Advances on the catalytic hydrogenation of biomass-derived furfural and 5-hydroxymethylfurfural
-
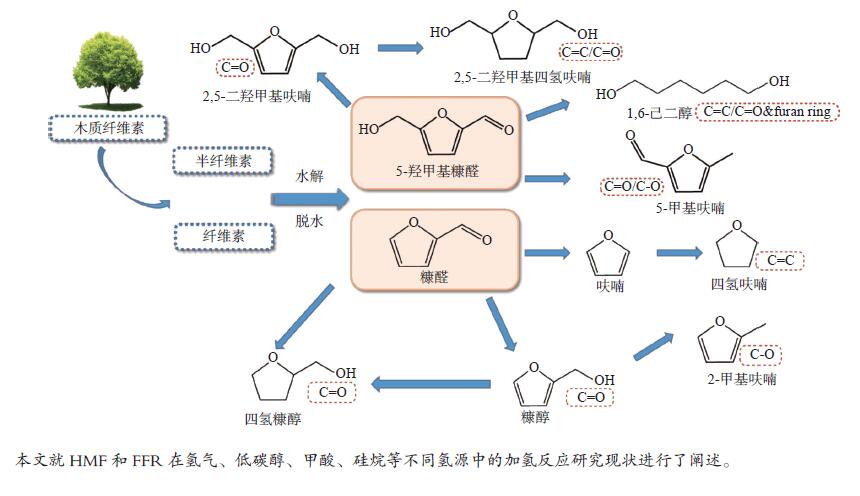
摘要: 近年来,利用生物质基平台化合物转化制备各种燃料及高值化学品引起研究人员的广泛关注。5-羟甲基糠醛(HMF)和糠醛(FFR)作为一类重要的生物质衍生平台化合物,分子结构中醛基和呋喃环等官能团赋予其独特的化学性质。本综述针对HMF和FFR在氢气、低碳醇、甲酸和硅烷等不同氢源中的催化加氢反应研究现状进行了阐述,对加氢转化过程中的主要影响因素如催化剂类型和反应条件以及反应机理等进行了详细分析,同时对HMF/FFR加氢转化应用研究前景进行了展望。Abstract: In recent years, the conversion of biomass-derived platform compounds into a variety of high value fuel and chemical products has attracted increasing attention from researchers. 5-Hydroxymethylfurfural (HMF) and furfural (FFR) belong to a class of important biomass-derived platform chemicals. The molecular structure of HMF and FFR is consisted of aldehyde group, furan ring and other functional groups, which endow them with unique chemical properties. In this paper, the research advances on the catalytic hydrogenation of HMF and FFR using various hydrogen sources, such as hydrogen, alcohol, silanes and formic acid, have been reviewed in detail. In addition, the main influencing factors like catalyst type and reaction conditions on the hydrogenation process as well as the reaction mechanism are discussed in depth. Meanwhile, research foreground in the catalytic hydrogenation of HMF/FFR has been prospected.
-
Key words:
- biomass /
- catalytic hydrogenation /
- 5-hydroxymethylfurfural /
- furfural
1) #共同第一作者 -
表 1 FFR和HMF各项物化性质[24]
Table 1 Physicochemical properties of furfural (FFR) and 5-hydroxymethylfurfural (HMF)[24]
Chemical Furfural (FFR) 5-hydroxym ethylfurfural (HMF) Molecular formula C5H4O2 C6H6O3 Molecular weight/(g·mol−1) 96.08 126.11 Boiling point/K 435 387−389 Melting point/K 237 301−307 Density/(g·cm−3) 1.16 1.24 表 2 分子H2作为氢源催化FFR和HMF加氢转化
Table 2 Hydrogenation of FFR and HMF using molecular H2 as hydrogen source
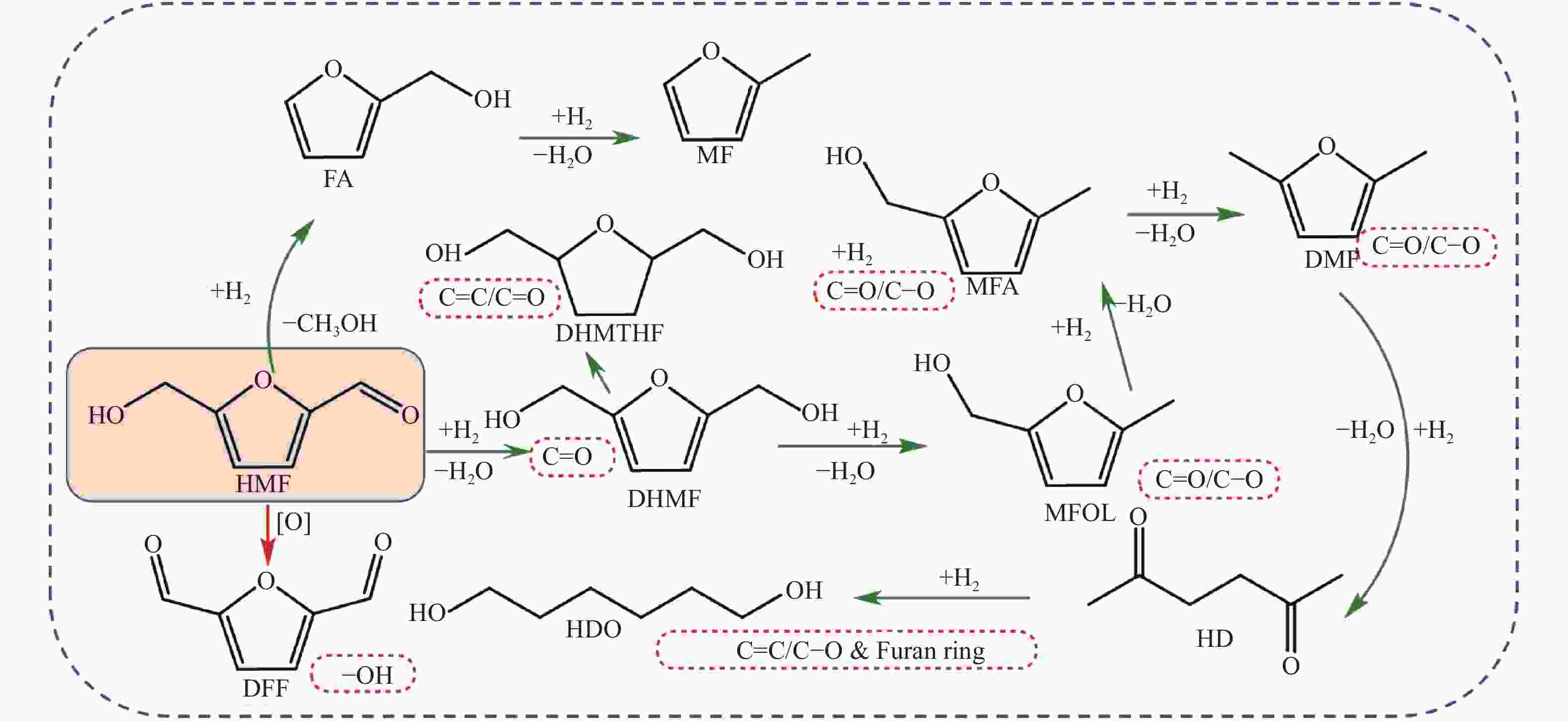
Entry Catalyst Substrate Solvent Time /h Temp./K Pres./MPa Conv./% Product Yield/% Ref. 1 Pt/HT FFR isopropanol 4 423 3 > 99 1,2-PeD 73 [29] 2 Pt/MCM-41 HMF H2O 2 308 0.8 100 BHMF 98.9 [30] 3 Ru/Al2O3 HMF 1-butanol-H2O 2 403 2.7 92 BHMF 74.5 [31] 4 Pt/C HMF ethanol 18 296 1.4 − BHMF 82 [32] 5 Pt/Al2O3 HMF ethanol 18 333 1.4 − BHMF 85 [32] 6 Pt1Sn1/Al2O3 HMF ethanol 5 333 1.4 − BHMF 82 [32] 7 Pt/Co2AlO4 FFR ethanol 24 423 1.5 − 1,5-PeD 31.9 [33] 8 Cu/AC-SO3H FFR isopropanol 2 378 4 100 FA > 99.9 [34] 9 Ni/NCNTs FFR H2O 7 373 4 100 THFA 100 [35] 10 Cu-Fe (1:2) HMF isopropanol 4 443 2 97 DMF 90 [36] 11 RuSn0. 4/C FFR H2O 5 363 1.25 95 FA 94.7 [37] 12 Ru(CO)/rGO FFR H2O 5 293 1 93.3 FA 91 [38] 13 Ni/CN FFR isopropanol 4 473 1 96 FA 91 [39] 14 Pd/Cu/MgO FFR H2O 0.9 403 0.8 100 FA 99 [40] 15 Pd-Ir-ReOx/SiO2 FFR H2O 80 313−373 6 > 99.9 1,5-PeD 83 [41] 16 Rh-Ir-ReOx/SiO2 FFR H2O 40 313−373 8 > 99.9 1,5-PeD 71 [42] 17 Ir-ReOx/SiO2 FFR H2O 6 403 0.8 >99 FA >99 [43] 18 Cu∶Zn∶Cr∶Zr(3∶2∶1∶3) FFR isopropanol 3.5 443 1 100 FA 96 [44] 19 CoAl HMF methanol 4 393 4 89.4 BHMF 83 [45] 20 CuZr HMF 1-butanol 2 473 1.5 100 DMF 60.6 [46] 21 Ni(40)/MgO(30)-M FFR 1-butanol 4 413 4 100 THFA 100 [47] 22 5Ni-12Cu/SBA-16 HMF THF 4 483 2 100 DMF 60.7 [48] 23 NiFeMgAl FFR ethanol 3 443 4 99.7 1,5-PeD 31 [49] 表 3 FFR、HMF加氢反应汇总(醇作氢供体)
Table 3 Hydrogenation of FFR and HMF over various catalysts using alcohol as hydrogen donor
Entry Catalyst Substrate Hydrogen donor Time
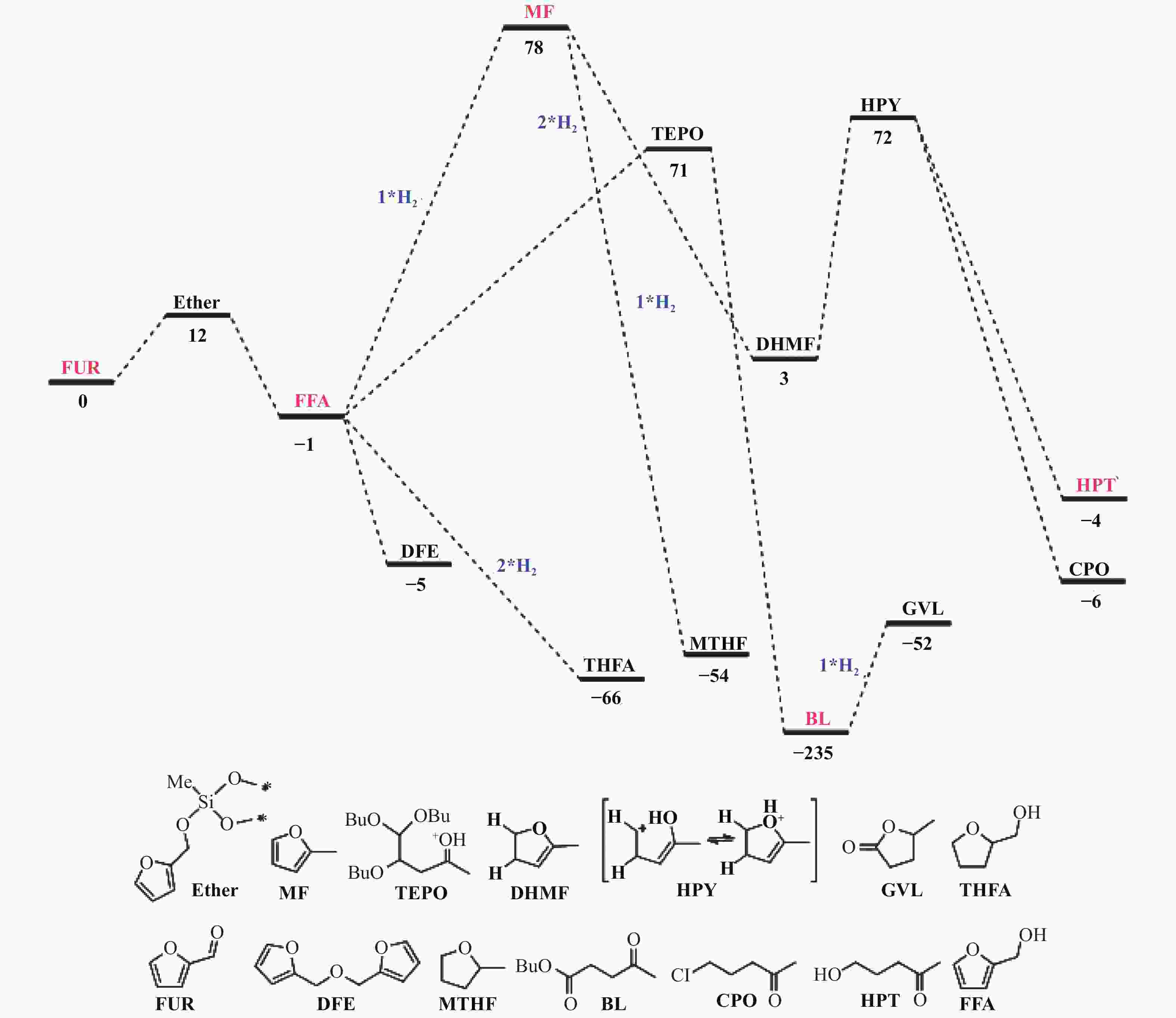
/hTemperature
/KConversion
/%Product Yield
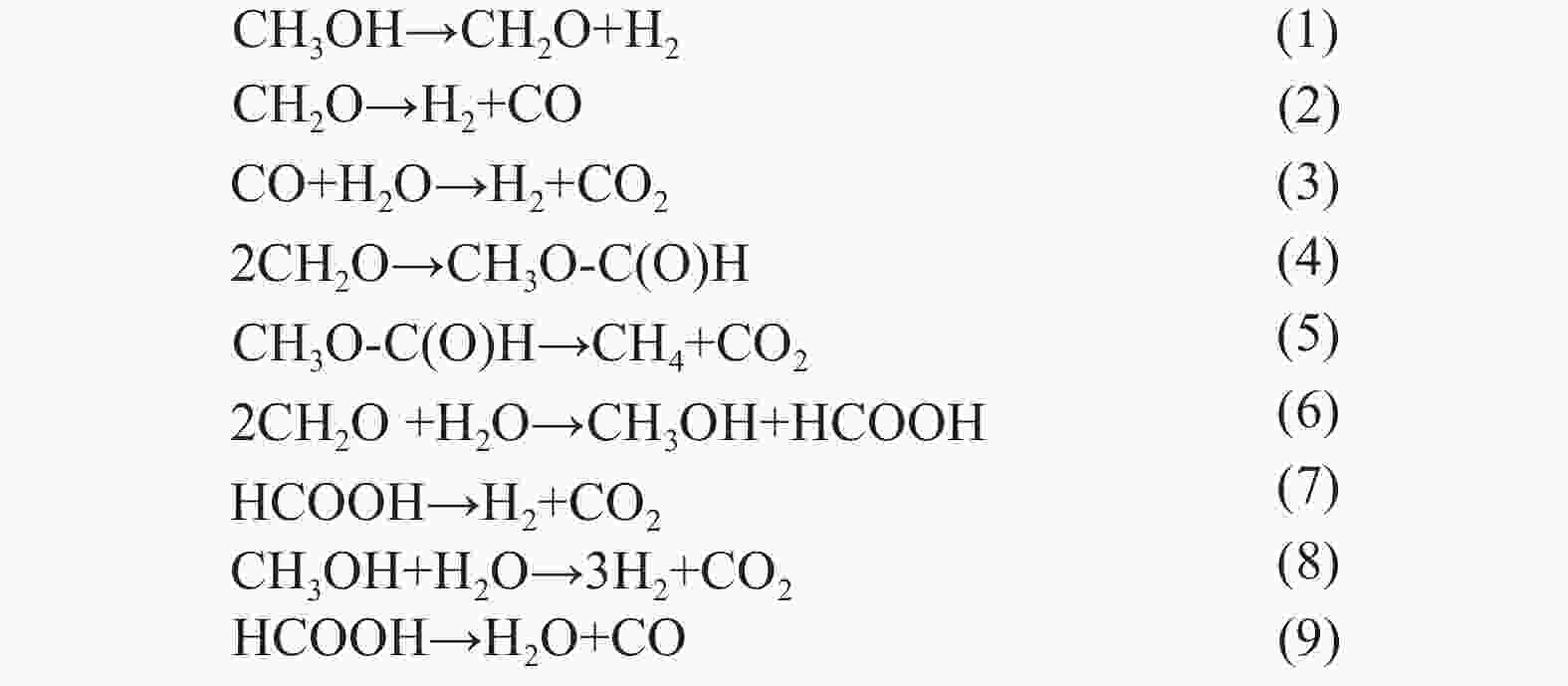
/%Ref. 1 Cu/AC-SO3H FFR isopropanol 5 423 − FA > 99.9 [34] 2 Cu2Al FFR methanol 2.5 473 100 FA 94 [16] 3 Cu3Al-A FFR methanol 1.5 513 100 MF 94.1 [16] 4 MgO HMF methanol 3 433 100 BHMF 100 [58] 5 Fe-L1/C-800 FFR 2-butanol 15 433 91.6 FA 76 [59] 6 ZrO(OH)2 HMF ethanol 2.5 423 94.1 BHMF 83.7 [60] 7 Ru/NiFe2O4 FFR isopropanol 6 453 > 97 MF 83 [61] 8 Mg/Fe/O FFR methanol 1 653 93 MF 83 [62] 9 Cu-PMO HMF methanol 3 533 100 DMF 48 [63] 10 Zr-LS FFR 2-propanol 1 373 92.2 FA 91.6 [55] 11 Zr1Fe1-150 FFR 2-propanol 2 453 100 FA 99.1 [64] 12 Ru/RuO2/C FFR 2-butanol 10 453 − MF 76 [66] 13 MZH(Zr/Fe = 2) HMF 2-butanol 5 423 98.4 BHMF 89.6 [66] 14 Ru/RuO2/C FFR 2-pentanol 10 453 − MF 76 [65] 15 Co3O4@MC HMF isopropanol 12 413 100 BHMF 97 [67] 16 Ru/Co3O4 HMF isopropanol 6 463 100 BHMF 82.8 [68] 17 Pd/Fe2O3 FFR isopropanol 7.5 453 87 FA 57 [69] 18 Au/ZrO2 FFR isopropanol 3 393 100 FA 100 [70] 19 Ni-SAs/NC FFR isopropanol 3 403 85.1 FA 82.6 [71] 20 Zr@Co-2 FFR isopropanol 4 433 93.9 FA 91.4 [72] 表 4 FFR、HMF加氢反应(甲酸作氢供体)
Table 4 Hydrogenation of FFR and HMF using formic acid as hydrogen donor
表 5 FFR、HMF加氢反应(硅烷作氢供体)
Table 5 Hydrogenation of FFR and HMF with silanes as hydrogen donor
-
[1] TANG X, WEI J, DING N, SUN Y, ZENG X H, HU L, LIU S J, LEI T Z, LIN L. Chemoselective hydrogenation of biomass derived 5-hydroxymethylfurfural to diols: Key intermediates for sustainable chemicals, materials and fuels[J]. Renewable Sustainable Energy Rev,2017,77:287−296. doi: 10.1016/j.rser.2017.04.013 [2] WANG Y T, ZHAO D Y, RODRÍGUEZ-PADRÓN D, LEN C. Recent advances in catalytic hydrogenation of furfural[J]. Catalysts,2019,9(10):796. doi: 10.3390/catal9100796 [3] AYUDE M A, DOUMIC L I, CASSANELLO M C, NIGAM K D P. Clean catalytic oxidation for derivatization of key biobased platform chemicals: Ethanol, glycerol, and hydroxymethyl furfural[J]. Ind Eng Chem Res,2019,58(35):16077−16095. doi: 10.1021/acs.iecr.9b00977 [4] LI H, HE J, RIISAGER A, SARAVANAMURUGAN S, SONG B A, YANG S. Acid-base bifunctional zirconium n-alkyltriphosphate nanohybrid for hydrogen transfer of biomass-derived carboxides[J]. ACS Catal,2016,6(11):7722−7727. doi: 10.1021/acscatal.6b02431 [5] JIN X, YIN B, XIA Q, FANG T, SHEN J, KUANG L, YANG C. Catalytic transfer hydrogenation of biomass-derived substrates to value-added chemicals on dual-function catalysts: opportunities and challenges[J]. ChemSusChem,2019,12(1):71−92. doi: 10.1002/cssc.201801620 [6] YE L, HAN Y W, FENG J, LU X B. A review about GVL production from lignocellulose: Focusing on the full components utilization[J]. Ind Crop Prod,2020,144:112031. doi: 10.1016/j.indcrop.2019.112031 [7] ARSLANOĞLU A, SERT M. Direct conversion of biomass to platform chemicals, catalyzed using a deep eutectic solvent of N, N diethyl ethanol ammonium chloride-oxalic acid in a microwave reactor[J]. Fuel,2019,258:116142. doi: 10.1016/j.fuel.2019.116142 [8] 常俊丽. 碳水化合物在乙醇介质中催化转化规律的研究[D]. 郑州: 郑州大学, 2015.CHANG Jun-li. The research on the law of carbohydrates catalyzed by the extremely low acid concentration in ethanol medium[D]. Zhengzhou: Zhengzhou University, 2015. [9] GAUDINO E C, CRAVOTTO G, MANZOLI M, TABASSO S. From waste biomass to chemicals and energy via microwave-assisted processes[J]. Green Chem,2019,21(6):1202−1235. doi: 10.1039/C8GC03908A [10] ŠIVEC R, GRILIC M, HUŠ M, LIKOZAR B. Multiscale modeling of (Hemi) cellulose hydrolysis and cascade hydrotreatment of 5-hydroxymethylfurfural, furfural, and levulinic acid[J]. Ind Eng Chem Res,2019,58(35):16018−16032. doi: 10.1021/acs.iecr.9b00898 [11] ZHAO P P, ZHANG Y Y, WANG Y, CUI H Y, SONG F, SUN X Y, ZHANG L P. Conversion of glucose into 5-hydroxymethylfurfural catalyzed by acid-base bifunctional heteropolyacid-based ionic hybrids[J]. Green Chem,2018,20(7):261−268. [12] MARISCAL R, MAIRELES-TORRES P, OJEDA M S, SADABA I, GRANADOS M L. Furfural: A renewable and versatile platform molecule for the synthesis of chemicals and fuels[J]. Energy Environ Sci,2016,9(4):1144−1189. doi: 10.1039/C5EE02666K [13] TOMISHIGE K, NAKAGAWA Y, TAMURA M. Selective hydrogenolysis of C-O bonds using the interaction of the catalyst surface and -OH groups[J]. Topics Curr Chem,2014,353:127−162. [14] HU L, XU J X, ZHOU S Y, HE A Y, TANG X, LIN L, XU J M, ZHAO Y J. Catalytic advances in the production and application of biomass-derived 2,5-dihydroxymethylfuran[J]. ACS Catal,2018,8(4):2959−2980. doi: 10.1021/acscatal.7b03530 [15] WANG Z W, LI H, FANG C J, ZHAO W F, YANG T T, YANG S. Simply assembled acidic nanospheres for efficient production of 5-ethoxymethylfurfural from 5-hydromethylfurfural and fructose[J]. Energy Technol-Ger,2017,5(11):2046−2054. doi: 10.1002/ente.201700153 [16] ZHANG J, CHEN J. Selective transfer hydrogenation of biomass-based furfural and 5-hydroxymethylfurfural over hydrotalcite-derived copper catalysts using methanol as a hydrogen donor[J]. ACS Sustainable Chem Eng,2017,5(7):5982−5993. doi: 10.1021/acssuschemeng.7b00778 [17] SATO S, IGARASHI J, YAMADA Y. Stable vapor-phase conversion of tetrahydrofurfuryl alcohol into 3, 4-2H-dihydropyran[J]. Appl Catal A:Gen,2013,453:213−218. doi: 10.1016/j.apcata.2012.12.017 [18] YAN K, CHEN A. Efficient hydrogenation of biomass-derived furfural and levulinic acid on the facilely synthesized noble-metal-free Cu-Cr catalyst[J]. Energy,2013,58:357−363. doi: 10.1016/j.energy.2013.05.035 [19] ROMAN-LESHKOV Y, BARETT C J, LIU Z Y, DUMESIC J A. Production of dimethylfuran for liquid fuels from biomass-derived carbohydrates[J]. Nature,2007,447(7147):982−985. doi: 10.1038/nature05923 [20] CAI H L, LI C Z, WANG A Q, ZHANG T. Biomass into chemicals: one-pot production of furan-based diols from carbohydrates via tandem reactions[J]. Catal Today,2014,234:59−65. doi: 10.1016/j.cattod.2014.02.029 [21] SAIKIA K, RATHANKUMAR A K, KUMAR P S, VARJANI S, NIZAR M, LENIN R, GEORGR J, VAIDYANATHAN V K. Recent advances in biotransformation of 5-hydroxymethylfurfural: Challenges and future aspects[J]. J Chem Technol Biot, 2021. [22] TONG X L, MA Y, LI Y D. Biomass into chemicals: Conversion of sugars to furan derivatives by catalytic processes[J]. Appl Catal A: Gen,2010,385(1/2):1−13. doi: 10.1016/j.apcata.2010.06.049 [23] SONG H, KONG X, WEI X J, BUTLER I A, XU L J, FANG Z, KOZINSKI J A, ZHU Y F. Catalytic conversion of 5-hydroxymethylfurfural to some value-added derivatives[J]. Green Chem,2018,20(16):3657−3682. doi: 10.1039/C8GC00234G [24] CHEN S, WOJCIESZAK R, DUMEIGNIL F, MARCEAU E, ROYER S. How catalysts and experimental conditions determine the selective hydroconversion of furfural and 5-hydroxymethylfurfural[J]. Chem Rev,2018,118(22):11023−11117. doi: 10.1021/acs.chemrev.8b00134 [25] YIN S S, SUN J, LIU B, ZHANG Z H. Magnetic material grafted cross-linked imidazolium based polyionic liquids: An efficient acid catalyst for the synthesis of promising liquid fuel 5-ethoxymethylfurfural from carbohydrates[J]. J Mater Chem A,2015,3(9):4992−4999. doi: 10.1039/C4TA06135G [26] YU W T, POROSOFF M D, CHEN J G. Review of Pt-based bimetallic catalysis: from model surfaces to supported catalysts[J]. Chem Rev,2012,112(11):5780−5817. doi: 10.1021/cr300096b [27] XU C, PAONE E, RODRÍGUEZ-PADRÓN D, LUQUE R, MAURIELLO F. Recent catalytic routes for the preparation and the upgrading of biomass derived furfural and 5-hydroxymethylfurfural[J]. Chem Soc Rev,2020,49:4273−4306. doi: 10.1039/D0CS00041H [28] ZHU Y F, KONG X, ZHENG H Y, DING G Q, ZHU Y L, LI Y W. Efficient synthesis of 2,5-dihydroxymethylfuran and 2,5-dimethylfuran from 5-hydroxymethylfurfural using mineral-derived Cu catalysts as versatile catalysts[J]. Catal Sci Technol,2015,5(8):4208−4217. doi: 10.1039/C5CY00700C [29] MIZUGAKI, T, AMAKAWA T, NAGATSU Y, MAENO Z, MITSUDOME T, JITSUKAWA K, KANEDA K. Direct transformation of furfural to 1, 2-pentanediol using a hydrotalcite-supported platinum nanoparticle catalyst[J]. ACS Sustainable Chem Eng,2014,2(10):2243−2247. doi: 10.1021/sc500325g [30] CHATTERJEE, M, ISHIZAKA T, KAWANAMI K. Selective hydrogenation of 5-hydroxymethylfurfural to 2,5-bis-(hydroxymethyl)furan using Pt/MCM-41 in an aqueous medium: A simple approach[J]. Green Chem,2014,16(11):4734−4739. doi: 10.1039/C4GC01127A [31] ALAMILLO R, TUCKER M H, CHIA M, PAGAN-TORRES Y, DUMESIC J. The selective hydrogenation of biomass-derived 5-hydroxymethylfurfural using heterogeneous catalysts[J]. Green Chem,2012,14(5):1413−1419. doi: 10.1039/c2gc35039d [32] BALAKRISHNAN M, SACIA E R, BELL A T. Etherification and reductive etherification of 5-(hydroxymethyl)furfural: 5-(alkoxymethyl)furfurals and 2,5-bis(alkoxymethyl)furans as potential bio-diesel candidates[J]. Green Chem,2012,14(6):1626−1634. doi: 10.1039/c2gc35102a [33] Xu W, WANG H, LIU X, REN J, WANG Y, LU G. Direct catalytic conversion of furfural to 1,5-pentanediol by hydrogenolysis of the furan ring under mild conditions over Pt/Co2AlO4 catalyst[J]. Chem Commun,2011,47(13):3924−3926. doi: 10.1039/c0cc05775d [34] GONG W B, CHEN C, ZHANG Y, ZHOU H J, WANG H M. Efficient synthesis of furfuryl alcohol from H2-hydrogenation/transfer hydrogenation of furfural using sulfonate group modified Cu catalyst[J]. ACS Sustainable Chem Eng,2017,5(3):2172−2180. doi: 10.1021/acssuschemeng.6b02343 [35] GONG W B, ZHANG H M, WANG G Z, ZHAO H J. Highly dispersed Co and Ni nanoparticles encapsulated in N-doped carbon nanotubes as efficient catalysts for the reduction of unsaturated oxygen compounds in aqueous phase[J]. Catal Sci Technol,2018,8(21):5506−5514. doi: 10.1039/C8CY01488D [36] SOLANKI B S, RODE C V. Selective hydrogenation of 5-HMF to 2,5-DMF over a magnetically recoverable non-noble metal catalyst[J]. Green Chem,2019,21(23):6390−6406. doi: 10.1039/C9GC03091C [37] MUSCI J J, MERLO A B, CASELLA M L. Aqueous phase hydrogenation of furfural using carbon-supported Ru and RuSn catalysts[J]. Catal Today,2017,296:43−50. doi: 10.1016/j.cattod.2017.04.063 [38] RAMIREZ-BARRIA C, LSAACS M, WILSON K, GUERRERO-RUIZ A, RODRÍGUEZ-RAMOS I. Optimization of ruthenium based catalysts for the aqueous phase hydrogenation of furfural to furfuryl alcohol[J]. Appl Catal A: Gen,2018,563:177−184. doi: 10.1016/j.apcata.2018.07.010 [39] KOTBAGI, T. V, GURAV H, NAGPURE A S, CHILUKURI S, BAKKER G. Highly efficient nitrogen-doped hierarchically porous carbon supported Ni nanoparticles for the selective hydrogenation of furfural to furfuryl alcohol[J]. RSC Adv,2016,6(72):67662−67668. doi: 10.1039/C6RA14078E [40] FULAJTÁROVA K, SOTÁK K, HRONEC M, VÁVRA L, DOBROČKA E, OMASTOVÁ M. Aqueous phase hydrogenation of furfural to furfuryl alcohol over Pd-Cu catalysts[J]. Appl Catal A: Gen,2015,502:78−85. doi: 10.1016/j.apcata.2015.05.031 [41] LIU S B, AMADA Y, TAMURA M, NAKAGAWA Y, TOMISHIGE K. One-pot selective conversion of furfural into 1,5-pentanediol over a Pd-added Ir–ReOx/SiO2 bifunctional catalyst[J]. Green Chem,2014,16(2):617−626. doi: 10.1039/C3GC41335G [42] LIU S B, AMADA Y, TAMURA M, NAKAGAWA Y, TOMISHIGE K. Performance and characterization of rhenium-modified Rh-Ir alloy catalyst for one-pot conversion of furfural into 1,5-pentanediol[J]. Catal Sci Technol,2014,4(8):2535−2549. doi: 10.1039/C4CY00161C [43] TAMURA M, TOKONAMI K, NAKAGAWA Y, TOMISHIGE K. Rapid synthesis of unsaturated alcohols under mild conditions by highly selective hydrogenation[J]. Chem Commun,2013,49(63):7034−7036. doi: 10.1039/c3cc41526k [44] SHARMA R V, DAS U, SAMMYNAIKEN S, DALAI A K. Liquid phase chemo-selective catalytic hydrogenation of furfural to furfuryl alcohol[J]. Appl Catal A: Gen,2013,454:127−136. doi: 10.1016/j.apcata.2012.12.010 [45] YAO S X, WANG X C, JIANG Y J, WU F, CHEN X G, MU X D. One-Step Conversion of biomass-derived 5-hydroxymethylfurfural to 1, 2, 6-hexanetriol over Ni-Co-Al mixed oxide catalysts under mild conditions[J]. ACS Sustainable Chem Eng,2013,2(2):173−180. [46] IRIONDO A, MENDIGUREN A, GÜEMEZ M B, REQUIES J, CAMBRA J F. 2,5-DMF production through hydrogenation of real and synthetic 5-HMF over transition metal catalysts supported on carriers with different nature[J]. Catal Today,2017,279:286−295. doi: 10.1016/j.cattod.2016.02.019 [47] SUNYOL C, OWEN R E, GONZÁLEZ M D, SALAGRE P, CESTEROS Y. Catalytic hydrogenation of furfural to tetrahydrofurfuryl alcohol using competitive nickel catalysts supported on mesoporous clays[J]. Appl Catal A: Gen,2021,611:117903. doi: 10.1016/j.apcata.2020.117903 [48] UMASANKAR S, TAMIZHDURAI P, KRISHNAN P S, NARAYANAN, S, MANGESH V L, SHANTHI K. Effect of copper on NiCu bimetallic catalyst supported on SBA-16 for the catalytic hydrogenation of 5-hydroxymethylfurfural to 2,5-dimethylfuran[J]. Biomass Bioenergy,2020,143:105868. doi: 10.1016/j.biombioe.2020.105868 [49] SHAO Y W, WANG J Z, SUN K, GAO G M, LI C, ZHANG L J, ZHANG S, XU L L, HE G Z, HU X. Selective hydrogenation of furfural and its derivative over bimetallic NiFe-based catalysts: Understanding the synergy between Ni sites and Ni-Fe alloy[J]. Renewable Energy,2021,170:1114−1128. doi: 10.1016/j.renene.2021.02.056 [50] ROMANO P N, ALMEIDA J M A R, CARVALHO Y, PRIECEL P, SOUSA-AGUIAR E F, JOSE A LOPEZ-SANCHEZ J A. Microwave-assisted selective hydrogenation of furfural to furfuryl alcohol employing a green and noble metal-free copper catalyst[J]. ChemSusChem,2016,9(24):3387−3392. doi: 10.1002/cssc.201601398 [51] NAGARAJA B M, PADMASRI A H, RAJU B D, RAO K S P. Production of hydrogen through the coupling of dehydrogenation and hydrogenation for the synthesis of cyclohexanone and furfuryl alcohol over different promoters supported on Cu-MgO catalysts[J]. Int J Hydrogen Energy,2011,36(5):3417−3425. doi: 10.1016/j.ijhydene.2010.12.013 [52] BERTOLINI G R, JIMÉNEZ-GÓMEZ C P, CECILIA J A, MAIRELES-TORRES P. Gas-phase hydrogenation of furfural to furfuryl alcohol over Cu-ZnO-Al2O3 catalysts prepared from layered double hydroxides[J]. Catalysts,2020,10(5):486. doi: 10.3390/catal10050486 [53] JIMÉNEZ-GÓMEZ C P, CECILIA J A, MORENO-TOST R, MAIRELES-TORRES P. Nickel phosphide/silica catalysts for the gas-phase hydrogenation of furfural to high-added-value chemicals[J]. ChemCatChem,2017,9:2881−2889. doi: 10.1002/cctc.201700312 [54] JIMÉNEZ-GÓMEZ C P, CECILIA J A, MORENO-TOST R, MAIRELES-TORRES P . Selective production of 2-methylfuran by gas-phase hydrogenation of furfural on copper incorporated by complexation in mesoporous silica catalysts[J]. ChemSusChem,2017,10:1448−1459. doi: 10.1002/cssc.201700086 [55] ZHOU S H, DAI F L, XIANG Z Y, SONG T, LIU D T, LV F C, QI H S. Zirconium-lignosulfonate polyphenolic polymer for highly efficient hydrogen transfer of biomass-derived oxygenates under mild conditions[J]. Appl Catal B: Environ,2019,248:31−43. doi: 10.1016/j.apcatb.2019.02.011 [56] MADERUELO-SOLERA R, LÓPEZ-ASENSIO R, CECILIA J A, JIMÉNEZ-GÓMEZ C P, MAIRELES-TORRES P. Catalytic Transfer hydrogenation of furfural to furfuryl alcohol over calcined MgFe hydrotalcites[J]. Appl Clay Sci,2019,183:105351. doi: 10.1016/j.clay.2019.105351 [57] ZHANG J, DONG K J, LUO W M, GUAN H F. Selective transfer hydrogenation of furfural into furfuryl alcohol on Zr-containing catalysts using lower alcohols as hydrogen donors[J]. ACS Omega,2018,3(6):6206−6216. doi: 10.1021/acsomega.8b00138 [58] PASINI T, LOLLI A, ALBONETTI S, CAVANI F, MELLA M. Methanol as a clean and efficient H-transfer reactant for carbonyl reduction: Scope, limitations, and reaction mechanism[J]. J Catal,2014,317:206−219. doi: 10.1016/j.jcat.2014.06.023 [59] LI J, LIU J, ZHOU H, YAO F. Catalytic transfer hydrogenation of furfural to furfuryl alcohol over nitrogen-doped carbon-supported iron catalysts[J]. ChemSusChem,2016,9(11):1339−1347. doi: 10.1002/cssc.201600089 [60] HAO W W, LI W F, TANG X, ZENG X H. Catalytic transfer hydrogenation of biomass-derived 5-hydroxymethyl furfural to the building block 2,5-bishydroxymethyl furan[J]. Green Chem,2016,18(4):1080−1088. doi: 10.1039/C5GC01221J [61] WANG B W, LI C, HE B, QI J, LIANG C H. Highly stable and selective Ru/NiFe2O4 catalysts for transfer hydrogenation of biomass-derived furfural to 2-methylfuran[J]. J Energy Chem,2017,26(4):799−807. doi: 10.1016/j.jechem.2017.04.008 [62] GRAZIA L, LOLLI A, FOLCO L, ZHANG Y, ALBONETTI S, CAVANI F. Gas-phase cascade upgrading of furfural to 2-methylfuran using methanol as a H-transfer reactant and MgO based catalysts[J]. Catal Sci Technol,2016,6(12):4418−4427. doi: 10.1039/C5CY02021B [63] HANSEN T S, BARTA K, ANASTA P T, FORD P C, RIISAGER A. One-pot reduction of 5-hydroxymethylfurfural via hydrogen transfer from supercritical methanol[J]. Green Chem,2012,14(9):2457−2461. doi: 10.1039/c2gc35667h [64] GU J, ZHANG J, WANG Y Z, LI D N, HUANG H Y, YUAN H R, CHEN Y. Efficient transfer hydrogenation of biomass derived furfural and levulinic acid via magnetic zirconium nanoparticles: Experimental and kinetic study[J]. Ind Crops Products,2020,145:112133. doi: 10.1016/j.indcrop.2020.112133 [65] PANAGIOTOPOULOU P, MARTIN P N, VLACHOS D G. Effect of hydrogen donor on liquid phase catalytic transfer hydrogenation of furfural over a Ru/RuO2/C catalyst[J]. J Mol Catal A: Chem,2014,392:223−228. doi: 10.1016/j.molcata.2014.05.016 [66] HU L, YANG M, XU N, XU J X, ZHOU S Y, CHU X Z, ZHAO Y J. Selective transformation of biomass-derived 5-hydroxymethylfurfural into 2,5-dihydroxymethylfuran via catalytic transfer hydrogenation over magnetic zirconium hydroxides[J]. Korean J Chem Eng,2017,35(1):99−109. [67] WANG G H, DENG X H, GU D, CHEN K, TÜYSÜZ H, SPLIETHOFF B, BONGARD H, WEIDENTHALER C, SCHMIDT W, SCHÎTH F. Co3O4 nanoparticles supported on mesoporous carbon for selective transfer hydrogenation of alpha, beta-unsaturated aldehydes[J]. Angew Chem Int Ed,2016,55(37):11101−11105. doi: 10.1002/anie.201604673 [68] WANG T, ZHANG J H, XIE W X, TANG Y J, GUO D L, NI Y H. Catalytic transfer hydrogenation of biobased HMF to 2,5-bis-(hydroxymethyl)furan over Ru/Co3O4[J]. Catalyst,2017,7(3):92. doi: 10.3390/catal7030092 [69] ADDIS D, DAS S, JUNGE K, BELLER M. Selective reduction of carboxylic acid derivatives by catalytic hydrosilylation[J]. Angew Chem Int Ed,2011,50(27):6004−6011. doi: 10.1002/anie.201100145 [70] ZHU S H, XUE Y F, GUO J, CEN Y L, WANG J G, FAN W B. Integrated conversion of hemicellulose and furfural into γ-valerolactone over Au/ZrO2 catalyst combined with ZSM-5[J]. ACS Catal,2016,6(3):2035−2042. doi: 10.1021/acscatal.5b02882 [71] FAN Y F, ZHUANG C F, LI S J, WANG Y, ZOU X Q, LIU X T, HUANG W M, ZHU G S. Efficient single-atom Ni for catalytic transfer hydrogenation of furfural to furfuryl alcohol[J]. J Mater Chem A,2021,9(2):1110−1118. doi: 10.1039/D0TA10838C [72] HOU P, MA M W, ZHANG P, CAO J J, LIU H, XU X L, YUE H J, TANG G, FANG S H. Catalytic transfer hydrogenation of furfural to furfuryl alcohol using easy-to-separate core–shell magnetic zirconium hydroxide[J]. New J Chem,2021,45(5):2715−2722. doi: 10.1039/D0NJ05638C [73] GRASEMANN M, LAURENCZY G. Formic acid as a hydrogen source-recent developments and future trends[J]. Energ Environ Sci,2012,5(8):8171−8181. doi: 10.1039/c2ee21928j [74] DU J, ZHAN J R, SUN Y, JIA W L, SI Z H, GAO H, TANG X, ZENG X H, LEI T Z, LIU S J, LIN L. Catalytic transfer hydrogenation of biomass-derived furfural to furfuryl alcohol over in-situ prepared nano Cu-Pd/C catalyst using formic acid as hydrogen source[J]. Chin J Catal,2018,368:69−78. doi: 10.1016/j.jcat.2018.09.025 [75] TUTEJA J, CHOUDARY H, NISHIMURA S, EBITANI K. Direct synthesis of 1,6-hexanediol from HMF over a heterogeneous Pd/ZrP catalyst using formic acid as hydrogen source[J]. ChemSusChem,2014,7(1):96−100. doi: 10.1002/cssc.201300832 [76] NAGAIAH P, GIDYONU P, ASHOKRAJU M, RAO M V, CHALLA P, BURRI D R, KAMARAJU S R R. Magnesium aluminate supported Cu catalyst for selective transfer hydrogenation of biomass derived furfural to furfuryl alcohol with formic acid as hydrogen donor[J]. ChemistrySelect,2019,4(1):145−151. doi: 10.1002/slct.201803645 [77] SUN Y, XIONG C X, ZHANG J R, TANG X, ZENG X H, LIU S J, LIN L. Catalytic transfer hydrogenolysis/hydrogenation of biomass-derived 5-formyloxymethylfurfural to 2,5-dimethylfuran over Ni-Cu bimetallic catalyst with formic acid as a hydrogen donor[J]. Ind Eng Chem Res,2019,58(14):5414−5422. doi: 10.1021/acs.iecr.8b05960 [78] BASU B, DAS P, DAS S. Transfer hydrogenation using recyclable polymer-supported formate (PSF): efficient and chemoselective reduction of nitroarenes[J]. Mol Divers,2005,9(4):259−262. doi: 10.1007/s11030-005-8106-1 [79] ABBINA S, DU G D. Scope and mechanistic studies of catalytic hydrosilylation with a high-valent nitridoruthenium(VI)[J]. ACS Catal,2013,3(4):678−684. doi: 10.1021/cs300848h [80] SOMMER L H, PIEYRUSZA E W, WHITMORE F C. Peroxide-catalyzed addition of trichlorosilane to 1-octene[J]. J Am Chem Soc,1947,69(1):188. [81] ROY S R, SAU S C, MANDAL S K. Chemoselective reduction of the carbonyl functionality through hydrosilylation: integrating click catalysis with hydrosilylation in one pot[J]. J Org Chem,2014,79(19):9150−9160. doi: 10.1021/jo501505j [82] LI H, ZHAO W F, SARAVANAMURUGAN S, DAI W S, HE J, MEIER S, YANG S, RIISAGER A. Control of selectivity in hydrosilane-promoted heterogeneous palladium-catalysed reduction of furfural and aromatic carboxides[J]. Commun Chem,2018,1(32):1−11. [83] LI H, ZHAO W F, FANG Z. Hydrophobic Pd nanocatalysts for one-pot and high-yield production of liquid furanic biofuels at low temperatures[J]. Appl Catal B: Environ,2017,215:18−27. doi: 10.1016/j.apcatb.2017.05.039 [84] ZHANG J, DONG K J, LUO W M. PdCl2-catalyzed hydrodeoxygenation of 5-hydroxymethylfurfural into 2,5-dimethylfuran at room-temperature using polymethylhydrosiloxane as the hydrogen donor[J]. Chem Eng Sci,2019,201:467−474. doi: 10.1016/j.ces.2019.03.011 [85] QIU M, GUO T M, X R, LI D N, QI X H. Highly efficient catalytic transfer hydrogenation of biomass-derived furfural to furfuryl alcohol using UiO-66 without metal catalysts[J]. Appl Catal A: Gen,2020,602:117719. doi: 10.1016/j.apcata.2020.117719 -





 下载:
下载: