Oxidation characteristics of soot in different entrained flow gasification processes
-
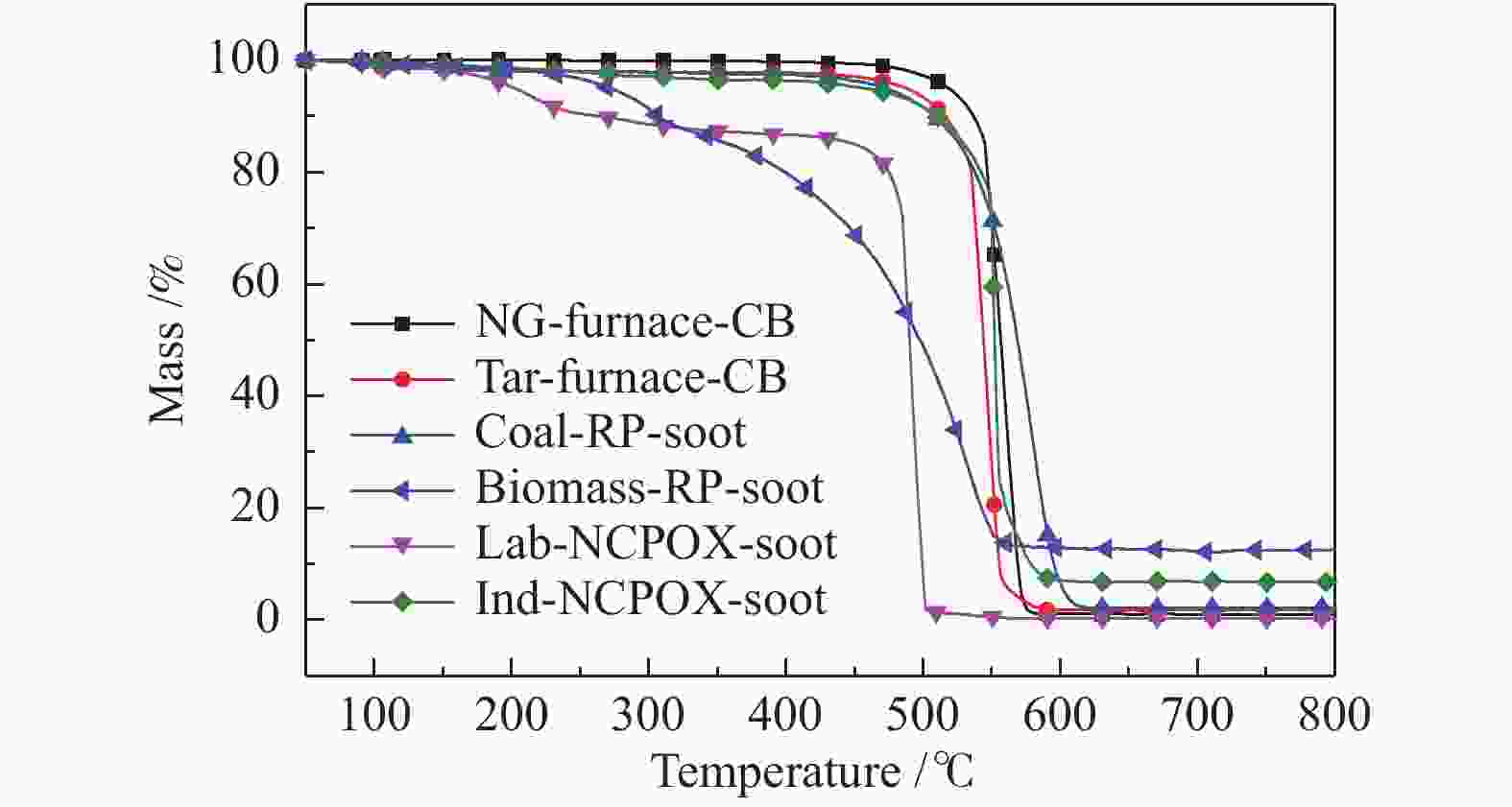
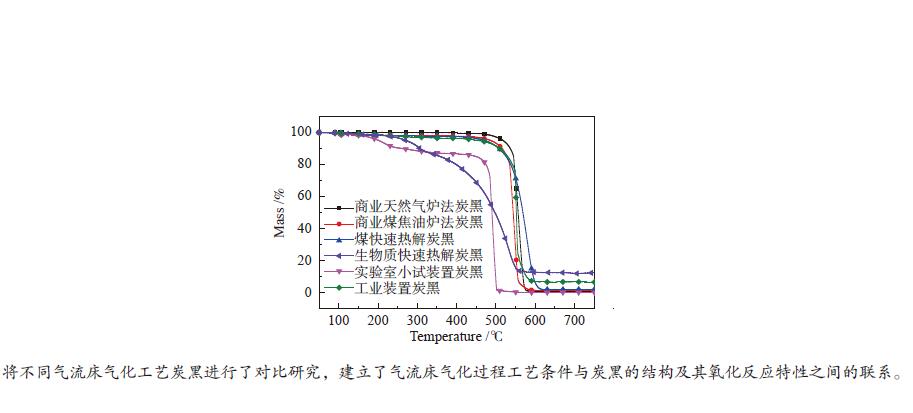
摘要: 利用高分辨透射显微镜分别对煤和生物质快速热解炭黑、天然气非催化部分氧化小试装置炭黑和工业装置炭黑、商业天然气炉法炭黑和煤焦油炉法炭黑等六种样品的形貌结构进行了表征;基于常压热重分析仪非等温法(50−800 ℃)对炭黑的着火点、氧化反应速率进行了研究,获得了炭黑的氧化反应动力学参数。研究表明,不同的炭黑理化性质差异较大,煤和生物质快速热解炭黑的球形度更高,粒径较大;天然气非催化部分氧化小试装置炭黑在较低温度下形成,呈现被碳囊包裹的形态;天然气非催化部分氧化工业装置炭黑呈现中空结构,粒径较小。非催化部分氧化小试装置和工业装置炭黑的氧化反应性接近,是天然气炉法炭黑的3.1倍,是煤焦油炉法炭黑的3.2倍;非催化部分氧化炭黑的反应性是煤快速热解炭黑的9.0倍,是生物质快速热解炭黑的26.6倍。两种非催化部分氧化炭黑和两种商业炉法炭黑的活化能随温度变化呈现分段形式;两种快速热解炭黑的活化能随温度升高基本不变。Abstract: The morphological structure of six samples including the rapid pyrolysis soot of solid fuels (coal, biomass), the soot from non-catalytic partial oxidation (NCPOX) of natural gas in a laboratory pilot plant and an industrial plant, the commercial carbon black in natural gas furnace/coal tar furnace, were characterized by using a transmission electron microscope. Based on atmospheric thermogravimetric analyzer, the non-isothermal method (50–800 ℃) was adopted to study the ignition point and the oxidation reaction rate of soot, and the oxidation reaction kinetic parameters of soot was obtained. Studies showed that the physical and chemical properties of various soot were quite different. The soot from the rapid pyrolysis of coal and biomass presented a higher sphericity and a larger particle size. The Lab-NCPOX-soot was formed at a lower temperature which caused the particle being wrapped by a carbon capsule. The Ind-NCPOX-soot had a hollow structure and a small particle size. The reactivity of the Lab-NCPOX-soot is close to that of the Ind-NCPOX-soot, which is 3.1 times that of the commercial natural gas furnace carbon black and 3.2 times that of the commercial coal tar furnace carbon black; the reactivity of NCPOX-soot is 9.0 times of the rapid pyrolysis soot of coal, and 26.6 times of the rapid pyrolysis soot of biomass. The activation energy of 2 kinds of NCPOX-soot and 2 kinds of commercial carbon blacks present staged forms with increasing temperature. The activation energy of the 2 rapid pyrolysis soot was basically unchanged with increasing the temperature.
-
Key words:
- soot /
- rapid pyrolysis /
- non-catalytic partial oxidation /
- structure /
- reactivity /
- kinetic
-
Sample Proximate analysis wd/% Ultimate analysis wd/% V FC A C H N S O Coal 35.48 59.06 5.46 76.85 4.63 1.23 1.34 10.49 Saw dust 90.92 7.74 1.34 42.39 5.64 0.79 0.44 49.40 表 2 天然气非催化部分氧化实验的反应工况
Table 2 Reaction conditions of the NCPOX experiment of natural gas
O2/CH4 CH4 flow rate/ (L∙min−1) O2 flow rate/ (L∙min−1) CH4 velocity/ (m∙s−1) O2 velocity/ (m∙s−1) Residence time/s 0.80 18.89 15.11 123.34 142.51 0.929 表 3 炭黑的氧化反应特性参数
Table 3 Oxidation reaction characteristic parameters of soot
Sample Ti0/℃ Temperature of
wmax/℃Tf/℃ Reaction time/
minwmax/
(%·min−1)wmean/
(%·min−1)S/
(× 10−9·%2·min−2·℃−3)NG- furnace-CB 496.7 559.9 584.3 8.76 3.51 1.09 26.6 Tar-furnace-CB 488.6 548.3 588.4 9.98 3.87 0.93 25.5 Coal-RP-soot 518.6 580.7 614.5 9.59 1.70 0.88 9.1 Biomass-RP-soot 460.8 531.2 572.0 11.12 0.79 0.47 3.1 Lab-NCPOX-soot 453.7 493.7 503.3 4.96 5.18 1.63 81.5 Ind-NCPOX-soot 531.6 550.9 594.6 6.30 9.63 1.12 63.9 表 4 炭黑样品的氧化动力学参数
Table 4 Oxidation kinetic parameters of soot samples
Sample Temperature range/℃ b a E/(kJ·mol−1) A/min−1 R2 NG-furnace-CB stage 1 496.7−542.8 11.42 −21924 182 2.15 × 1010 0.9926 stage 2 542.8−577.3 71.65 −70943 590 9.53 × 1036 0.9970 stage 3 577.3−584.3 4.98 −14327 119 2.36 × 107 0.9759 Tar-furnace-CB stage 1 488.6−533.8 5.49 −16575 138 4.41 × 107 0.9851 stage 2 533.8−558.7 76.08 −73433 611 8.23 × 1038 0.9978 stage 3 558.7−588.4 10.00 −18630 155 4.49 × 109 0.9932 Coal-RP-soot 518.6−614.5 18.41 −26972 224 2.83 × 1013 0.9789 Biomass-RP-soot 460.8−572.0 1.17 −11222 93 4.14 × 105 0.9654 Lab-NCPOX-soot stage 1 453.7−483.9 −2.47 −9144 76 9.21 × 103 0.9941 stage 2 483.9−503.3 91.53 −80272 667 4.62 × 1045 0.9509 Ind-NCPOX-soot stage 1 531.6−547.6 5.37 −16459 137 3.91 × 107 0.9981 stage 2 547.6−556.3 201.19 −177362 1475 4.25 × 1093 0.9874 stage 3 556.3−594.6 16.06 −24071 200 2.44 × 1012 0.9777 -
[1] UMEMOTO S, KAJITANI S, MIURA K, WATANABE H, KAWASE M. Extension of the chemical percolation devolatilization model for predicting formation of tar compounds as soot precursor in coal gasification[J]. Fuel Process Technol,2017,159:256−265. doi: 10.1016/j.fuproc.2017.01.037 [2] MIURA K, NAKAGAWA H, NAKAI S-I, KAJITANI S. Analysis of gasification reaction of coke formed using a miniature tubing-bomb reactor and a pressurized drop tube furnace at high pressure and high temperature[J]. Chem Eng Sci,2004,59(22/23):5261−5268. doi: 10.1016/j.ces.2004.08.025 [3] GÖKTEPE B, UMEKI K, GEBART R. Does distance among biomass particles affect soot formation in an entrained flow gasification process?[J]. Fuel Process Technol,2016,141:99−105. doi: 10.1016/j.fuproc.2015.06.038 [4] 王辅臣, 李伟锋, 代正华, 陈雪莉, 刘海峰, 于遵宏. 天然气非催化部分氧化制合成气过程的研究[J]. 石油化工,2006,1:47−51.WANG Fu-chen, LI Wei-feng, DAI Zheng-hua, CHEN Xue-li, LIU Hai-feng, YU Zun-hong. Preparation of syngas from natural gas by non-catalytic partial oxidation[J]. Petrochem Technol,2006,1:47−51. [5] 王辅臣, 代正华, 刘海峰, 龚欣, 于广锁, 于遵宏. 焦炉气非催化部分氧化与催化部分氧化制合成气工艺比较[J]. 煤化工,2006,34(2):4−9. doi: 10.3969/j.issn.1005-9598.2006.02.002WANG Fu-chen, DAI Zheng-hua, LIU Hai-feng, GONG Xin, YU Guang-suo, YU Zun-hong. COG based syngas production process with catalytic and non- catalytic partial oxidation[J]. Coal Chem Ind,2006,34(2):4−9. doi: 10.3969/j.issn.1005-9598.2006.02.002 [6] VERMA P, PICKERING E, SAVIC N, ZARE A, BROWN R, RISTOVSKI Z. Comparison of manual and automatic approaches for characterisation of morphology and nanostructure of soot particles[J]. J Aerosol Sci,2019,136:91−105. doi: 10.1016/j.jaerosci.2019.07.001 [7] UMEMOTO S, KAJITANI S, HARA S, KAWASE M. Proposal of a new soot quantification method and investigation of soot formation behavior in coal gasification[J]. Fuel,2016,167:280−287. doi: 10.1016/j.fuel.2015.11.074 [8] CHANG Q, GAO R, GAO M, YU G, WANG F. The structural evolution and fragmentation of coal-derived soot and carbon black during high-temperature air oxidation[J]. Combust Flame,2020,216:111−125. doi: 10.1016/j.combustflame.2019.11.045 [9] SEPTIEN S, VALIN S, PEYROT M, DUPONT C, SALVADOR S. Characterization of char and soot from millimetric wood particles pyrolysis in a drop tube reactor between 800 °C and 1400 °C[J]. Fuel,2014,121:216−224. doi: 10.1016/j.fuel.2013.12.026 [10] TRUBETSKAYA A, JENSEN P A, JENSEN A D, GARCIA LLAMAS A D, UMEKI K, GARDINI D, KLING J, BATES R B, GLARBORG P. Effects of several types of biomass fuels on the yield, nanostructure and reactivity of soot from fast pyrolysis at high temperatures[J]. Appl Energy,2016,171:468−482. doi: 10.1016/j.apenergy.2016.02.127 [11] TRUBETSKAYA A, LARSEN F H, SHCHUKAREV A, STÅHL K, UMEKI K. Potassium and soot interaction in fast biomass pyrolysis at high temperatures[J]. Fuel,2018,225:89−94. doi: 10.1016/j.fuel.2018.03.140 [12] TRUBETSKAYA A, BROWN A, TOMPSETT G A, TIMKO M T, KLING J, BROSTRöM M, ANDERSEN M L, UMEKI K. Characterization and reactivity of soot from fast pyrolysis of lignocellulosic compounds and monolignols[J]. Appl Energy,2018,212:1489−1500. doi: 10.1016/j.apenergy.2017.12.068 [13] 袁帅. 煤、生物质及其混合物的快速热解及过程中氮的迁移[D]. 上海: 华东理工大学, 2012.YUAN Shuai. Rapid pyrolysis of coal, biomass, and coal/biomass blends, and nitrogen evolution during rapid pyrolysis[D]. Shanghai: East China University of Science and Technology, 2012. [14] CHANG Q, GAO R, LI H, YU G, LIU X, WANG F. Understanding of formation mechanisms of fine particles formed during rapid pyrolysis of biomass[J]. Fuel,2018,216:538−547. doi: 10.1016/j.fuel.2017.12.036 [15] CHANG Q, GAO R, LI H, YU G, WANG F. Effect of CO2 on the characteristics of soot derived from coal rapid pyrolysis[J]. Combust Flame,2018,197:328−339. doi: 10.1016/j.combustflame.2018.05.033 [16] 李炳炎主编. 炭黑生产与应用手册[M]. 北京: 化学工业出版社, 2000.LI Bing-yan. Carbon Black Production and Application Manual[M]. Beijing: Chemical Industry Press, 2000. [17] WANG X, JIN Q, WANG L, BAI S, MIKULČIĆ H, VUJANOVIĆ M, TAN H. Synergistic effect of biomass and polyurethane waste co-pyrolysis on soot formation at high temperatures[J]. J Environ Manage,2019,239:306−315. doi: 10.1016/j.jenvman.2019.03.073 [18] 吕建燚, 石晓斌. 生物质燃烧碳烟的物化特性及生成机理研究[J]. 燃料化学学报,2013,41(10):1184−1190.LÜ Jian-yi, SHI Xiao-bin. Physicochemical properties and formation mechanism of soot during biomass burning[J]. J Fuel Chem Technol,2013,41(10):1184−1190. [19] AL-OMARI S B, KAWAJIRI K, YONESAWA T. Soot processes in a methane-fueled furnace and their impact on radiation heat transfer to furnace walls[J]. Int J Heat Mass Transf,2001,44:2567−2581. doi: 10.1016/S0017-9310(00)00288-X [20] BELTRAME A, PORSHNEV P, MERCHAN-MERCHAN W, SAVELIEV A, FRIDMAN A, KENNEDY L A, PETROVA O, ZHDANOK S, AMOURI F, CHARON O. Soot and NO formation in methane-oxygen enriched diffusion flames[J]. Combust Flame,2001,124(1):295−310. [21] SHI Y, MURR L E, SOTO K F, LEE W Y, GUERRERO P A, RAMIREZ D A. Characterization and comparison of speciated atmospheric carbonaceous particulates and their polycyclic aromatic hydrocarbon contents in the context of the paso del norte airshed along the U. S. -mexico border[J]. Polycycl Aromat Compd,2007,27(5):361−400. doi: 10.1080/10406630701624333 [22] 谢广录, 范卫东, 徐宾, 章明川. 天然气炭黑燃烧特性的热天平研究[J]. 热能动力工程,2005,5:521−526, 554−555. doi: 10.3969/j.issn.1001-2060.2005.05.018XIE Guang-lu, FAN Wei-dong, XU Bin, ZHANG Ming-chuan. Thermogravimetric study of the combustion characteristics of natural-gas soot[J]. J Eng Therm Energy Power,2005,5:521−526, 554−555. doi: 10.3969/j.issn.1001-2060.2005.05.018 [23] 范卫东, 谢广录, 徐宾, 于娟, 章明川. 氧体积分数对炭黑燃烧特性影响的热天平研究[J]. 燃料化学学报,2005,33(5):550−555.FAN Wei-dong, XIE Guang-lu, XU Bin, YU Juan, ZHANG Ming-chuan. Thermogravimetric study of the effect of oxygen concentrations on combustion characteristics of natural gas soot[J]. J Fuel Chem Technol,2005,33(5):550−555. [24] 杨冬. 柴油机颗粒氧化动力学特性研究[D]. 成都: 西华大学, 2015.YANG Dong. Investigation on the oxidation kinetics of diesel particulate[D]. Chengdu: Xihua University, 2015. [25] 唐子君, 岑超平, 方平. 城市污水污泥与煤混烧的热重试验研究[J]. 动力工程学报,2012,32(11):878−884, 897. doi: 10.3969/j.issn.1674-7607.2012.11.010TANG Zi-jun, CEN Chao-ping, FANG Ping. Thermogravimetric experiment on co-firing characteristics of coal with municipal sewage sludge[J]. J Chin Soc Power Eng,2012,32(11):878−884, 897. doi: 10.3969/j.issn.1674-7607.2012.11.010 [26] HE Q, HUANG Y, DING L, GUO Q, GONG Y, YU G. Effect of partial rapid pyrolysis on bituminous properties: From structure to reactivity[J]. Energy Fuels,2020,34(5):5476−5484. [27] 周志杰, 范晓雷, 张薇, 王辅臣, 于遵宏. 非等温热重分析研究煤焦气化动力学[J]. 煤炭学报,2006,2:219−222. doi: 10.3321/j.issn:0253-9993.2006.02.019ZHOU Zhi-jie, FAN Xiao-lei, ZHANG Wei, WANG Fu-chen, YU Zun-hong. Char gasification kinetics using non-isothermal TGA[J]. J China Coal Soc,2006,2:219−222. doi: 10.3321/j.issn:0253-9993.2006.02.019 [28] 梁斌, 冯强, 白浩隆, 武琼, 宋华, 杨晓辉, 蓝天. 煤泥干粉在流化床中燃烧特性的实验研究[J]. 煤炭学报,2018,43(z2):560−567.LIANG Bin, FENG Qiang, BAI Hao-long, WU Qiong, SONG Hua, YANG Xiao-hui, LAN tian. Combustion characteristics of dry coal slime powders in a fluidized bed[J]. J China Coal Soc,2018,43(z2):560−567. [29] DING L, ZHOU Z, GUO Q, WANG Y, YU G. In situ analysis and mechanism study of char-ash/slag transition in pulverized coal gasification[J]. Energy Fuels,2015,29(6):3532−3544. doi: 10.1021/acs.energyfuels.5b00322 [30] CHANG Q, GAO R, GAO M, YU G, MATHEWS J P, WANG F. Experimental analysis of the evolution of soot structure during CO2 gasification[J]. Fuel,2020,265:111−125. [31] YE D P, AGNEW J B, ZHANG D K. Gasification of a South Australian low-rank coal with carbon dioxide and steam: Kinetics and reactivity studies[J]. Fuel,1998,77(11):1209−1219. doi: 10.1016/S0016-2361(98)00014-3 -




 下载:
下载: