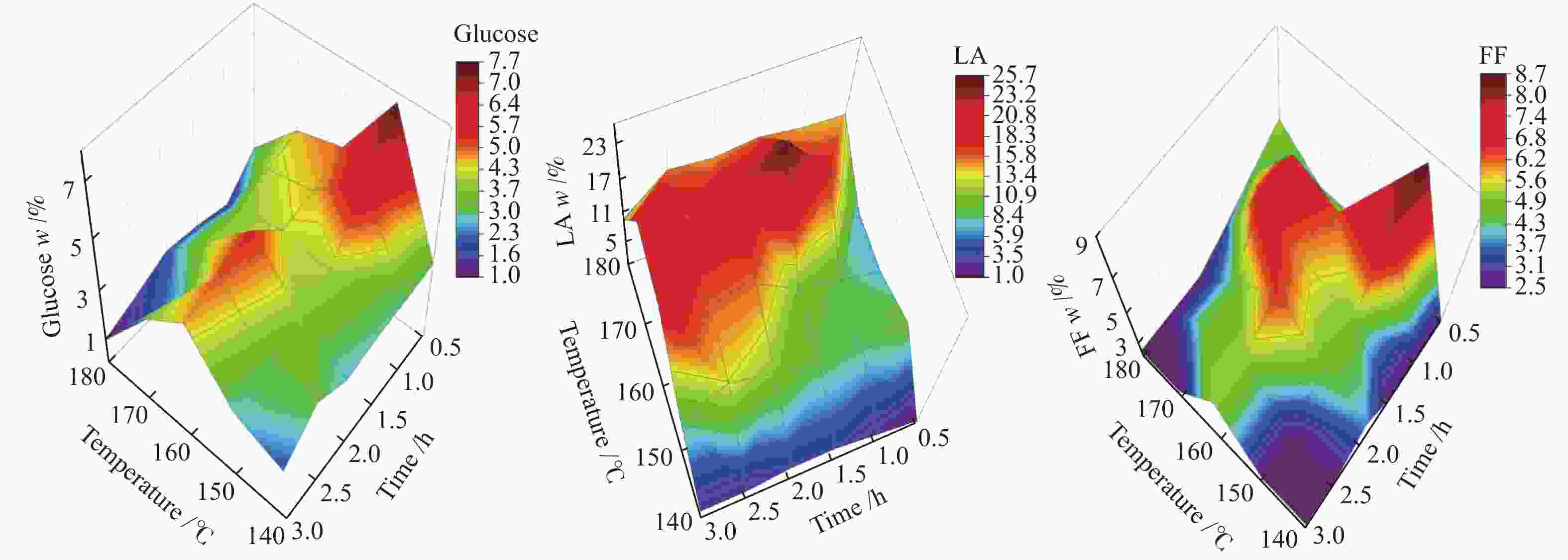
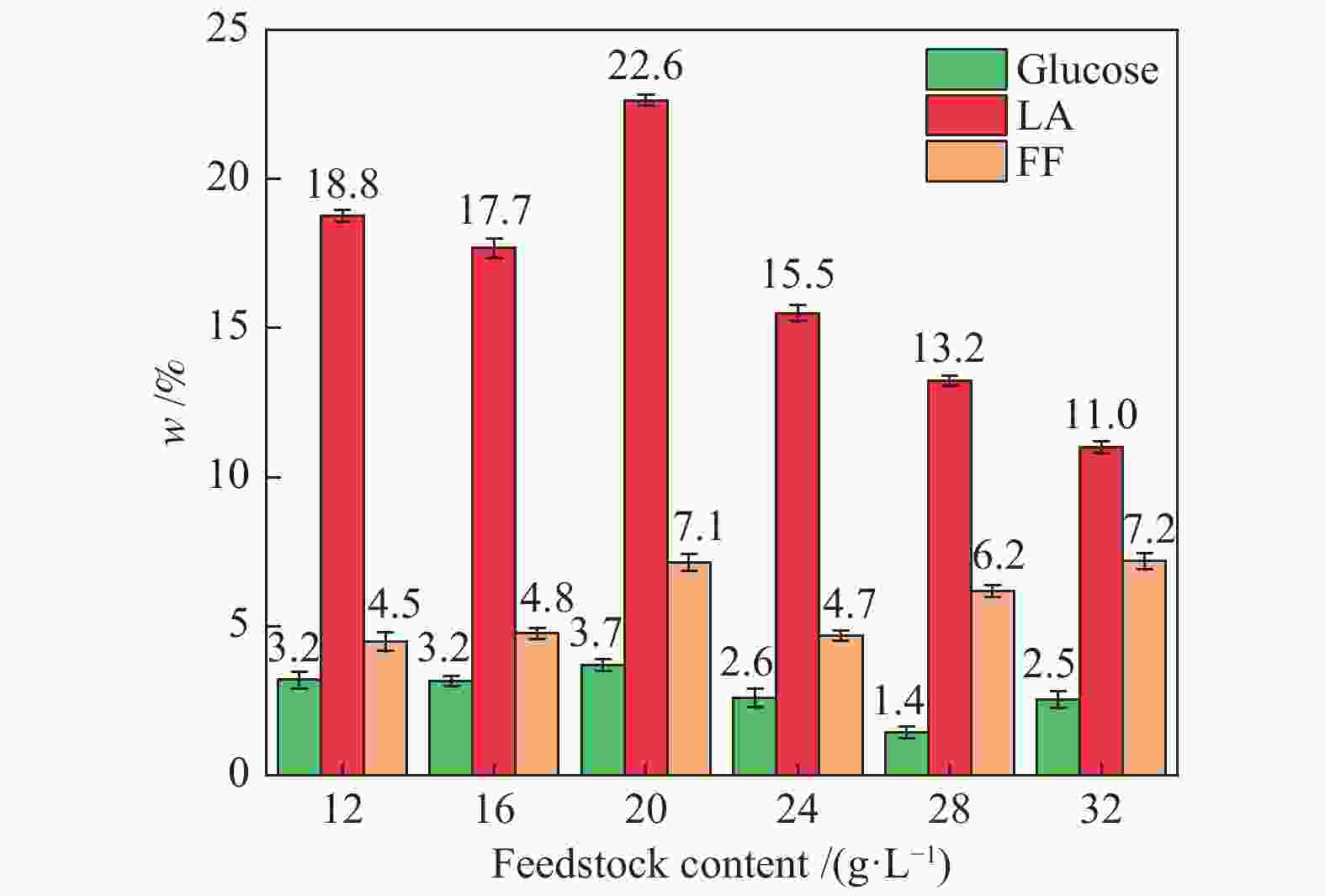
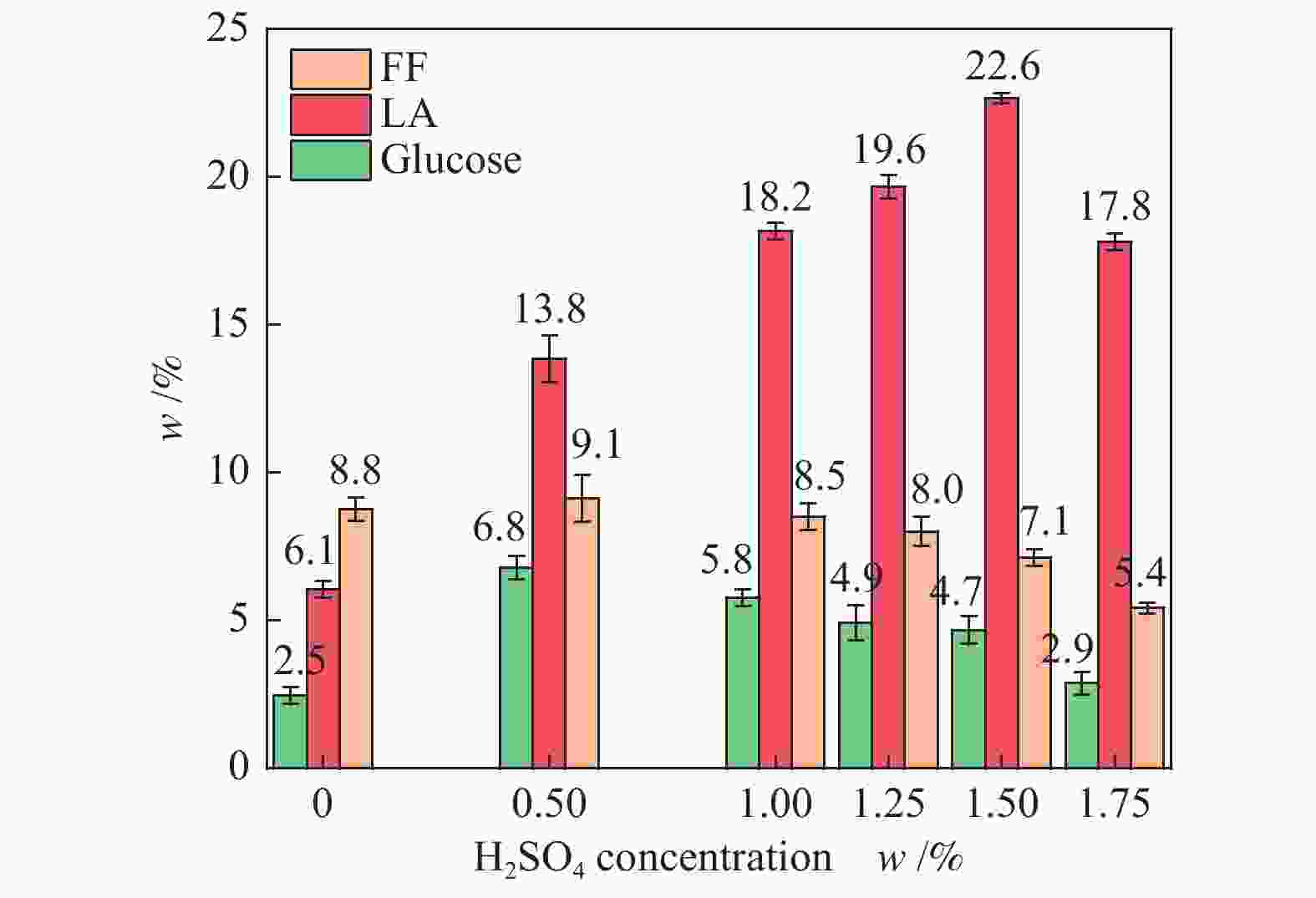
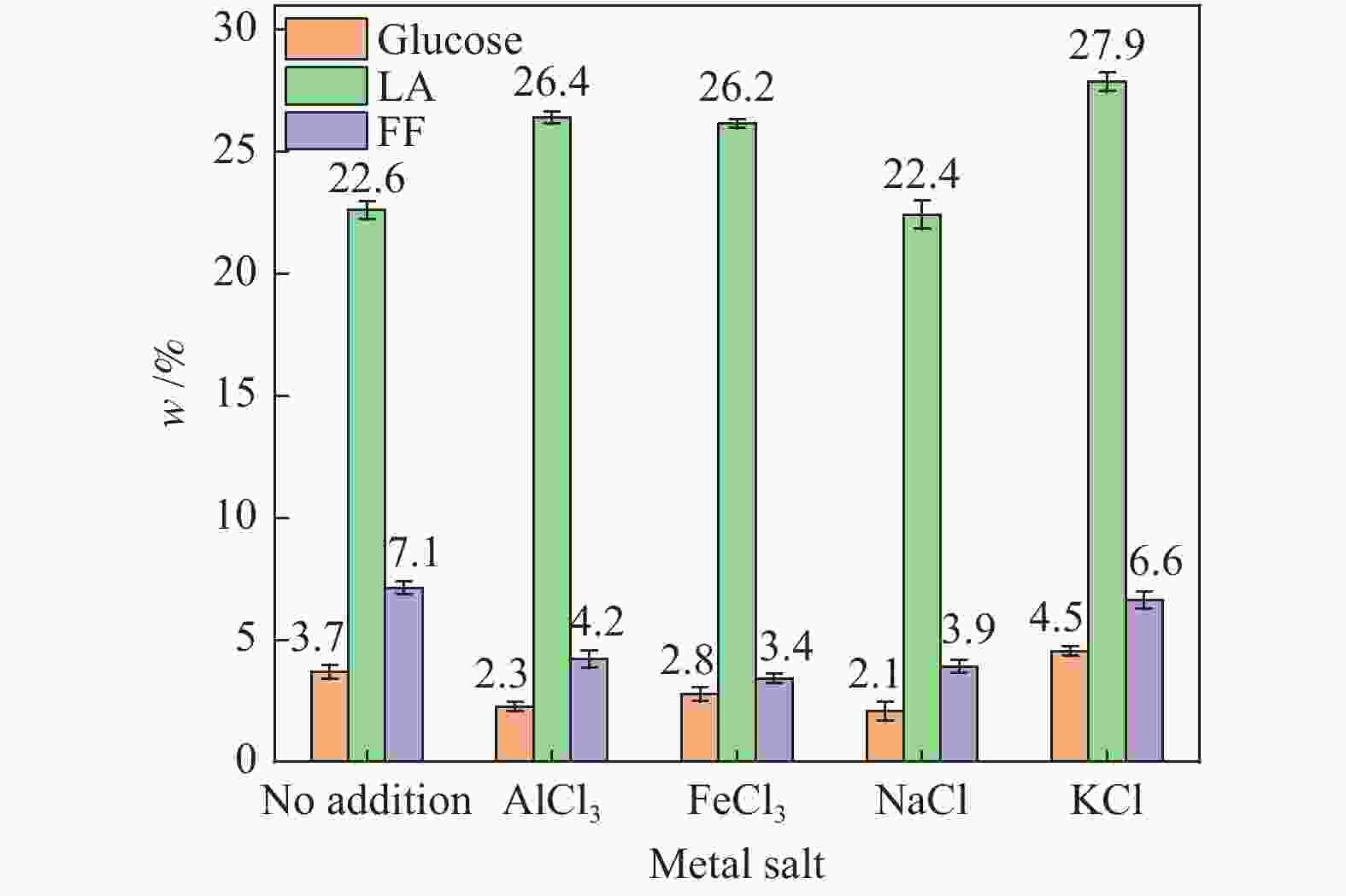
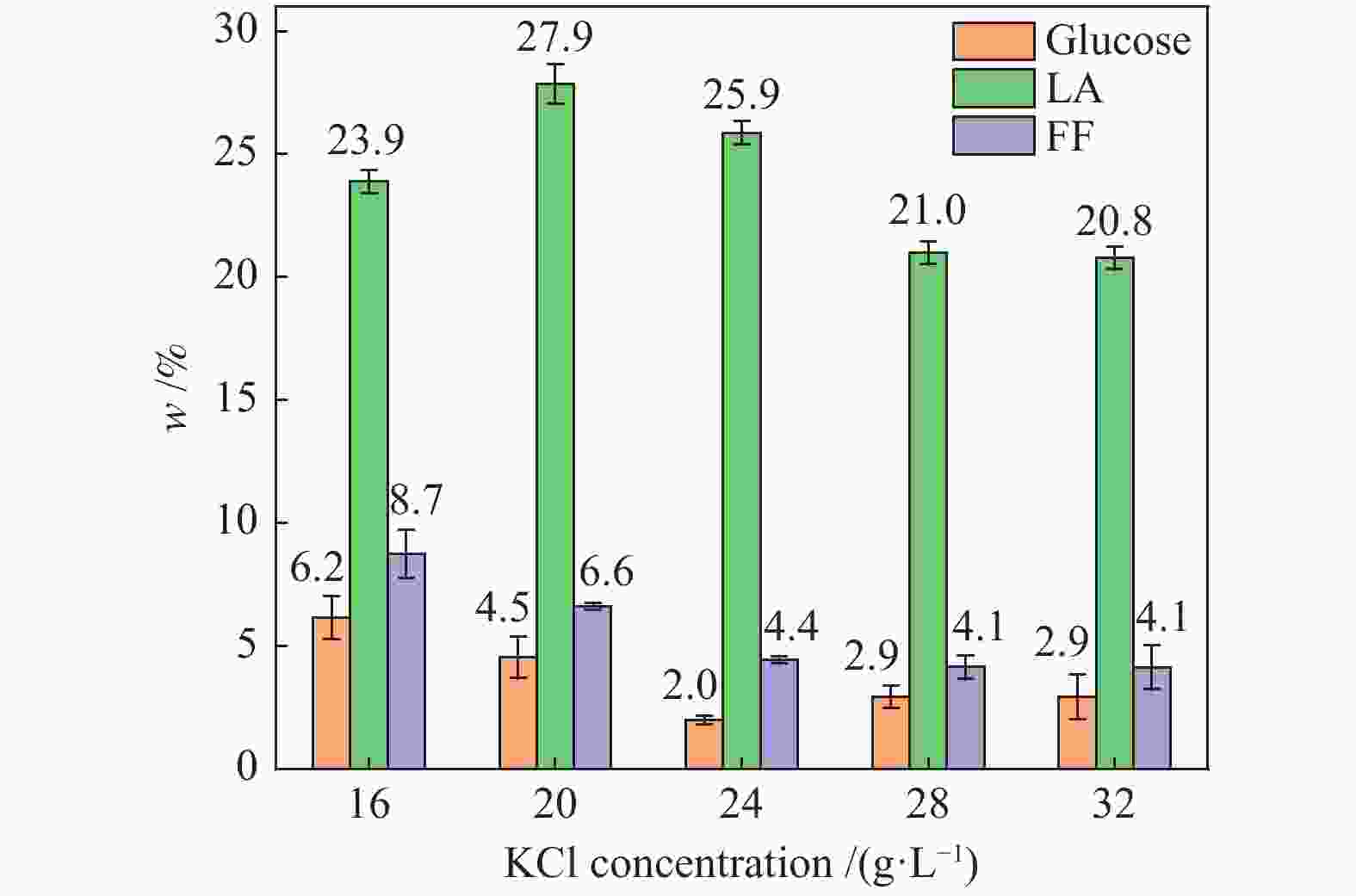
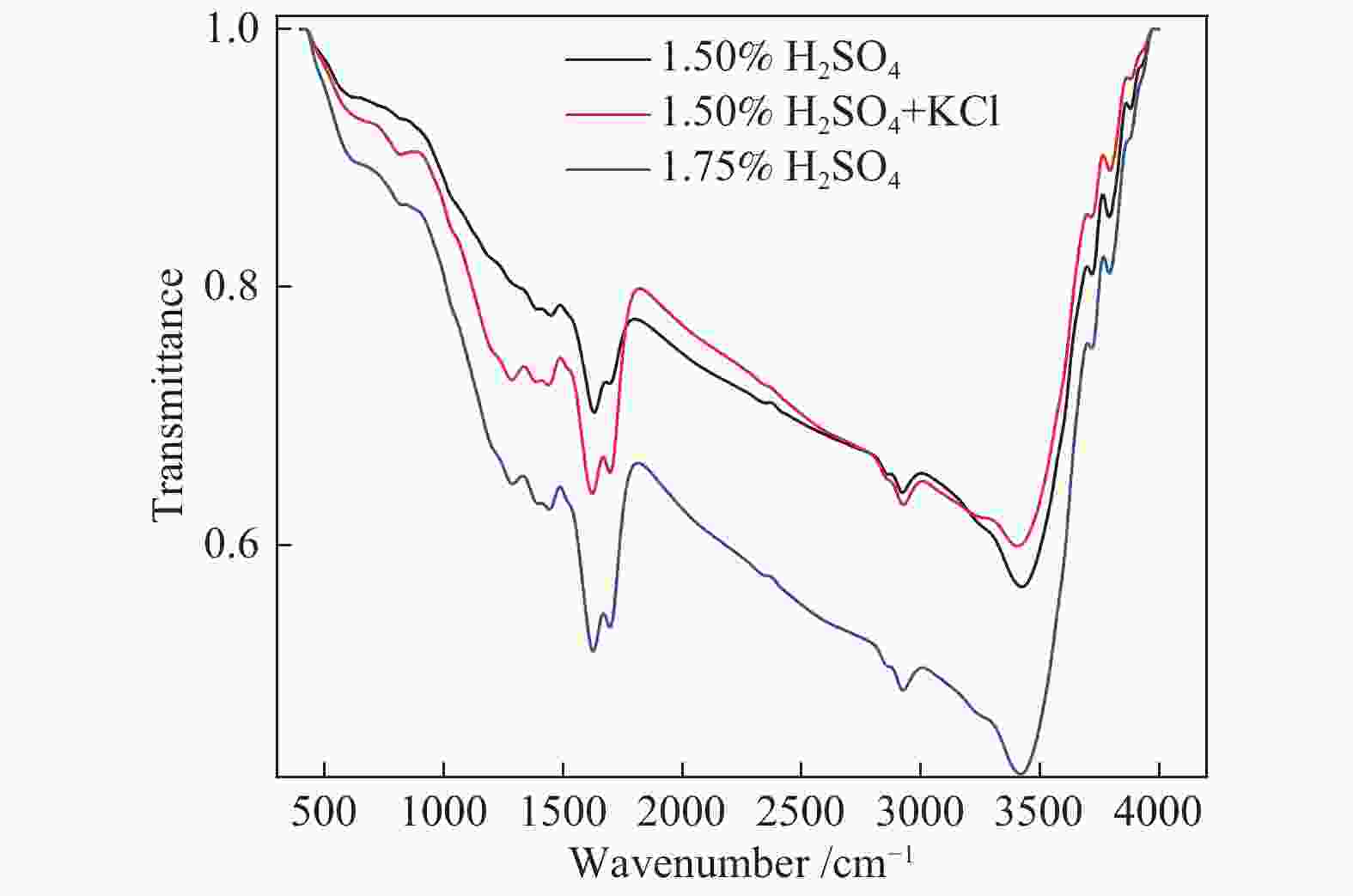
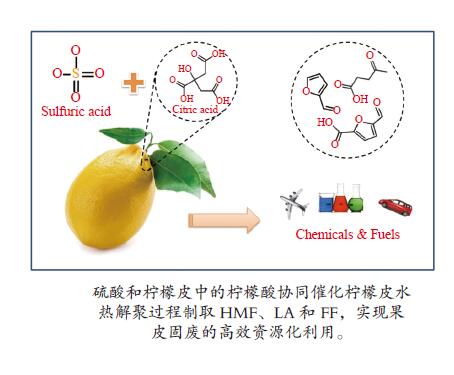
| [1] |
ZHANG Z, SONG J, HAN B. Catalytic transformation of lignocellulose into chemicals and fuel products in ionic liquids[J]. Chem Rev,2017,117(10):6834−6880. doi: 10.1021/acs.chemrev.6b00457
|
| [2] |
周琦, 藕志强, 饶鑫, 杜学禹. 椰子果皮废弃物的高值化利用现状及发展趋势[J]. 广州化工,2020,48(18):16−19. doi: 10.3969/j.issn.1001-9677.2020.18.007ZHOU Qi, OU Zhi-qiang, RAO Xing, DU Xue-yu. Current situation and development trend of high−value utilization of coconut peel waste[J]. Guangzhou Chem Ind,2020,48(18):16−19. doi: 10.3969/j.issn.1001-9677.2020.18.007
|
| [3] |
邓理, 廖兵, 郭庆祥. 纤维素选择性催化转化为重要平台化合物的研究进展[J]. 化工进展,2013,32(2):245−254.DENG Li, LIAO Bing, GUO Qing-xiang. Advances in the selectively catalyzed catalytic conversion of cellulose into important platform compounds[J]. Chem Prog,2013,32(2):245−254.
|
| [4] |
林海周. 生物质碳水化合物液相催化转化制取呋喃类平台化合物研究[D]. 杭州: 浙江大学, 2017.LING Hai-zhou. Preparation of furan platform compounds by liquid phase catalytic conversion of biomass carbohydrates[D]. Hangzhou: Zhejiang University, 2017.
|
| [5] |
THIRUMAVALAVAN M, LAI Y, LIN L, LEE J. Cellulose-based native and surface modified fruit peels for the adsorption of heavy metal ions from aqueous solution: Langmuir adsorption isotherms[J]. J Chem Eng Data,2010,55(3):1186−1192. doi: 10.1021/je900585t
|
| [6] |
MAX B, SALGADO J M, RODRÍGUEZ N, CORTÉS S, CONVERTI A, DOMÍNGUEZ J M. Biotechnological production of citric acid[J]. Braz J Microb,2010,41(4):862−875. doi: 10.1590/S1517-83822010000400005
|
| [7] |
章凯, 黄国林, 陈中胜, 黄德娟, 傅哪哪. 微波辅助萃取柠檬皮中果胶动力学及热力学研究[J]. 食品科学,2010,(15):107−111.ZHANG Kai, HUANG Guo-lin, CHEN Zhong-sheng, HUANG De-juan, FU Na-na. Study on kinetics and thermodynamics in microwave−assisted pectin extraction[J]. Food Sci,2010,(15):107−111.
|
| [8] |
章凯, 黄国林, 黄小兰, 张高飞. 响应面法优化微波辅助萃取柠檬皮中果胶的研究[J]. 精细化工,2010,(01):52−56.ZHANG Kai, HUANG Guo-lin, HUANG Xiao-lan, ZHANG Gao-fei. Optimization of microwave-assisted extraction of pectin in lemon peel by response surface method[J]. Ind Fine Chem,2010,(01):52−56.
|
| [9] |
张苒, 刘咏, 王军辉. 不同工艺提取柠檬皮果胶的理化及流变学特性研究[C]//中国食品科学技术学会第十五届年会论文摘要集. 2018.ZHANG Ran, LIU Yong, WANG Jun-hui. Study on the physical, chemical and rheological properties of lemon peel for pectin extracted by different processes[C]//Sum Papers 15th Annual Meeting Chinese Society Food Science Technol, 2018.
|
| [10] |
ZHAO Y, LU K, XU H, ZHU L, WANG S. A critical review of recent advances in the production of furfural and 5-hydroxymethylfurfural from lignocellulosic biomass through homogeneous catalytic hydrothermal conversion[J]. Renewable Sustainable Energy Rev,2021,139:110706−110733. doi: 10.1016/j.rser.2021.110706
|
| [11] |
ZHENG X, ZHI Z, GU X, LI X, ZHANG R, LU X. Kinetic study of levulinic acid production from corn stalk at mild temperature using FeCl3 as catalyst[J]. Fuel (Guildford),2017,187:261−267. doi: 10.1016/j.fuel.2016.09.019
|
| [12] |
XIANG Q, LEE Y Y, TORGET R W. Kinetics of glucose decomposition during dilute-acid hydrolysis of lignocellulosic biomass[J]. Appl Biochem Biotechnol, 2004, 113−116(1): 1127−1138.
|
| [13] |
HU X, WANG S, WU L, DONG D, MAHMUDUL HASAN M, LI C. Acid-treatment of C5 and C6 sugar monomers/oligomers: Insight into their interactions[J]. Fuel Proc Technol,2014,126:315−323. doi: 10.1016/j.fuproc.2014.05.024
|
| [14] |
ZHAO Y, XU H, WANG K, LU K, QU Y, ZHU L, WANG S. Enhanced furfural production from biomass and its derived carbohydrates in the renewable butanone-water solvent system[J]. Sustainable Energy Fuels,2019,3(11):3208−3218. doi: 10.1039/C9SE00459A
|
| [15] |
ZHAO Y, XU H, LU K, QU Y, ZHU L, WANG S. Experimental and kinetic study of arabinose conversion to furfural in renewable butanone water solvent mixture catalyzed by Lewis acidic ionic liquid catalyst[J]. Ind Eng Chem Res,2019,58(36):17088−17097. doi: 10.1021/acs.iecr.9b03420
|
| [16] |
LI X, PENG K, LIU X, XIA Q, WANG Y. Comprehensive understanding of the role of Brønsted and Lewis acid sites in glucose conversion into 5-hydromethylfurfural[J]. ChemCatChem,2017,9(14):2739−2746. doi: 10.1002/cctc.201601203
|
| [17] |
ENSLOW K R, BELL A T. The role of metal halides in enhancing the dehydration of xylose to furfural[J]. ChemCatChem,2015,7(3):479−489. doi: 10.1002/cctc.201402842
|
| [18] |
KAWAMURA K, SAKO K, OGATA T, TANABE K. Environmentally friendly, hydrothermal treatment of mixed fabric wastes containing polyester, cotton, and wool fibers: Application for HMF production[J]. Bioresour Technol Rep, 2020, 11: 100478−100486.
|
| [19] |
QI T, HE M, ZHU L, LYU Y, YANG H, HU C. Cooperative catalytic performance of Lewis and Brønsted acids from AlCl3 salt in aqueous solution toward glucose-to-fructose isomerization[J]. J Phys Chem C, 2019, 123(8): 4879−4891.
|
| [20] |
XIN H, ZHANG T, LI W, SU M, LI S, SHAO Q, MA L. Dehydration of glucose to 5-hydroxymethylfurfural and 5-ethoxymethylfurfural by combining Lewis and Brønsted acid[J]. RSC Adv,2017,7(66):41546−41551.
|
| [21] |
王兰英. 生物质糖类制备5-羟甲基糠醛和乙酰丙酸的研究[D]. 广州: 华南理工大学, 2018.WANG Lan-ying. Study on preparation of 5-hydroxymethylfurfural and acetylpropionic acid in biomass sugars[D]. Guangzhou: Institutes of Technology of South China, 2018.
|
| [22] |
尉慰奇. 碳水化合物催化转化制备羟甲基糠醛及乙酰丙酸的研究[D]. 广州: 华南理工大学, 2018.WEI Wei-qi. Preparation of hydroxymethylfurfural and levulinic acid by catalytic conversion of carbohydrate[D]. Guangzhou: Institutes of Technology of South China, 2018.
|
| [23] |
QU Y, ZHAO Y, XIONG S, WANG C, WANG S, ZHU L, MA L. Conversion of glucose into 5-hydroxymethylfurfural and levulinic acid catalyzed by $ {\rm{SO}}_{4}^{2-} $ /ZrO2 in a biphasic solvent system[J]. Energy Fuels,2020,34(9):11041−11049. doi: 10.1021/acs.energyfuels.0c01823 /ZrO2 in a biphasic solvent system[J]. Energy Fuels,2020,34(9):11041−11049. doi: 10.1021/acs.energyfuels.0c01823
|
| [24] |
陆强, 张栋, 朱锡锋. 四种金属氯化物对纤维素快速热解的影响(Ⅱ)机理分析[J]. 化工学报,2010,61(4):1025−1032.LU Qiang, ZHANG Dong, ZHU Xi-feng. Effect of four metal chlorides on rapid pyrolysis of cellulose (ⅱ) Mechanism analysis[J]. Chem J,2010,61(4):1025−1032.
|
| [25] |
徐志磊. 葡萄糖催化转化制备5-羟甲基糠醛的研究[D]. 长春: 长春工业大学, 2016.XU Zhi−lei. Preparation of 5-hydroxymethylfurfural by catalytic conversion of glucose[D]. Changchun: Changchun University of Technology, 2016.
|
| [26] |
袁永朋. 降解植物纤维素制备5-羟甲基糠醛[D]. 长春: 长春工业大学, 2013.YUAN Yong-peng. Preparation of 5-hydroxymethyl furfural by degraded plant cellulose[D]. Changchun: Changchun University of Technology, 2013.
|
| [27] |
GUO Y, ZENG Z, ZHU Y, HUANG Z, CUI Y, YANG J. Catalytic oxidation of aqueous organic contaminants by persulfate activated with sulfur-doped hierarchically porous carbon derived from thiophene[J]. Appl Catal B:Environ,2017,635−644.
|
| [28] |
SIVANANTHAM M, KESAVAMOORTHY R, SAIRAM T N, SABHARWAL K N, RAJ B. Stimulus response and molecular structural modification of polyacrylamide gel in nitric acid: A study by Raman, FTIR, and photoluminescence techniques[J]. J Poly Sci Part B, Polym Phys,2008,46(7):710−720. doi: 10.1002/polb.21402
|




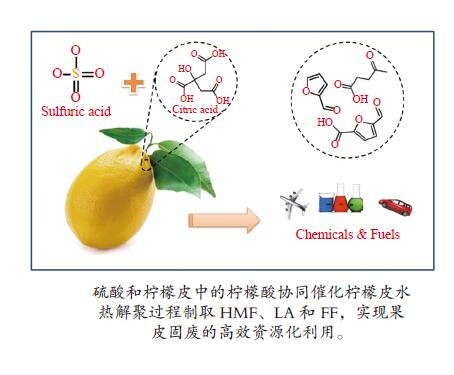
 下载:
下载: