidinones catalyzed with binary ionic liquids[J]. ChemCatChem,2014,6:278−283. doi: 10.1002/cctc.201300801 [99] WANG B, LUO Z, ELAGEED E H M, WU S, ZHANG Y, WU X, XIA F, ZHANG G, GAO G. DBU and DBU-derived ionic liquid synergistic catalysts for the conversion of carbon dioxide/carbon disulfide to 3-aryl-2-oxazolidinones/[1, 3]dithiolan-2-ylidenephenyl- amine[J]. ChemCatChem,2016,8:830−838. doi: 10.1002/cctc.201500928 [100] LV M, WANG P, YUAN D, YAO Y. Conversion of carbon dioxide into oxazolidinones mediated by quaternary ammonium salts and DBU[J]. ChemCatChem,2017,9:4451−4455. doi: 10.1002/cctc.201700594 [101] SEO U R, CHUNG Y K. Potassium phosphate-catalyzed one-pot synthesis of 3-aryl-2-oxazolidinones from epoxides, amines, and atmospheric carbon dioxide[J]. Green Chem,2017,19:803−808. doi: 10.1039/C6GC02934E [102] SADEGHZADEH S M, ZHIANI R, EMRANI S. Spirulina (arthrospira) platensis supported ionic lquid as a catalyst for the synthesis of 3-aryl-2-oxazolidinones from carbon sioxide, epoxide, anilines[J]. Catal Lett,2017,148:119−124. [103] XIE Y F, GUO C, SHI L, PENG B H, LIU N. Bifunctional organocatalysts for the conversion of CO2, epoxides and aryl amines to 3-aryl-2-oxazolidinones[J]. Org Biomol Chem,2019,17:3497−3506. doi: 10.1039/C9OB00224C [104] WU F-T, WU L, CUI C-N. The catalytic system ‘Rhodamine B/additive’ for the chemical fixation of CO2[J]. Tetrahedron,2021,83:131965. doi: 10.1016/j.tet.2021.131965 [105] XU B, WANG P, LV M, YUAN D, YAO Y. Transformation of carbon dioxide into oxazolidinones and cyclic carbonates catalyzed by rare-earth-metal phenolates[J]. ChemCatChem,2016,8:2466−2471. doi: 10.1002/cctc.201600534 [106] CHEN F, LI M, WANG J, DAI B, LIU N. Fe(II) complexes: Reservoirs for Lewis acids and carbenes and their utility in the conversion of CO2 to oxazolidinones[J]. J CO2 Util,2018,28:181−188. doi: 10.1016/j.jcou.2018.09.025 [107] HELAL A, CORDOVA K E, ARAFAT M E, USMAN M, YAMANI Z H. Defect-engineering a metal-organic framework for CO2 fixation in the synthesis of bioactive oxazolidinones[J]. Inor Chem Front,2020,7:3571−3577. doi: 10.1039/D0QI00496K [108] FENG L, LI X, XU C, SADEGHZADEH S M. Green synthesis of Dy2Ce2O7 nanoparticles immobilized on fibrous nano-silica for synthesis of 3-Aryl-2-oxazolidinones from alkenes amines, and carbon dioxide[J]. Catal Letters,2019,150:1729−1740. [109] HELAL A, FETTOUHI M, ARAFAT M E, KHAN M Y, SANHOOB M A. Nickel based metal-organic framework as catalyst for chemical fixation of CO2 in oxazolidinone synthesis[J]. J CO2 Util,2021,50:101603. doi: 10.1016/j.jcou.2021.101603 [110] YAMANAKA N, HARA T, ICHIKUNI N, SHIMAZU S. Chemoselective hydrogenation of unsaturated nitro compounds to unsaturated amines by Ni-Sn alloy catalysts[J]. Chem Lett,2018,47:971−974. doi: 10.1246/cl.180458 [111] SARTILLO-PISCIL F, ROMERO-IBAÑEZ J, FUENTES L. Transition-metal-free functionalization of saturated and unsaturated amines to bioactive alkaloids mediated by sodium chlorite[J]. Synlett,2020,32:1385−1261. [112] MITSUDO T-A, HORI Y, YAMAKAWA Y, WATANABE Y. Ruthenium catalyzed selective synthesis of enol carbamates by fixation of carbon dioxide[J]. Tetrahedron Lett,1987,28:4417−4418. doi: 10.1016/S0040-4039(00)96526-2 [113] SHI M, SHEN Y-M. Transition-metal-catalyzed reactions of propargylamine with carbon dioxide and carbon disulfide[J]. The J Org Chem,2002,67:16−21. doi: 10.1021/jo0014966 [114] GARCÍA-DOMÍNGUEZ P, FEHR L, RUSCONI G, NEVADO C. Palladium-catalyzed incorporation of atmospheric CO2: Efficient synthesis of functionalized oxazolidinones[J]. Chem Sci,2016,7:3914−3918. doi: 10.1039/C6SC00419A [115] GHOSH S, RIYAJUDDIN S, SARKAR S, GHOSH K, ISLAM S M. Pd NPs decorated on POPs as recyclable catalysts for the synthesis of 2-oxazolidinones from propargylic amines via atmospheric cyclizative CO2 incorporation[J]. ChemNanoMat,2019,6:160−172. [116] PÉREZ P J. Silver in Organic Chemistry. Edited by Michael Harmata[J]. Angew Chem Int Ed,2010,49:9040−9040. doi: 10.1002/anie.201006002 [117] YOSHIDA S, FUKUI K, KIKUCHI S, YAMADA T. Silver-catalyzed preparation of oxazolidinones from carbon dioxide and propargylic amines[J]. Chem Lett,2009,38:786−787. doi: 10.1246/cl.2009.786 [118] YOSHIDA M, MIZUGUCHI T, SHISHIDO K. Synthesis of oxazolidinones by efficient fixation of atmospheric CO2 with propargylic amines by using a ailver/1, 8-diazabicyclo[5.4. 0]undec-7-ene (DBU) dual-catalyst system[J]. Chem Eur J,2012,18:15578−15581. doi: 10.1002/chem.201203366 [119] WANG M-Y, SONG Q-W, MA R, XIE J-N, HE L-N. Efficient conversion of carbon dioxide at atmospheric pressure to 2-oxazolidinones promoted by bifunctional Cu(II)-substituted polyoxometalate-based ionic liquids[J]. Green Chem,2016,18:282−287. doi: 10.1039/C5GC02311D [120] LIU X, WANG M-Y, WANG S-Y, WANG Q, HE L-N. In situ generated Zinc(II) catalyst for incorporation of CO2 into 2-oxazolidinones with propargylic amines at atmospheric pressure[J]. ChemSusChem,2017,10:1210−1216. doi: 10.1002/cssc.201601469 [121] GHOSH S, KHAN T S, GHOSH A, CHOWDHURY A H, HAIDER M A, KHAN A, ISLAM S M. Utility of silver nanoparticles embedded covalent organic frameworks as recyclable catalysts for the sustainable synthesis of cyclic carbamates and 2-oxazolidinones via atmospheric cyclizative CO2 capture[J]. ACS Sustainable Chem Eng,2020,8:5495−5513. doi: 10.1021/acssuschemeng.9b06704 [122] SADEGHZADEH S M. A green approach for the synthesis of 2-oxazolidinones using gold(I) complex immobilized on KCC-1 as nanocatalyst at room temperature[J]. Appl Organomet Chem,2016,30:835−842. doi: 10.1002/aoc.3511 [123] HASE S, KAYAKY I, IKARIYA T. Mechanistic aspects of the carboxylative cyclization of propargylamines and carbon dioxide catalyzed by Gold(I) complexes bearing an N-heterocyclic carbene ligand[J]. ACS Catal,2015,5:5135−5140. doi: 10.1021/acscatal.5b01335 [124] COSTA M, CHIUSOLI G P, RIZZARDI M. Base-catalysed direct introduction of carbon dioxide into acetylenic amines[J]. Chem Commun,1996,14:1699−1700. [125] YOSHIDA M, KOMATSUZAKI Y, IHARA M. Synthesis of 5-vinylideneoxazolidin-2-ones by DBU-mediated CO2-fixation reaction of 4-(benzylamino)-2-butynyl carbonates and benzoates[J]. Org Lett,2008,10:2083−2086. doi: 10.1021/ol800663v [126] ZHANG Z, WU C, MA J, SONG J, FAN H, LIU J, ZHU Q, HAN B. A strategy to overcome the thermodynamic limitation in CO2 conversion using ionic liquids and urea[J]. Green Chem,2015,17:1633−1639. doi: 10.1039/C4GC02199A [127] HU J, MA J, ZHANG Z, ZHU Q, ZHOU H, LU W, HAN B. A route to convert CO2: Synthesis of 3, 4, 5-trisubstituted oxazolones[J]. Green Chem,2015,17:1219−1225. doi: 10.1039/C4GC02033B [128] MACÉ A, TOUCHET S, ANDRES P, COSSÍO F, DORCET V, CARREAUX F, CARBONI B. [3, 3]-Sigmatropic rearrangement/allylboration/cyclization sequence: Enantioenriched seven-membered-ring carbamates and ring contraction to pyrrolidines[J]. Angew Chem Int Ed,2016,55:1025−1029. doi: 10.1002/anie.201509824 [129] MEI C, ZHAO Y, CHEN Q, CAO C, PANG G, SHI Y. Synthesis of oxazolidinones and derivatives through three-component fixation of carbon dioxide[J]. ChemCatChem,2018,10:3057−3068. doi: 10.1002/cctc.201800142 [130] CHEN X-C, ZHAO K-C, YAO Y-Q, LU Y, LIU Y. Synergetic activation of CO2 by the DBU-organocatalyst and amine substrates towards stable carbamate salts for synthesis of oxazolidinones[J]. Catal Sci Technol,2021,11:7072−7082. doi: 10.1039/D1CY01298C -





 下载:
下载: