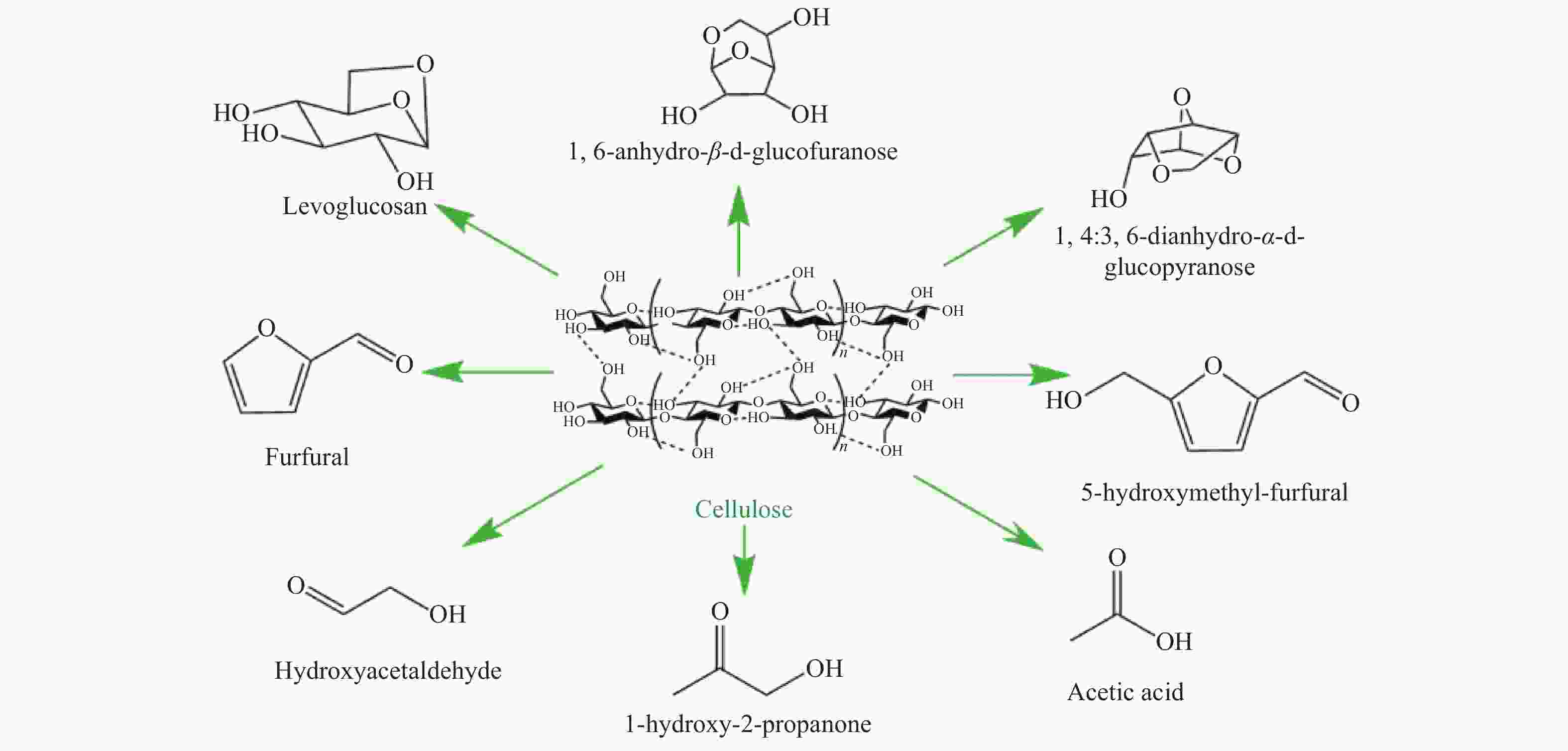
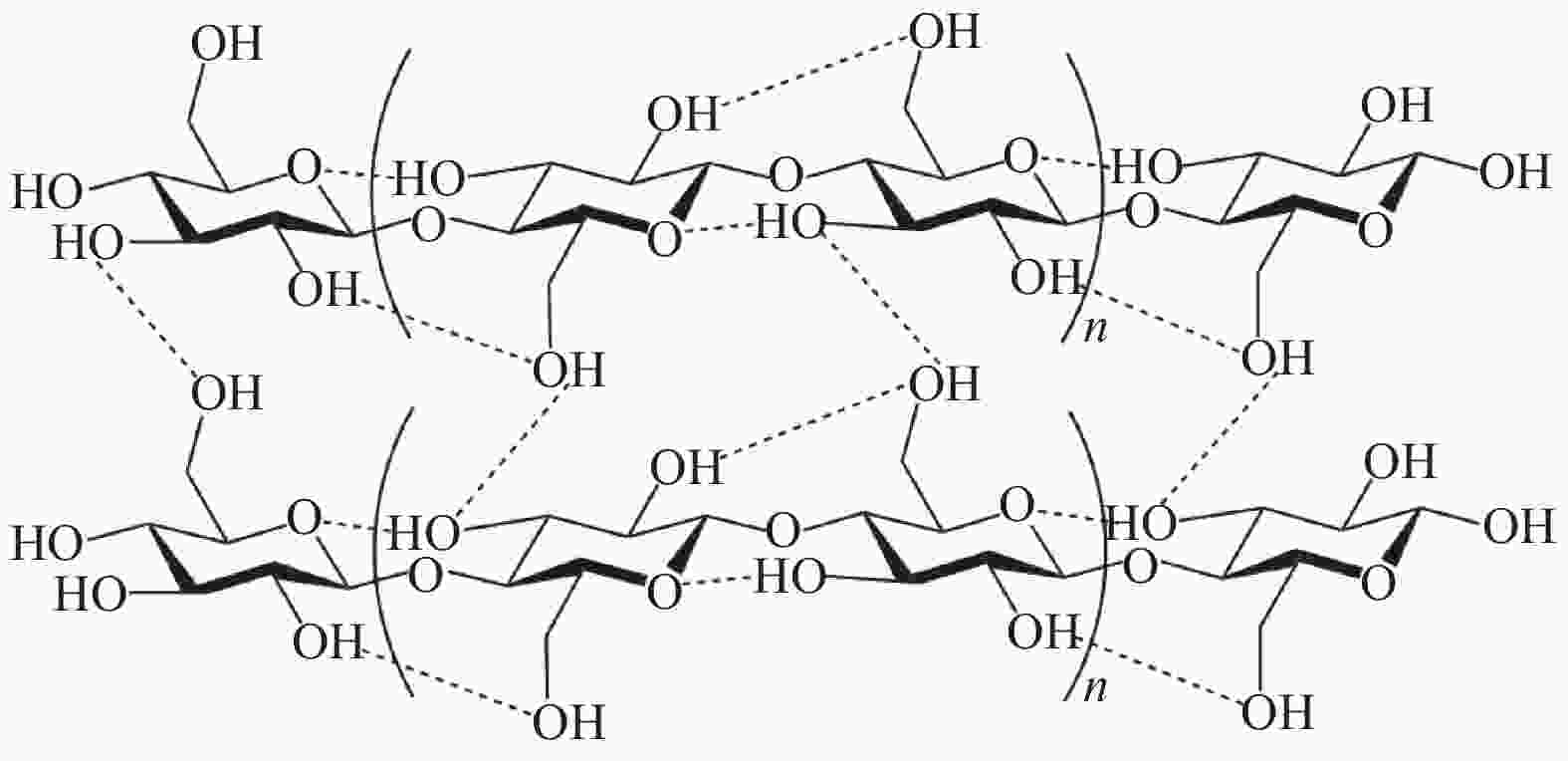
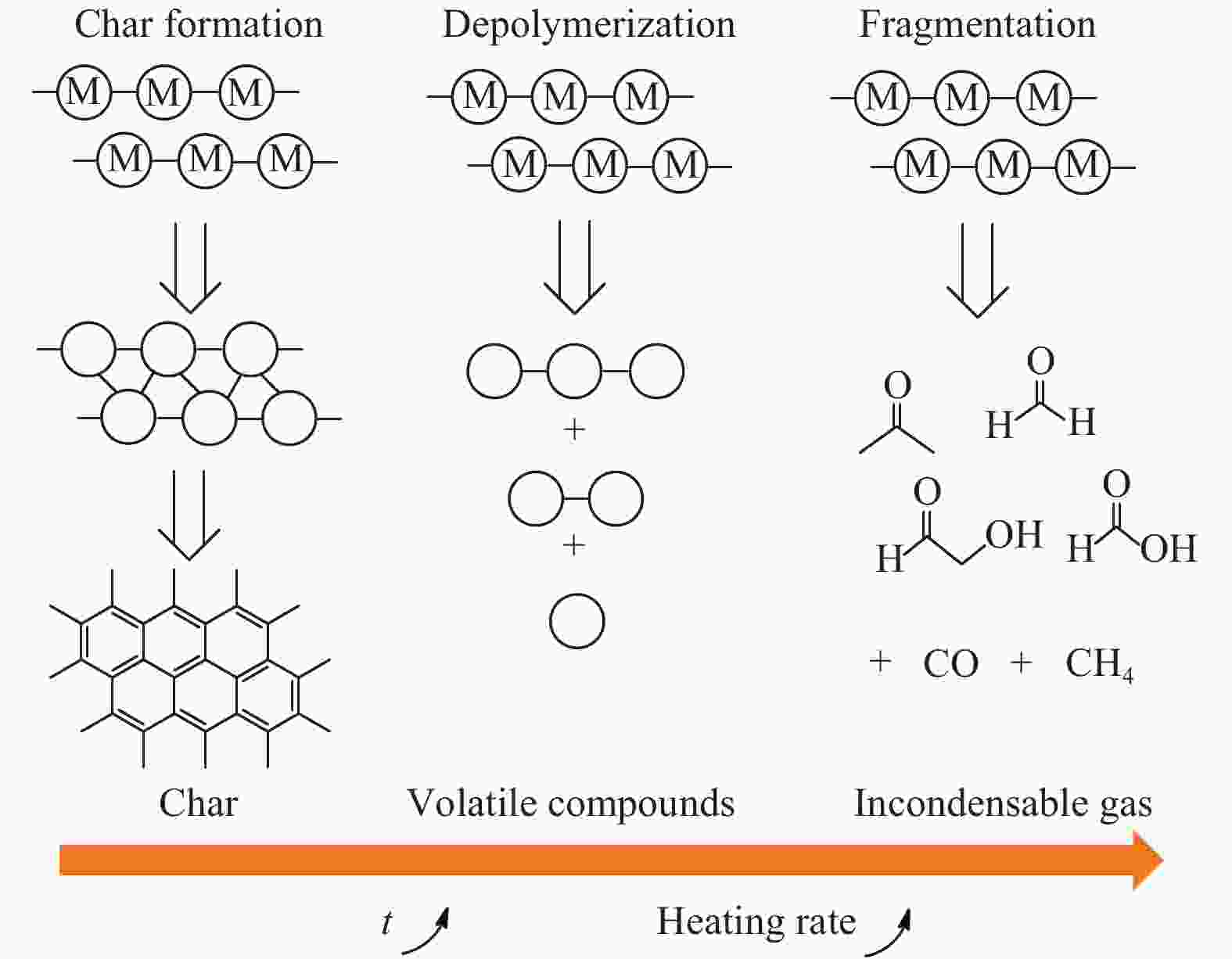
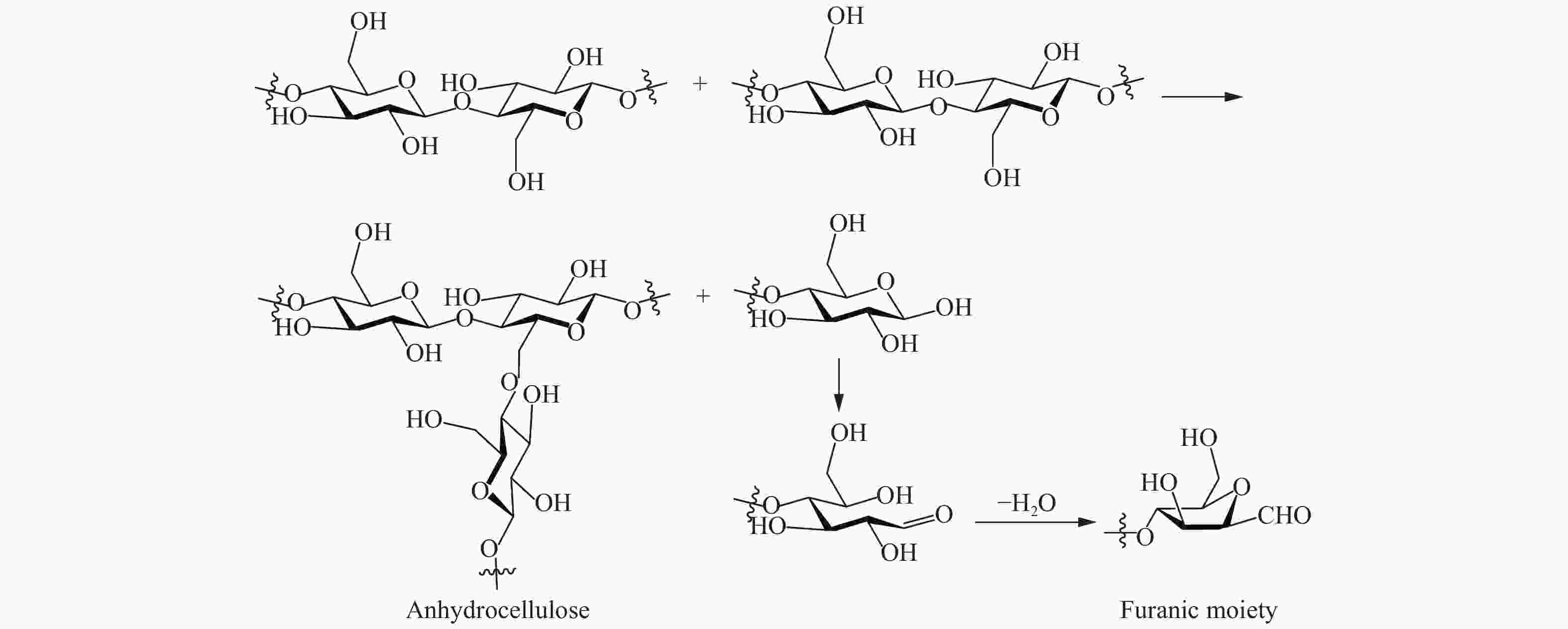
Advance on the pyrolytic transformation of cellulose
-
摘要: 纤维素的热解技术是一种非常有应用前景的高值转化技术。本综述系统地介绍了纤维素的基础特性,深入讨论了纤维素热解机制、研究方法、催化剂类型及其他影响纤维素热解产物分布的因素。其中,不同类型催化剂的添加和反应装置结构的设计优化可以显著提高纤维素热解转化效率,改善产物种类分布和提高特定高值化学品的选择性,从而有效地提高纤维素热解产物的资源、能源化利用价值。最后,对纤维素热解未来技术研究的发展方向进行了展望。Abstract: Pyrolysis technology has a great application prospect in the future. The essential properties of cellulose, pyrolysis mechanism, research tools, catalyst type and other important factors affecting the products distribution are discussed in detail. Particularly, the addition of a variety of catalysts and the optimum design of reaction device can significantly accelerate the cellulose pyrolysis, improve the product distribution and increase the selectivity of some high-value chemicals, thus effectively enhancing the resource and energy utilization value of pyrolysis products. Last but not the least, the future research orientation of cellulose pyrolysis technology is put forward based on some key questions to be solved.
-
Key words:
- cellulose /
- pyrolysis /
- bio-oil /
- catalytic upgrading
1) #共同第一作者 -
表 1 四种不同动力学方法计算出的纤维素热解表观活化能[76]
Table 1 Ea of cellulose pyrolysis calculated by different kinetic methods[76]
α Ekissinger/(kJ·mol−1) EFWO/(kJ·mol−1) EKAS/(kJ·mol−1) Estarink/(kJ·mol−1) 0.1 165 180.81 180.82 181.09 0.2 165 172.22 171.43 171.72 0.3 165 161.95 160.54 160.80 0.4 165 159.95 158.79 158.61 0.5 165 158.06 156.13 156.41 0.6 165 155.35 153.31 153.63 0.7 165 159.88 157.96 158.34 Average 165 164.03 162.71 162.94 表 2 不同类型的无机盐离子催化剂对纤维素热解产物分布的影响[87, 96]
Table 2 Effect of different types of inorganic salt ion catalysts on the distribution of cellulose pyrolysis products[87, 96]
Catalyst Temperature t/℃ Yield w/% char gas tar compounds Blank 500 5.35 7.07 79.6 LG 59% 1.0%NaCl 500 9.77 23.45 61.55 LG 16% 1.0%KCl 500 18.37 28.72 51.97 LG 12% 1.0%MgCl2 500 13.66 33.65 63.59 LG 21% 1.0%CaCl2 500 12.67 40.12 49.15 LG 18% 1.0%Ca(OH)2 500 − − − LG 30% 1.0%Ca(NO3)2 500 − − − LG 23% 1.0%CaCO3 500 − − − LG 41% 1.0%CaHPO4 500 − − − LG 38% Blank 600 13.2 45.7 41.1 − 0.26 mmol/g Fe(NO3)3 600 16.9 51.6 31.5 − 0.26 mmol/g Ni(NO3)2 600 15.2 50.7 34.1 − 8.3 mg/g Fe2(SO4)3 500 − − − LG 3.2%, LGO 40.7% 95.6 mg/g Fe2(SO4)3 500 − − − LG 44.8%, LGO 1.7% 表 3 不同类型的金属氧化物对纤维素热解产物分布的影响[1, 105]
Table 3 Effects of various metal oxide catalysts on the product distribution for cellulose pyrolysis[1, 105]
Catalyst Temperature t/℃ Yield w/% char gas tar compounds SiO2 500 22.89 18.35 58.76 aromatics 0.1%, CO 6.5%,
heavy oil 11.7%SiO2-Al2O3 500 29.62 25.03 45.39 aromatics 0.7%, CO 10.5%,
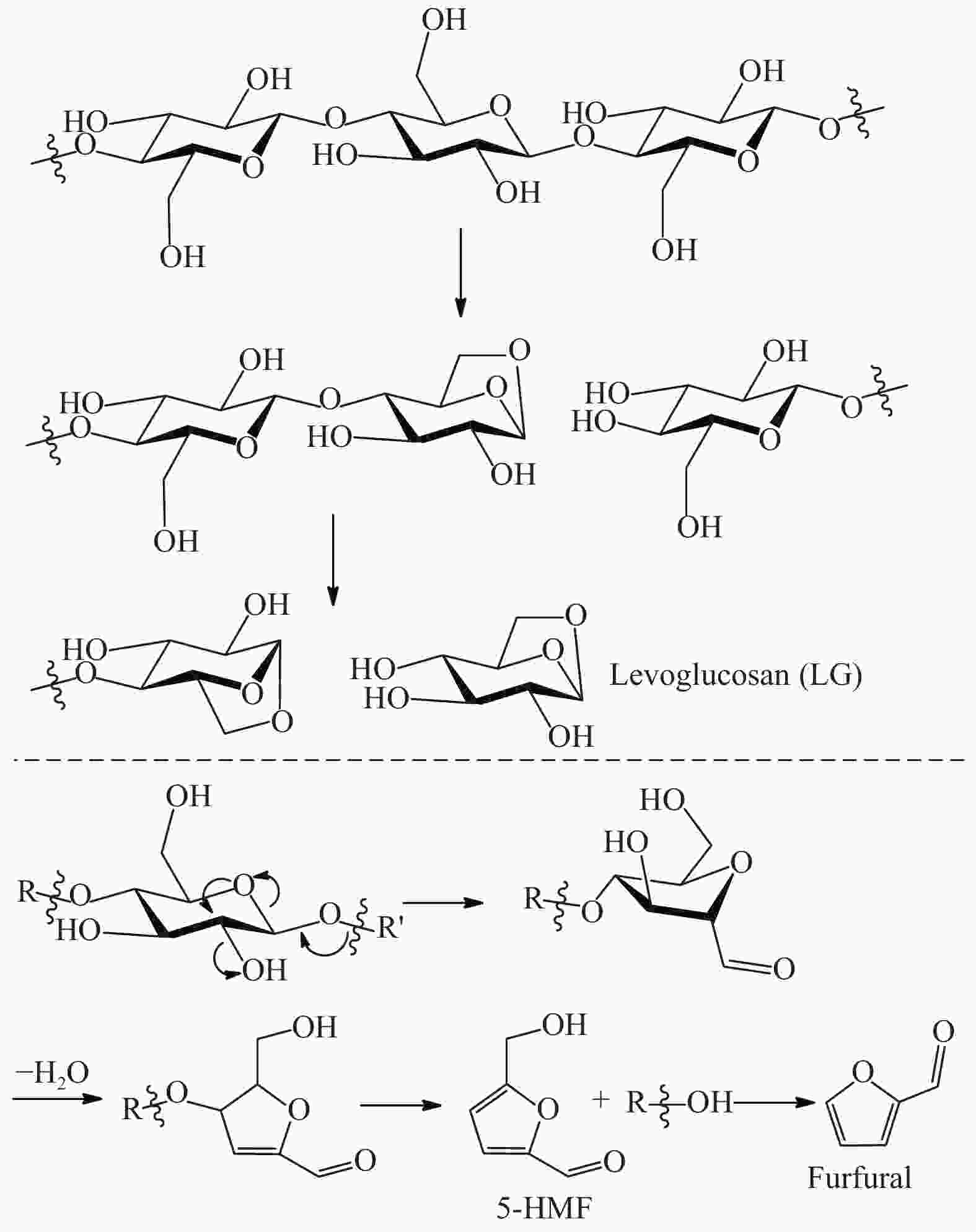
heavy oil 8.0%Al2O3 500 33.85 28.23 37.39 aromatics 10.8%, CO 11.1%,
heavy oil 0.0MgO 500 27.50 28.30 44.23 aromatics 0.6%, CO 9.6%,
heavy oil 13.5%NiO 500 24.84 27.73 47.46 aromatics 0.1%, CO 8.9%,
heavy oil 9.8%TiO2 500 24.71 18.10 57.19 aromatics 0.0, CO 7.0%,
heavy oil 11.3%ZrO2 500 23.52 20.32 56.11 aromatics 0.9%, CO 7.5%,
heavy oil 13.1%TiO2-ZrO2 500 28.17 29.61 42.22 aromatics 7.3%, CO 10.1%,
heavy oil 18.3%Blank 500 − − − LG 10.5%, LGO 3.7% Nano Al2O3 500 − − − LG 9.1%, LGO 7.7% Nano MgO 500 − − − LG 11.0%, LGO 2.2% Nano SiO2 500 − − − LG 2.8%, LGO 0.2% Nano TiSiO4 500 − − − LG 7.0%, LGO 11.0% Nano TiO2-Al2O3 500 − − − LG 2.3%, LGO 19.0% TiO2-Al2O3 500 − − − LG 10.5%, LGO 3.13% 表 4 不同类型的沸石分子筛催化剂对纤维素热解产物分布的影响[121, 122, 125]
Table 4 Effect of different types of zeolite catalysts on the distribution of cellulose pyrolysis products[121, 122, 125]
Catalyst Temperature t/℃ Yield w/% char gas tar compounds Blank 450 24 19 57 furans 9.2%, hydrocarbons 12%, acids 4.5%,
aromatics 1.94%,
aldehydes 5.43%, ketones 2.48%H-ZSM-5 450 23 20 57 furans 2.2%, hydrocarbons 29%, acids 0.5% Mg-ZSM-5 450 20 21 59 furans 2.4%, hydrocarbons 29%, acids 0.8% Ni-ZSM-5 450 25 18 57 furans 2.5%, hydrocarbons 35%, acids 0.4% Cu-ZSM-5 450 27 17 56 furans 2.6%, hydrocarbons 31%, acids 0.4% Ga-ZSM-5 450 22 19 59 furans 2.4%, hydrocarbons 34%, acids 0.6% Sn-ZSM-5 450 24 20 56 furans 2.7%, hydrocarbons 33%, acids 0.4% Hierarchical H-ZSM-5 450 22 21 57 aromatics 8.97%, aldehydes 0.22%,
ketones 2.48%Hierarchical Mg-ZSM-5 450 21 19 60 aromatics 6.92%, aldehydes 0.27%,
ketones 3.02%Hierarchical Ni-ZSM-5 450 21 20 59 aromatics 7.65%, aldehydes 0.26%,
ketones 2.63%Hierarchical Cu-ZSM-5 450 18 22 60 aromatics 7.00%, aldehydes 0.39%,
ketones 2.73%Hierarchical Sn-ZSM-5 450 24 20 56 aromatics 7.98%, aldehydes 0.29%,
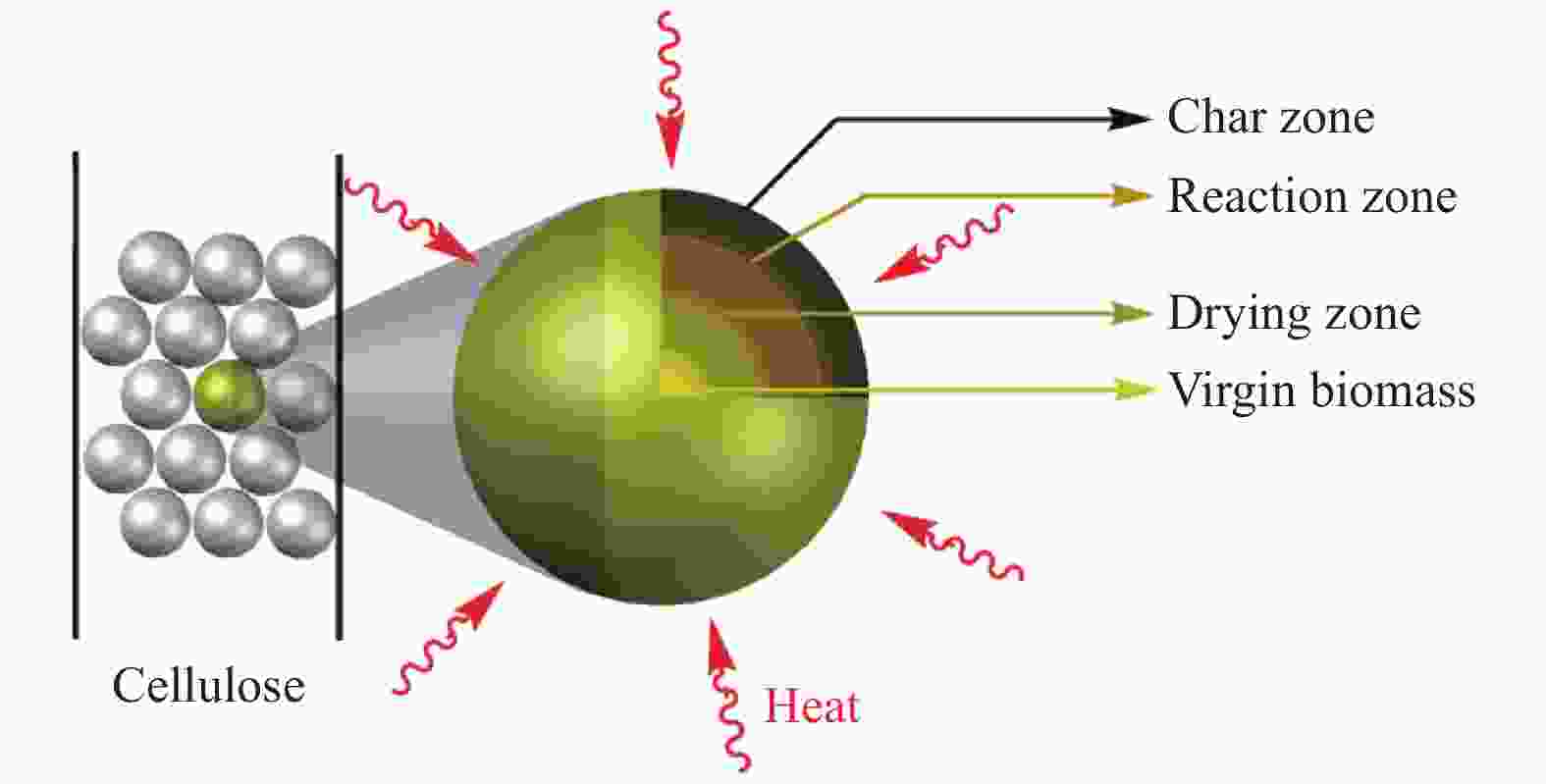
ketones 2.36%Blank 550 20 28 52 monocyclic aromatics 1.46%,
polycyclic aromatics 1.22%H-ZSM-5 550 24 31 45 monocyclic aromatics 13.84%,
polycyclic aromatics 15.10%Fe-ZSM-5 550 25 34 41 monocyclic aromatics 17.25%,
polycyclic aromatics 22.36%Zr-ZSM-5 550 24 33 43 monocyclic aromatics 23.96%,
polycyclic aromatics 17.84%Co-ZSM-5 550 26 36 38 monocyclic aromatics 10.62%,
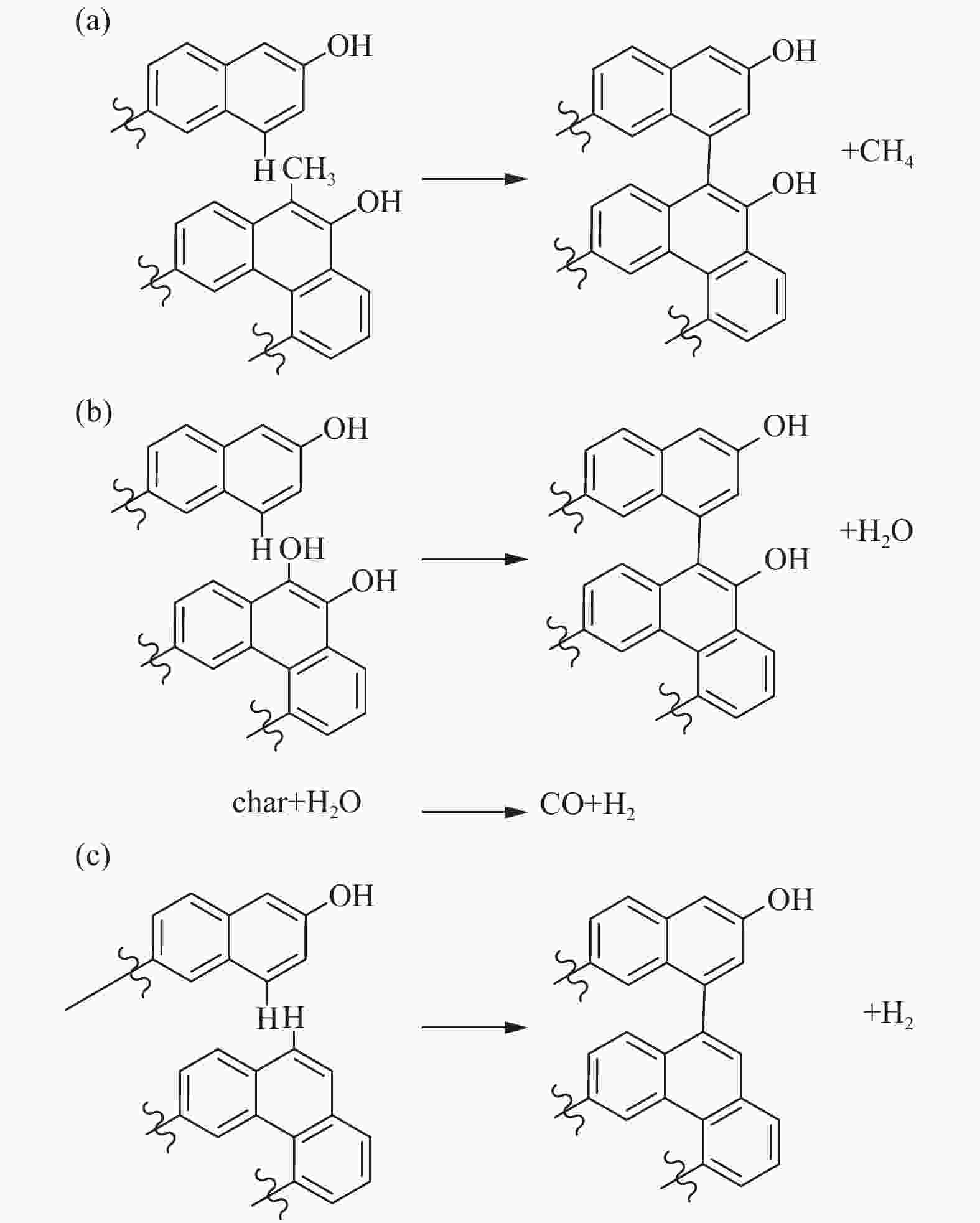
polycyclic aromatics 11.67% -
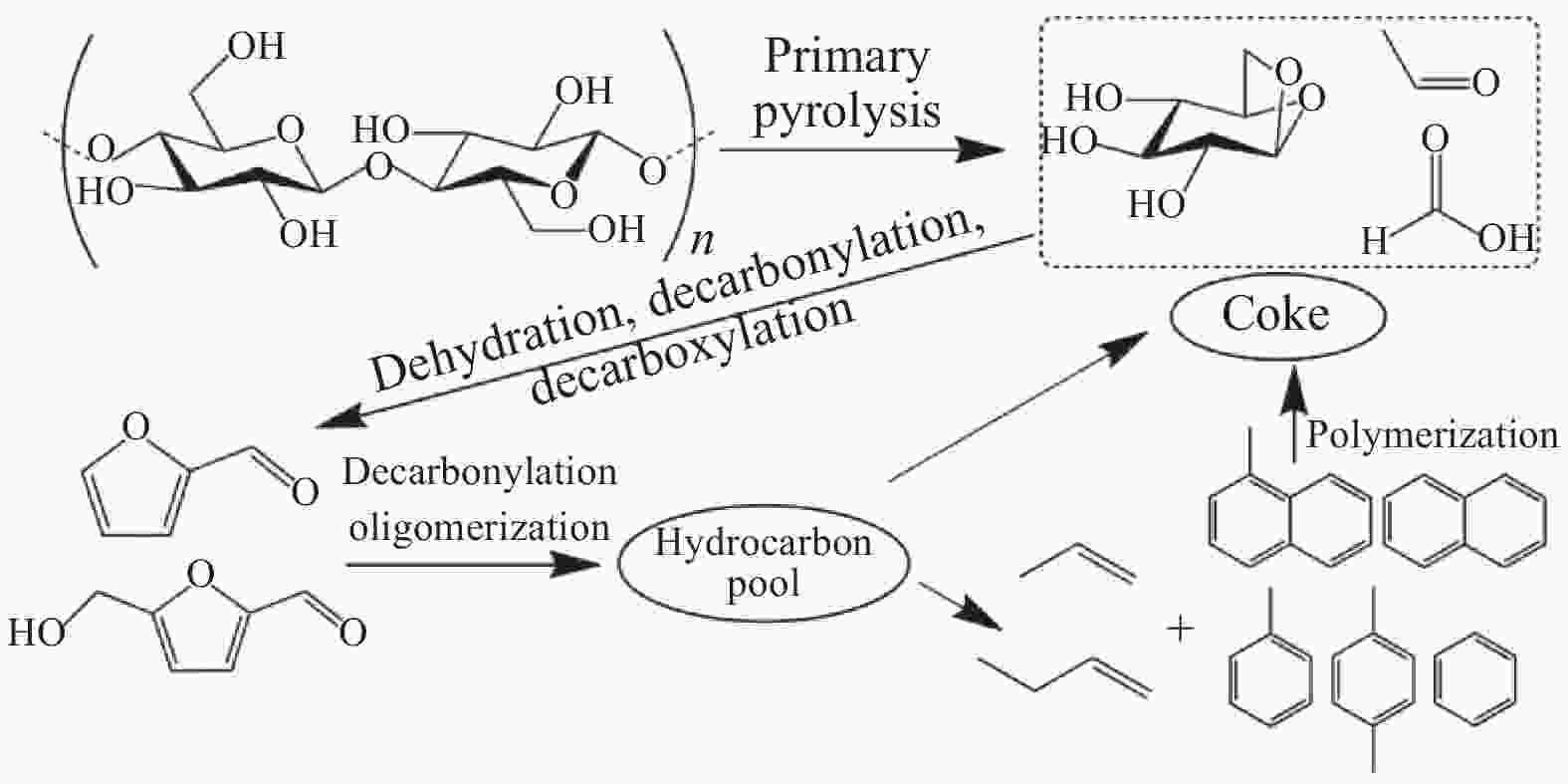
[1] STEFANIDIS S D, KALOGIANNIS K G, ILIOPOULOU E F, LAPPAS A A, PILAVACHI P A. In-situ upgrading of biomass pyrolysis vapors: Catalyst screening on a fixed bed reactor[J]. Bioresour Technol,2011,102(17):8261−8267. doi: 10.1016/j.biortech.2011.06.032 [2] SHELDON R A. Green and sustainable manufacture of chemicals from biomass: State of the art[J]. Green Chem,2014,16(3):95−963. [3] WANG S R, DAI G X, YANG H P, LUO Z Y. Lignocellulosic biomass pyrolysis mechanism: A state-of-the-art review[J]. Prog Energy Combust,2017,62:33−86. doi: 10.1016/j.pecs.2017.05.004 [4] GOYAL H B, SEAL D, SAXENA R C. Bio-fuels from thermochemical conversion of renewable resources: A review[J]. Renewable Sustainable Energy Rev,2008,12(2):504−517. doi: 10.1016/j.rser.2006.07.014 [5] LIMAYEM A, RICKE S C. Lignocellulosic biomass for bioethanol production: Current perspectives, potential issues and future prospects[J]. Prog Energy Combust,2012,38(4):449−467. doi: 10.1016/j.pecs.2012.03.002 [6] KWIETNIEWSKA E, TYS J. Process characteristics, inhibition factors and methane yields of anaerobic digestion process, with particular focus on microalgal biomass fermentation[J]. Renewable Sustainable Energy Rev,2014,34:491−500. doi: 10.1016/j.rser.2014.03.041 [7] BRIDGWATER A V. Principles and practice of biomass fast pyrolysis processes for liquids[J]. J Anal Appl Pyrolysisysis,1999,51(1):3−22. [8] NOMURA T, MIZUNO H, MINAMI E, KAWAMOTO H. Fast pyrolysis of cellulose by infrared heating[J]. Energies (Basel),2021,14(7):1842. doi: 10.3390/en14071842 [9] LU Q, YANG X C, DONG C Q, ZHANG Z F, ZHANG X M, ZHU X F. Influence of pyrolysis temperature and time on the cellulose fast pyrolysis products: Analytical Py-GC/MS study[J]. J Anal Appl Pyrolysis,2011,92(2):430−438. doi: 10.1016/j.jaap.2011.08.006 [10] WANG Y, FAN M, ZHU L, ZHANG Z F, ZHANG X M, ZHU X F. Enhancement of bio-based para-xylene selectivity in catalytic fast pyrolysis of cellulose using a surface-modified Mg/P/HZSM-5 catalyst[J]. Chem Res Chin Univ,2019,35(3):449−456. doi: 10.1007/s40242-019-9024-6 [11] ŞERBĂNESCU C. Kinetic analysis of cellulose pyrolysis: a short review[J]. Chem Pap, 2014, 68(7). [12] MALIEKKAL V, DAUENHAUER P J, NEUROCK M. Glycosidic C–O bond activation in cellulose pyrolysis: Alpha versus beta and condensed phase hydroxyl-catalytic scission[J]. ACS Catal,2020,10(15):8454−8464. doi: 10.1021/acscatal.0c02133 [13] HUBER G W, IBORRA S, CORMA A. Synthesis of transportation fuels from biomass: chemistry, catalysts, and engineering[J]. Chem Rev,2006,106(9):4044−4098. doi: 10.1021/cr068360d [14] ZHANG H Y, XIAO R, WANG D H, HE G Y, SHAO S S, ZHANG J B, ZHONG Z P. Biomass fast pyrolysis in a fluidized bed reactor under N2, CO2, CO, CH4 and H2 atmospheres[J]. Bioresour Technol,2011,102(5):4258−4264. doi: 10.1016/j.biortech.2010.12.075 [15] ZHENG J, WEI Q. Improving the quality of fast pyrolysis bio-oil by reduced pressure distillation[J]. Biomass Bioenergy,2011,35(5):1804−1810. doi: 10.1016/j.biombioe.2011.01.006 [16] MERCADER F D M, GROENEVELD M J, KERSTEN S R A, VENDERBOSCH R H, HOGENDOORN J A. Pyrolysis oil upgrading by high pressure thermal treatment[J]. Fuel,2010,89(10):2829−2837. doi: 10.1016/j.fuel.2010.01.026 [17] OASMAA A, KUOPPALA E, SELIN J F, GUST S, SOLANTAUSTA Y. Fast pyrolysis of forestry residue and pine. 4. Improvement of the product quality by solvent addition[J]. Energy Fuels,2004,18(5):1578−1583. doi: 10.1021/ef040038n [18] KAWAMOTO H. Review of reactions and molecular mechanisms in cellulose pyrolysis[J]. Curr Org Chem,2016,20(23):2444−2457. [19] ZHU Y F, CHEN J L, LI W B, WANG D C, LI S R, ZHENG Z F. A new method for long-chain alkanes under a condition without extra hydrogen source: Catalytic upgrading of cellulose pyrolysis vapors over Au/TS-1 catalyst[J]. J Anal Appl Pyrolysisysis,2020,151:104906. doi: 10.1016/j.jaap.2020.104906 [20] HABIBI Y, LUCIA L A, ROJAS O J. Cellulose Nanocrystals: Chemistry, self-assembly, and applications[J]. Chem Rev,2010,110(6):3479−3500. doi: 10.1021/cr900339w [21] ROWELL R M. Handbook of wood chemistry and wood composites[M]. Boca Raton: CRC Press, 2012. [22] POLETTO M, ORNAGHI H L, ZATTERA A J. Native Cellulose: Structure, characterization and thermal properties[J]. Materials (Basel),2014,7(9):6105−6119. doi: 10.3390/ma7096105 [23] CHEN X L, YU J, ZHANG Z B, LU C H. Study on structure and thermal stability properties of cellulose fibers from rice straw[J]. Carbohyd Polym,2011,85(1):245−250. doi: 10.1016/j.carbpol.2011.02.022 [24] MAZEAU K, HEUX L. Molecular dynamics simulations of bulk native crystalline and amorphous structures of cellulose[J]. J Phys Chem B,2003,107(10):2394−2403. doi: 10.1021/jp0219395 [25] KIM U, EOM S H, WADA M. Thermal decomposition of native cellulose: Influence on crystallite size[J]. Polym Degrad Stabil,2010,95(5):778−781. doi: 10.1016/j.polymdegradstab.2010.02.009 [26] PARK S, BAKER J O, HIMMEL M E, PARILLA P A, JOHNSON D K. Cellulose crystallinity index: measurement techniques and their impact on interpreting cellulase performance[J]. Biotechnol Biofuels,2010,3:10. doi: 10.1186/1754-6834-3-10 [27] VANDERHART D L, ATALLA R H. Studies of microstructure in native celluloses using solid-state carbon-13 NMR[J]. Macromolecules,1984,17(8):1465−1472. doi: 10.1021/ma00138a009 [28] 吴祺祺. 纤维素Ⅲ型纳米晶体的制备、表征及其结晶结构稳定性研究[D]. 广州: 华南理工大学, 2020.WU Qi-qi. Study on preparation, characterizations and crystalline stability of cellulose III nanocrystals[D]. Guangzhou: South China University of Technology, 2020. [29] 裴继诚. 植物纤维化学[M]. 第4版. 北京: 中国轻工业出版社, 2014: 12–20.PEI Ji-cheng. Lignocellulosic Chemistry[M]. Beijing:China Light Industry Press, 2014: 12–20. [30] MUKARAKATE C, MITTAL A, CIESIELSKI P N, BUDHI S, THOMPSON L, IISA K, NIMLOS M R, DONOHOE B S. Influence of crystal allomorph and crystallinity on the products and behavior of cellulose during fast pyrolysis[J]. ACS Sustainable Chem Eng,2016,4(9):4662−4674. doi: 10.1021/acssuschemeng.6b00812 [31] ZHANG J X, LUO J, TONG D M, ZHU L F, DONG L L, HU C W. The dependence of pyrolysis behavior on the crystal state of cellulose[J]. Carbohyd Polym,2010,79(1):164−169. doi: 10.1016/j.carbpol.2009.07.038 [32] LENG E, FERREIRO A I, LIU T, GONG X, COSTA M, LI X, XU M. Experimental and kinetic modelling investigation on the effects of crystallinity on cellulose pyrolysis[J]. J Anal Appl Pyrolysis,2020,152:104863. doi: 10.1016/j.jaap.2020.104863 [33] VAN DE VELDEN M, BAEYENS J, BREMS A, JANSSENS B, DEWIL R. Fundamentals, kinetics and endothermicity of the biomass pyrolysis reaction[J]. Renewable Energy,2010,35(1):232−242. doi: 10.1016/j.renene.2009.04.019 [34] COLLARD F, BLIN J. A review on pyrolysis of biomass constituents: Mechanisms and composition of the products obtained from the conversion of cellulose, hemicelluloses and lignin[J]. Renewable Sustainable Energy Rev,2014,38:594−608. doi: 10.1016/j.rser.2014.06.013 [35] WOOTEN J B, SEEMAN J I, HAJALIGOL M R. Observation and characterization of cellulose pyrolysis intermediates by 13C CPMAS NMR. A new mechanistic model[J]. Energy Fuels,2004,18(1):1−15. doi: 10.1021/ef0300601 [36] MAMLEEV V, BOURBIGOT S, LE BRAS M, YVON J. The facts and hypotheses relating to the phenomenological model of cellulose pyrolysis: Interdependence of the steps[J]. J Anal Appl Pyrolysis,2009,84(1):1−17. doi: 10.1016/j.jaap.2008.10.014 [37] COLLARD F, BLIN J, BENSAKHRIA A, VALETTE J. Influence of impregnated metal on the pyrolysis conversion of biomass constituents[J]. J Anal Appl Pyrolysis,2012,95:213−226. doi: 10.1016/j.jaap.2012.02.009 [38] MCGRATH T E, CHAN W G, HAJALIGOL M R. Low temperature mechanism for the formation of polycyclic aromatic hydrocarbons from the pyrolysis of cellulose[J]. J Anal Appl Pyrolysis, 2003, 66(1–2): 51–70. [39] LÓPEZ M C B, BLANCO C G, MARTÍNEZ-ALONSO A, TASCON J M D. Composition of gases released during olive stones pyrolysis[J]. J Anal Appl Pyrolysis,2002,65(2):313−322. doi: 10.1016/S0165-2370(02)00008-6 [40] MORF P, HASLER P, NUSSBAUMER T. Mechanisms and kinetics of homogeneous secondary reactions of tar from continuous pyrolysis of wood chips[J]. Fuel,2002,81(7):843−853. doi: 10.1016/S0016-2361(01)00216-2 [41] ANCA-COUCE A. Reaction mechanisms and multi-scale modelling of lignocellulosic biomass pyrolysis[J]. Prog Energy Combust,2016,53:41−79. doi: 10.1016/j.pecs.2015.10.002 [42] KILZER F J, BROIDO A. Speculations on the nature of cellulose pyrolysis[J]. Pyrodynamics.,1965,2:151−163. [43] SHAFIZADEH F, BRADBURY A. Thermal degradation of cellulose in air and nitrogen at low temperatures[J]. J Appl Polym Sci,1979,23(5):1431−1442. doi: 10.1002/app.1979.070230513 [44] PISKORZ J, RADLEIN D S A, SCOTT D S, CZERNIK S. Pretreatment of wood and cellulose for production of sugars by fast pyrolysis[J]. J Anal Appl Pyrolysis,1989,16(2):127−142. doi: 10.1016/0165-2370(89)85012-0 [45] BANYASZ J L, LI S, LYONS-HART J L, SHAFER K H. Gas evolution and the mechanism of cellulose pyrolysis[J]. Fuel,2001,80(12):1757−1763. doi: 10.1016/S0016-2361(01)00060-6 [46] BANYASZ J L, LI S, LYONS-HART J L, SHAFER K H. Cellulose pyrolysis: the kinetics of hydroxyacetaldehyde evolution[J]. J Anal Appl Pyrolysis,2001,57(2):223−248. doi: 10.1016/S0165-2370(00)00135-2 [47] SCHEIRS J, CAMINO G, TUMIATTI W. Overview of water evolution during the thermal degradation of cellulose[J]. Eur Polym J,2001,37(5):933−942. doi: 10.1016/S0014-3057(00)00211-1 [48] WORASUWANNARAK N, SONOBE T, TANTHAPANICHAKOON W. Pyrolysis behaviors of rice straw, rice husk, and corncob by TG-MS technique[J]. J Anal Appl Pyrolysis,2007,78(2):265−271. doi: 10.1016/j.jaap.2006.08.002 [49] PATWARDHAN P R, DALLUGE D L, SHANKS B H, BROWN R C. Distinguishing primary and secondary reactions of cellulose pyrolysis[J]. Bioresour Technol,2011,102(8):5265−5269. doi: 10.1016/j.biortech.2011.02.018 [50] SHEN D K, GU S. The mechanism for thermal decomposition of cellulose and its main products[J]. Bioresour Technol,2009,100(24):6496−6504. doi: 10.1016/j.biortech.2009.06.095 [51] YANG H P, YAN R, CHEN H P, LEE D H, ZHENG C G. Characteristics of hemicellulose, cellulose and lignin pyrolysis[J]. Fuel,2007,86(12/13):1781−1788. [52] PASTOROVA I, BOTTO R E, ARISZ P W, BOON J J. Cellulose char structure: A combined analytical Py-GC-MS, FTIR, and NMR study[J]. Carbohyd Res,1994,262(1):27−47. doi: 10.1016/0008-6215(94)84003-2 [53] ZHANG C T, CHAO L, ZHANG Z M, ZHANG L J, LI Q Y, FAN H L, ZHANG S, LIU Q, QIAO Y Y, TIAN Y Y, WANG Y, HU X. Pyrolysis of cellulose: Evolution of functionalities and structure of bio-char versus temperature[J]. Renewable Sustainable Energy Rev,2021,135:110416. doi: 10.1016/j.rser.2020.110416 [54] JENSEN A, DAM-JOHANSEN K, WÓJTOWICZ M A, SERIO M A. TG-FTIR study of the influence of potassium chloride on wheat straw pyrolysis[J]. Energy Fuels,1998,12(5):929−938. doi: 10.1021/ef980008i [55] BAUMLIN S, BROUST F, FERRER M, MEUNIER N, MARTY E, LEDE J. The continuous self stirred tank reactor: Measurement of the cracking kinetics of biomass pyrolysis vapours[J]. Chem Eng Sci,2005,60(1):41−55. doi: 10.1016/j.ces.2004.07.057 [56] NEVES D, THUNMAN H, MATOS A, TARELHO L, GOMEZ-BAREA A. Characterization and prediction of biomass pyrolysis products[J]. Prog Energy Combust,2011,37(5):611−630. doi: 10.1016/j.pecs.2011.01.001 [57] SHEN J, WANG X S, GARCIA-PEREZ M, MOURANT D, RHODES M J, LI C Z. Effects of particle size on the fast pyrolysis of oil mallee woody biomass[J]. Fuel,2009,88(10):1810−1817. doi: 10.1016/j.fuel.2009.05.001 [58] PAULSEN A D, HOUGH B R, WILLIAMS C L, TEIXEIRA A R, SCHWARTZ D T, PFAENDTNER J, DAUENHAUER P J. Fast pyrolysis of wood for biofuels: spatiotemporally resolved diffuse reflectance in situ spectroscopy of particles[J]. ChemSusChem,2014,7(3):765−776. doi: 10.1002/cssc.201301056 [59] PATWARDHAN P R, BROWN R C, SHANKS B H. Understanding the fast pyrolysis of lignin[J]. ChemSusChem,2011,4(11):1629−1636. doi: 10.1002/cssc.201100133 [60] BIAGINI E, BARONTINI F, TOGNOTTI L. Devolatilization of biomass fuels and biomass components studied by TG/FTIR technique[J]. Ind Eng Chem Res,2006,45(13):4486−4493. doi: 10.1021/ie0514049 [61] LV G, WU S. Analytical pyrolysis studies of corn stalk and its three main components by TG-MS and Py-GC/MS[J]. J Anal Appl Pyrolysis,2012,97:11−18. doi: 10.1016/j.jaap.2012.04.010 [62] SANCHEZ-SILVA L, LÓPEZ-GONZÁLEZ D, VILLASEÑOR J, SANCHEZ P, VALVERDE J L. Thermogravimetric-mass spectrometric analysis of lignocellulosic and marine biomass pyrolysis[J]. Bioresour Technol,2012,109:163−172. doi: 10.1016/j.biortech.2012.01.001 [63] HUANG Y F, KUAN W H, CHIUEH P T, LO S L. Pyrolysis of biomass by thermal analysis-mass spectrometry (TA-MS)[J]. Bioresour Technol,2011,102(3):3527−3534. doi: 10.1016/j.biortech.2010.11.049 [64] CHEN T, ZHANG J, WU J. Kinetic and energy production analysis of pyrolysis of lignocellulosic biomass using a three-parallel Gaussian reaction model[J]. Bioresour Technol,2016,211:502−508. doi: 10.1016/j.biortech.2016.03.091 [65] SIENGCHUM T, ISENBERG M, CHUANG S S. Fast pyrolysis of coconut biomass-An FTIR study[J]. Fuel,2013,105:559−565. doi: 10.1016/j.fuel.2012.09.039 [66] ZICKLER G A, WAGERMAIER W, FUNARI S S, BURGHAMMER M, PARIS O. In situ X-ray diffraction investigation of thermal decomposition of wood cellulose[J]. J Anal Appl Pyrolysis,2007,80(1):134−140. doi: 10.1016/j.jaap.2007.01.011 [67] DUFOUR A, CASTRO-DÍAZ M, MARCHAL P, BROSSE N, OLCESE R, BOUROUKBA M, SNAPE C. In situ analysis of biomass pyrolysis by high temperature rheology in relations with 1H NMR[J]. Energy Fuels,2012,26(10):6432−6441. doi: 10.1021/ef301310x [68] NODA I, DOWREY A E, MARCOTT C, STORY G M, OZAKI Y. Generalized two-dimensional correlation spectroscopy[J]. Appl Spectr,2000,54(7):236A−248A. doi: 10.1366/0003702001950454 [69] LÓPEZ-BECEIRO J, DÍAZ-DÍAZ A M, ÁLVAREZ-GARCÍA A, TARRIO-SAAVEDRA J, NAYA S, ARTIAGA R. A Logistic approach for kinetics of isothermal pyrolysis of cellulose[J]. Processes,2021,9(3):551. doi: 10.3390/pr9030551 [70] KOSTETSKYY P, BROADBELT L J. Progress in modeling of biomass fast pyrolysis: A review[J]. Energy Fuels,2020,34(12):15195−15216. doi: 10.1021/acs.energyfuels.0c02295 [71] KISSINGER H E. Reaction kinetics in differential thermal analysis[J]. Anal Chem,1957,29(11):1702−1706. doi: 10.1021/ac60131a045 [72] OZAWA T. A new method of analyzing thermogravimetric data[J]. Bull Agric Chem Soc Jpn,1965,38(11):1881−1886. doi: 10.1246/bcsj.38.1881 [73] FLYNN J H, WALL L A. A quick, direct method for the determination of activation energy from thermogravimetric data[J]. J Polym Sci Pol Phys,1966,4(5):323−328. [74] FRIEDMAN H L. Kinetics of thermal degradation of char-forming plastics from thermogravimetry. Application to a phenolic plastic[J]. J Polym Sci Pol Symp,1964,6(1):183−195. [75] STARINK M J. The determination of activation energy from linear heating rate experiments: a comparison of the accuracy of isoconversion methods[J]. Thermochim Acta,2003,404(1):163−176. [76] KHAN A S, MAN Z, BUSTAM MA, KAIT CF, KHAN M I, MUHAMMAD N, NASRULLAH A, ULLAH Z, AHMAD P. Impact of ball-milling pretreatment on pyrolysis behavior and kinetics of crystalline cellulose[J]. Waste Biomass Valori,2016,7(3):571−581. doi: 10.1007/s12649-015-9460-6 [77] LU Q, YE X N, ZHANG Z B, DONG C Q, ZHANG Y. Catalytic fast pyrolysis of cellulose and biomass to produce levoglucosenone using magnetic SO42−/TiO2-Fe3O4[J]. Bioresour Technol,2014,171:10−15. doi: 10.1016/j.biortech.2014.08.075 [78] BOZELL J J, PETERSEN G R. Technology development for the production of biobased products from biorefinery carbohydrates-the US Department of Energy's Top 10 revisited[J]. Green Chem,2010,12(4):539−554. doi: 10.1039/b922014c [79] DICKERSON T, SORIA J. Catalytic fast pyrolysis: a review[J]. Energies,2013,6(1):514−538. doi: 10.3390/en6010514 [80] REZAEI P S, SHAFAGHAT H, WAN M A W D. Production of green aromatics and olefins by catalytic cracking of oxygenate compounds derived from biomass pyrolysis: A review[J]. Appl Catal A: Gen,2014,469:490−511. doi: 10.1016/j.apcata.2013.09.036 [81] BJØRGEN M, SVELLE S, JOENSEN F, NERLOV J, KOLBOE S, BONINO F, PALUMBO L, BORDIGA S, OLSBYE U. Conversion of methanol to hydrocarbons over zeolite H-ZSM-5: On the origin of the olefinic species[J]. J Catal,2007,249(2):195−207. doi: 10.1016/j.jcat.2007.04.006 [82] LI J Z, WEI Y X, LIU G Y, QI Y, TIAN P, LI B, HE Y L, LIU Z M. Comparative study of MTO conversion over SAPO-34, H-ZSM-5 and H-ZSM-22: Correlating catalytic performance and reaction mechanism to zeolite topology[J]. Catal Today,2011,171(1):221−228. doi: 10.1016/j.cattod.2011.02.027 [83] STEFANIDIS S D, KARAKOULIA S A, KALOGIANNIS K G, ILIOPOULOU E F, DELIMITIS A, YIANNOULAKIS H, ZAMPETAKIS T, LAPPAS A A, TRIANTAFYLLIDIS K S. Natural magnesium oxide (MgO) catalysts: A cost-effective sustainable alternative to acid zeolites for the in situ upgrading of biomass fast pyrolysis oil[J]. Appl Catal B:Environ,2016,196:155−173. doi: 10.1016/j.apcatb.2016.05.031 [84] PACCHIONI G. Ketonization of carboxylic acids in biomass conversion over TiO2 and ZrO2 surfaces: A DFT perspective[J]. ACS Catal,2014,4(9):2874−2888. doi: 10.1021/cs500791w [85] NOLTE M W, SHANKS B H. A perspective on catalytic strategies for deoxygenation in biomass pyrolysis[J]. Energy Technol Ger,2017,5(1):7−18. doi: 10.1002/ente.201600096 [86] BU Q, LEI H, ZACHER AH, WANG L, REN S, LIANG J, WEI Y, LIU Y P, TANG J, ZHANG Q, RUAN R. A review of catalytic hydrodeoxygenation of lignin-derived phenols from biomass pyrolysis[J]. Bioresour Technol,2012,124:470−477. doi: 10.1016/j.biortech.2012.08.089 [87] PATWARDHAN P R, SATRIO J A, BROWN R C, SHANKS B H. Influence of inorganic salts on the primary pyrolysis products of cellulose[J]. Bioresour Technol,2010,101(12):4646−4655. doi: 10.1016/j.biortech.2010.01.112 [88] TRENDEWICZ A, EVANS R, DUTTA A, SYKES R, CARPENTER D, BRAUN R. Evaluating the effect of potassium on cellulose pyrolysis reaction kinetics[J]. Biomass Bioenergy,2015,74:15−25. doi: 10.1016/j.biombioe.2015.01.001 [89] BANKS S W, NOWAKOWSKI D J, BRIDGWATER A V. Impact of potassium and phosphorus in biomass on the properties of fast pyrolysis bio-oil[J]. Energy Fuels,2016,30(10):8009−8018. doi: 10.1021/acs.energyfuels.6b01044 [90] SADDAWI A, JONES J M, WILLIAMS A. Influence of alkali metals on the kinetics of the thermal decomposition of biomass[J]. Fuel Process Technol,2012,104:189−197. doi: 10.1016/j.fuproc.2012.05.014 [91] ZHOU X W, MAYES H B, BROADBELT L J, NOLTE M W, SHANKS B H. Fast pyrolysis of glucose‐based carbohydrates with added NaCl part 2: Validation and evaluation of the mechanistic model[J]. AIChE J,2016,62(3):778−791. doi: 10.1002/aic.15107 [92] LIU D, YU Y, LONG Y, WU H W. Effect of MgCl2 loading on the evolution of reaction intermediates during cellulose fast pyrolysis at 325 ℃[J]. Proc Combust Inst,2015,35(2):2381−2388. doi: 10.1016/j.proci.2014.05.026 [93] LIU D, YU Y, HAYASHI J, MOGHTADERI B, WU H W. Contribution of dehydration and depolymerization reactions during the fast pyrolysis of various salt-loaded celluloses at low temperatures[J]. Fuel (Guildford),2014,136:62−68. doi: 10.1016/j.fuel.2014.07.025 [94] CARVALHO W S, CUNHA I F, PEREIRA M S, ATAIDE C H. Thermal decomposition profile and product selectivity of analytical pyrolysis of sweet sorghum bagasse: Effect of addition of inorganic salts[J]. Ind Crop Prod,2015,74:372−380. doi: 10.1016/j.indcrop.2015.05.020 [95] BRANCA C, DI BLASI C, GALGANO A. Pyrolysis of corncobs catalyzed by zinc chloride for furfural production[J]. Ind Eng Chem Res,2010,49(20):9743−9752. doi: 10.1021/ie101067v [96] DOBELE G, ROSSINSKAJA G, DIZHBITE T, TELYSHEVA G, MEIER D, FAIX O. Application of catalysts for obtaining 1, 6-anhydrosaccharides from cellulose and wood by fast pyrolysis[J]. J Anal Appl Pyrolysis,2005,74(1/2):401−405. [97] MAYER Z A, APFELBACHER A, HORNUNG A. Effect of sample preparation on the thermal degradation of metal-added biomass[J]. J Anal Appl Pyrolysis,2012,94:170−176. doi: 10.1016/j.jaap.2011.12.008 [98] LI Y, LI K, HU B, ZHANG Z X, ZHANG G, FENG S Y, WANG T P, LU Q. Catalytic fast pyrolysis of cellulose for selective production of 1-hydroxy-3, 6-dioxabicyclo[3.2. 1]octan-2-one using nickel-tin layered double oxides[J]. Ind Crop Prod,2021,162:113269. doi: 10.1016/j.indcrop.2021.113269 [99] WANG Z, LU Q, ZHU X F, ZHANG Y. Catalytic fast pyrolysis of cellulose to prepare levoglucosenone using sulfated zirconia[J]. ChemSusChem,2011,4(1):79−84. doi: 10.1002/cssc.201000210 [100] LI F, LU T, CHEN B, HUANG Z J, YUAN G Q. Pt nanoparticles over TiO2-ZrO2 mixed oxide as multifunctional catalysts for an integrated conversion of furfural to 1, 4-butanediol[J]. Appl Catal A:Gen,2014,478:252−258. doi: 10.1016/j.apcata.2014.04.012 [101] KELKAR S, SAFFRON C M, ANDREASSI K, LI Z L, MURKUTE A, MILLER D J, PINNAVAIA T J, KRIEGEL R M. A survey of catalysts for aromatics from fast pyrolysis of biomass[J]. Appl Catal B: Environ, 2015, 174–175: 85–95. [102] PÜTÜN E. Catalytic pyrolysis of biomass: Effects of pyrolysis temperature, sweeping gas flow rate and MgO catalyst[J]. Energy,2010,35(7):2761−2766. doi: 10.1016/j.energy.2010.02.024 [103] VESES A, AZNAR M, MARTÍNEZ I, MARTINEZ J D, LOPEZ J M, NAVARRO M V, CALLEN M S, MURILLO R, GARCIA T. Catalytic pyrolysis of wood biomass in an auger reactor using calcium-based catalysts[J]. Bioresour Technol,2014,162:250−258. doi: 10.1016/j.biortech.2014.03.146 [104] LI J F, YAN R, XIAO B, LIANG D T, LEE D H. Preparation of Nano-NiO particles and evaluation of their catalytic activity in pyrolyzing biomass components[J]. Energy Fuels,2008,22(1):16−23. doi: 10.1021/ef700283j [105] FABBRI D, TORRI C, BARAVELLI V. Effect of zeolites and nanopowder metal oxides on the distribution of chiral anhydrosugars evolved from pyrolysis of cellulose: An analytical study[J]. J Anal Appl Pyrolysis,2007,80(1):24−29. doi: 10.1016/j.jaap.2006.12.025 [106] FABBRI D, TORRI C, MANCINI I. Pyrolysis of cellulose catalysed by nanopowder metal oxides: Production and characterisation of a chiral hydroxylactone and its role as building block[J]. Green Chem,2007,9(12):1374. doi: 10.1039/b707943e [107] DONAR Y O, SıNAĞ A. Catalytic effect of tin oxide nanoparticles on cellulose pyrolysis[J]. J Anal Appl Pyrolysis,2016,119:69−74. doi: 10.1016/j.jaap.2016.03.016 [108] LI Y, HU B, NAQVI S R, ZHANG Z X, LI K, LU Q. Selective preparation of 5-hydroxymethylfurfural by catalytic fast pyrolysis of cellulose over zirconium-tin mixed metal oxides[J]. J Anal Appl Pyrolysis,2021,155:105103. doi: 10.1016/j.jaap.2021.105103 [109] SUN L Z, ZHANG X D, CHEN L, XIE X P, YANG S X, ZHAO B F, SI H Y. Effect of preparation method on structure characteristics and fast pyrolysis of biomass with Fe/CaO catalysts[J]. J Anal Appl Pyrolysis,2015,116:183−189. doi: 10.1016/j.jaap.2015.09.011 [110] XU X W, JIANG E C, WANG M F, LI B S. Rich hydrogen production from crude gas secondary catalytic cracking over Fe/γ-Al2O3[J]. Renewable Energy,2012,39(1):126−131. doi: 10.1016/j.renene.2011.07.030 [111] KIM P, RIALS T G, LABBE N, CHMELY S C. Screening of mixed-metal oxide species for catalytic ex situ vapor-phase deoxygenation of cellulose by py-GC/MS coupled with multivariate analysis[J]. Energy Fuels,2016,30(4):3167−3174. doi: 10.1021/acs.energyfuels.6b00347 [112] DING Y L, WANG H Q, XIANG M, YU P, LI R Q, KE Q P. The effect of Ni-ZSM-5 catalysts on catalytic pyrolysis and hydro-pyrolysis of biomass[J]. Front Chem,2020,8:790. doi: 10.3389/fchem.2020.00790 [113] TAARNING E, OSMUNDSEN C M, YANG X, VOSS B, ANDERSEN S I, CHRISTENSEN C H. Zeolite-catalyzed biomass conversion to fuels and chemicals[J]. Energy Environ Sci,2011,4(3):793−804. doi: 10.1039/C004518G [114] ASADIERAGHI M, ASHRI WAN DAUD W M, ABBAS H F. Heterogeneous catalysts for advanced bio-fuel production through catalytic biomass pyrolysis vapor upgrading: a review[J]. RSC Adv,2015,5(28):22234−22255. doi: 10.1039/C5RA00762C [115] CARLSON T R, TOMPSETT G A, CONNER W C, HUBER G W Aromatic production from catalytic fast pyrolysis of biomass-derived feedstocks[J]. Top Catal, 2009, 52(3): 241–252. [116] WANG K, KIM K H, BROWN R C. Catalytic pyrolysis of individual components of lignocellulosic biomass[J]. Green Chem,2014,16(2):727−735. doi: 10.1039/C3GC41288A [117] GAYUBO A G, AGUAYO A T, ATUTXA A, AGUADO R, BILBAO J. Transformation of oxygenate components of biomass pyrolysis oil on a HZSM-5 Zeolite. I. Alcohols and phenols[J]. Ind Eng Chem Res,2004,43(11):2610−2618. doi: 10.1021/ie030791o [118] GAYUBO A G, AGUAYO A T, ATUTXA A, AGUADO R, BILBAO J. Transformation of oxygenate components of biomass pyrolysis oil on a HZSM-5 zeolite. II. Aldehydes, ketones, and acids[J]. Ind Eng Chem Res,2004,43(11):2619−2626. doi: 10.1021/ie030792g [119] JAE J, TOMPSETT G A, FOSTER A J, HAMMOND K D, AUERBACH S M, LOBO R F, HUBER G W. Investigation into the shape selectivity of zeolite catalysts for biomass conversion[J]. J Catal,2011,279(2):257−268. doi: 10.1016/j.jcat.2011.01.019 [120] YU Y Q, LI X Y, SU L, ZHANG Y, WANG Y J, ZHANG H Z. The role of shape selectivity in catalytic fast pyrolysis of lignin with zeolite catalysts[J]. Appl Catal A: Gen, 2012, 447–448: 115–123. [121] VESES A, PUÉRTOLAS B, CALLÉN M S, GARCIA T. Catalytic upgrading of biomass derived pyrolysis vapors over metal-loaded ZSM-5 zeolites: Effect of different metal cations on the bio-oil final properties[J]. Microporous Mesoporous Mater,2015,209:189−196. doi: 10.1016/j.micromeso.2015.01.012 [122] MULLEN C A, BOATENG A A. Production of aromatic hydrocarbons via catalytic pyrolysis of biomass over Fe-modified HZSM-5 zeolites[J]. ACS Sustainable Chem Eng,2015,3(7):1623−1631. doi: 10.1021/acssuschemeng.5b00335 [123] ENNAERT T, VAN AELST J, DIJKMANS J, DE CLERCQ R, SCHUTYSER W, DUSSELIER M, VERBOEKEND D, SELS B F. Potential and challenges of zeolite chemistry in the catalytic conversion of biomass[J]. Chem Soc Rev,2016,45(3):584−611. doi: 10.1039/C5CS00859J [124] KIM Y H, LEE K H, NAM C M, LEE J S. Formation of hierarchical pore structures in Zn/ZSM-5 to improve the catalyst stability in the aromatization of branched olefins[J]. ChemCatChem,2012,4(8):1143−1153. doi: 10.1002/cctc.201200007 [125] NEUMANN G T, HICKS J C. Novel hierarchical cerium-incorporated MFI zeolite catalysts for the catalytic fast pyrolysis of lignocellulosic biomass[J]. ACS Catal,2012,2(4):642−646. doi: 10.1021/cs200648q [126] VESES A, PUERTOLAS B, LOPEZ J M, CALLEN M S, SOLSONA B, GARCIA T. Promoting deoxygenation of bio-oil by metal-loaded hierarchical ZSM-5 zeolites[J]. ACS Sustainable Chem Eng,2016,4(3):1653−1660. doi: 10.1021/acssuschemeng.5b01606 [127] NISHU, LIU R, RAHMAN M M, LI C, CHAI M Y, SARKER M, WANG Y C, CAI J M. Catalytic pyrolysis of microcrystalline cellulose extracted from rice straw for high yield of hydrocarbon over alkali modified ZSM-5[J]. Fuel (Guildford),2021,285:119038. doi: 10.1016/j.fuel.2020.119038 [128] CHENG Y T, WANG Z, GILBERT C J, FAN W, HUBER G W. Production of p-xylene from biomass by catalytic fast pyrolysis using ZSM-5 catalysts with reduced pore openings[J]. Angew Chem Int Ed Eng,2012,51(44):11097−11100. doi: 10.1002/anie.201205230 [129] ZHANG B, ZHONG Z P, WANG X B, DING K, SONG Z W. Catalytic upgrading of fast pyrolysis biomass vapors over fresh, spent and regenerated ZSM-5 zeolites[J]. Fuel Process Technol,2015,138:430−434. doi: 10.1016/j.fuproc.2015.06.011 [130] WANG J, ZHONG Z, DING K, XUE Z Y. Catalytic fast pyrolysis of mushroom waste to upgraded bio-oil products via pre-coked modified HZSM-5 catalyst[J]. Bioresour Technol,2016,212:6−10. doi: 10.1016/j.biortech.2016.04.005 [131] WANG K G, ZHANG J, SHANKS B H, BROWN R C. The deleterious effect of inorganic salts on hydrocarbon yields from catalytic pyrolysis of lignocellulosic biomass and its mitigation[J]. Appl Energy,2015,148:115−120. doi: 10.1016/j.apenergy.2015.03.034 [132] SHAOLONG W Y W. A review on ex situ catalytic fast pyrolysis of biomass[J]. Front Chem Sci Eng,2014,8(3):280−294. doi: 10.1007/s11705-014-1436-8 [133] GALADIMA A, MURAZA O. In situ fast pyrolysis of biomass with zeolite catalysts for bioaromatics/gasoline production: A review[J]. Energy Convers Manage,2015,105:338−354. doi: 10.1016/j.enconman.2015.07.078 [134] LI B, OU L, DANG Q, MEYER P, JONES S, BROWN R, WRIGHT M. Techno-economic and uncertainty analysis of in situ and ex situ fast pyrolysis for biofuel production[J]. Bioresour Technol,2015,196:49−56. doi: 10.1016/j.biortech.2015.07.073 -




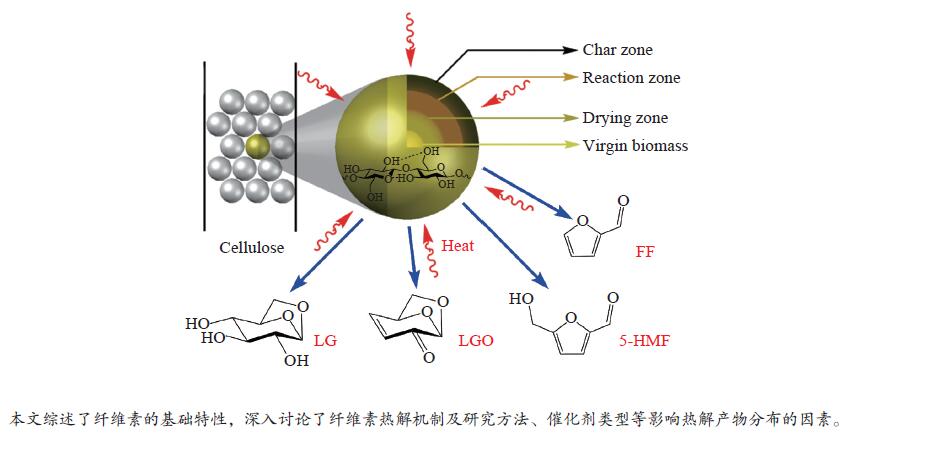
 下载:
下载: