Ash composition, structural characteristics and thermal conversion performance of Texaco gasifier based on Zhundong coal
-
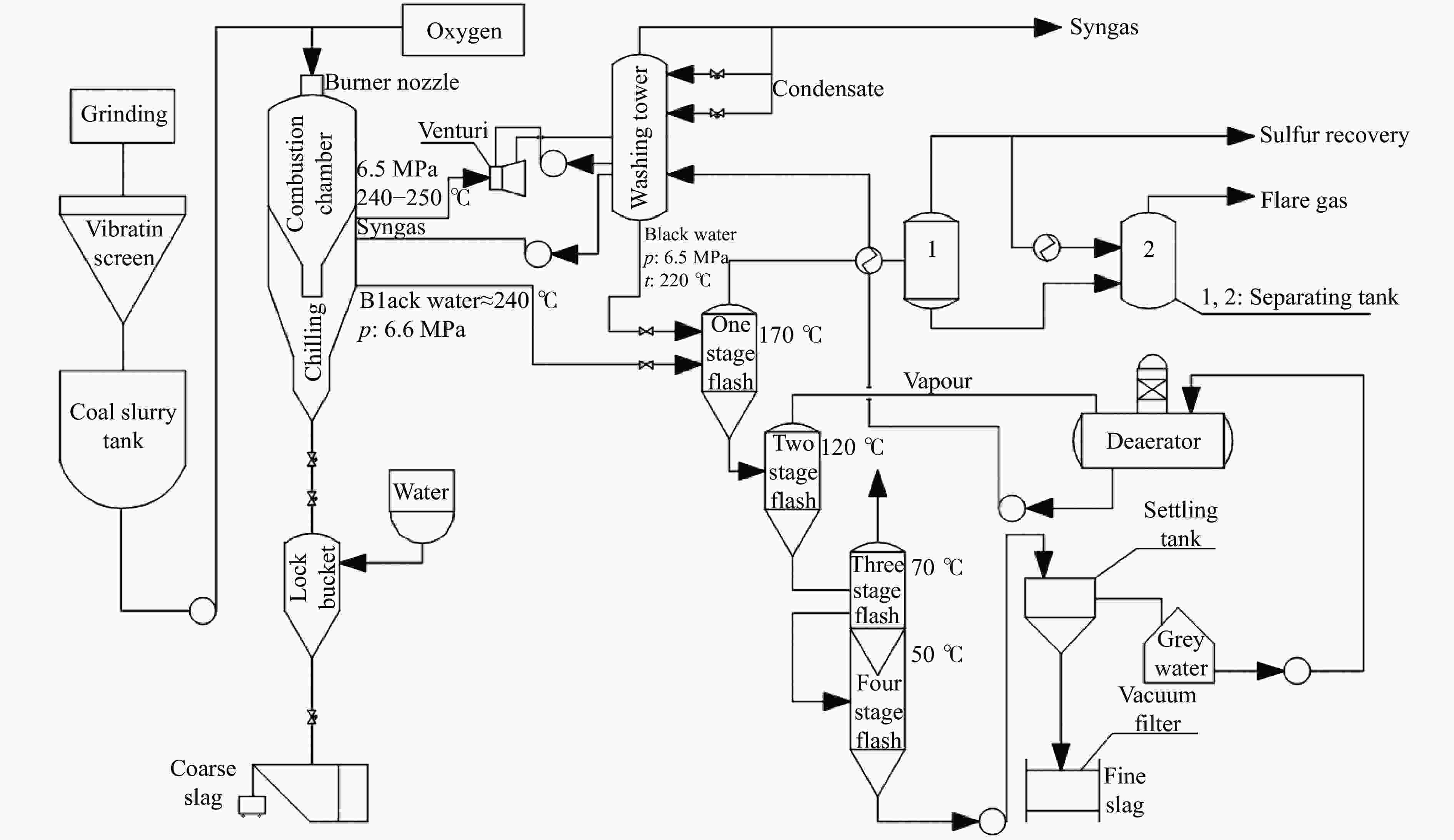
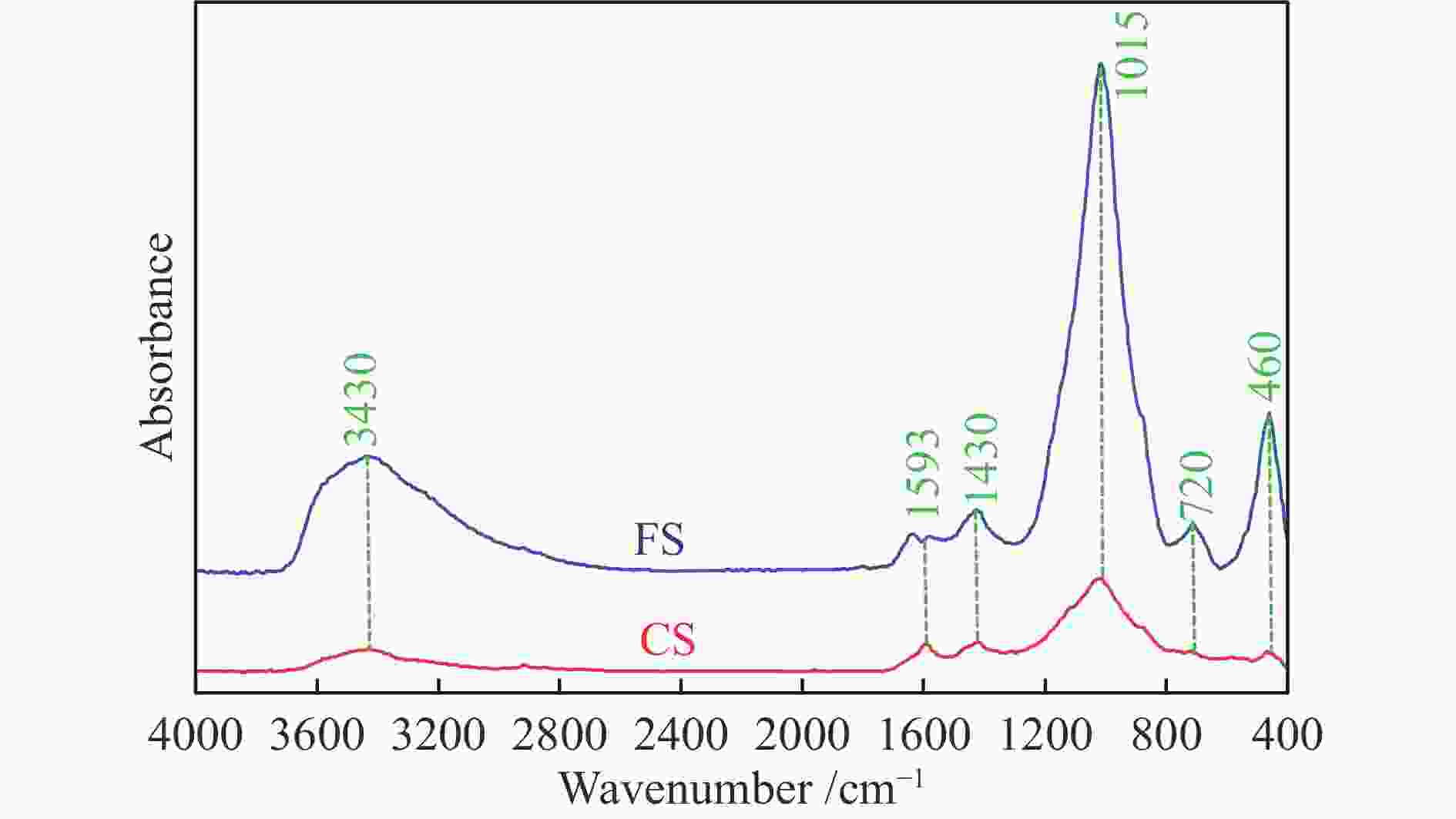
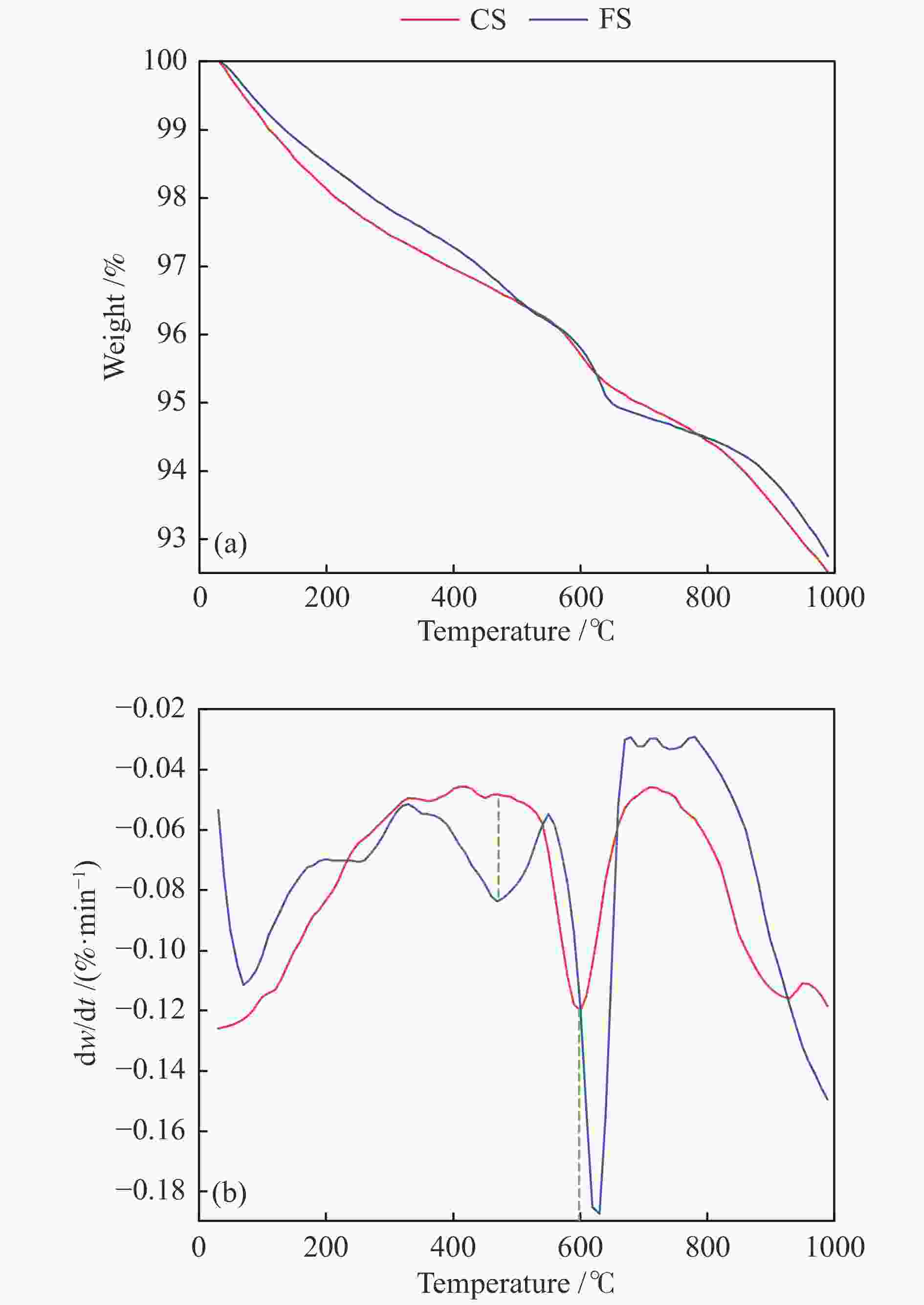
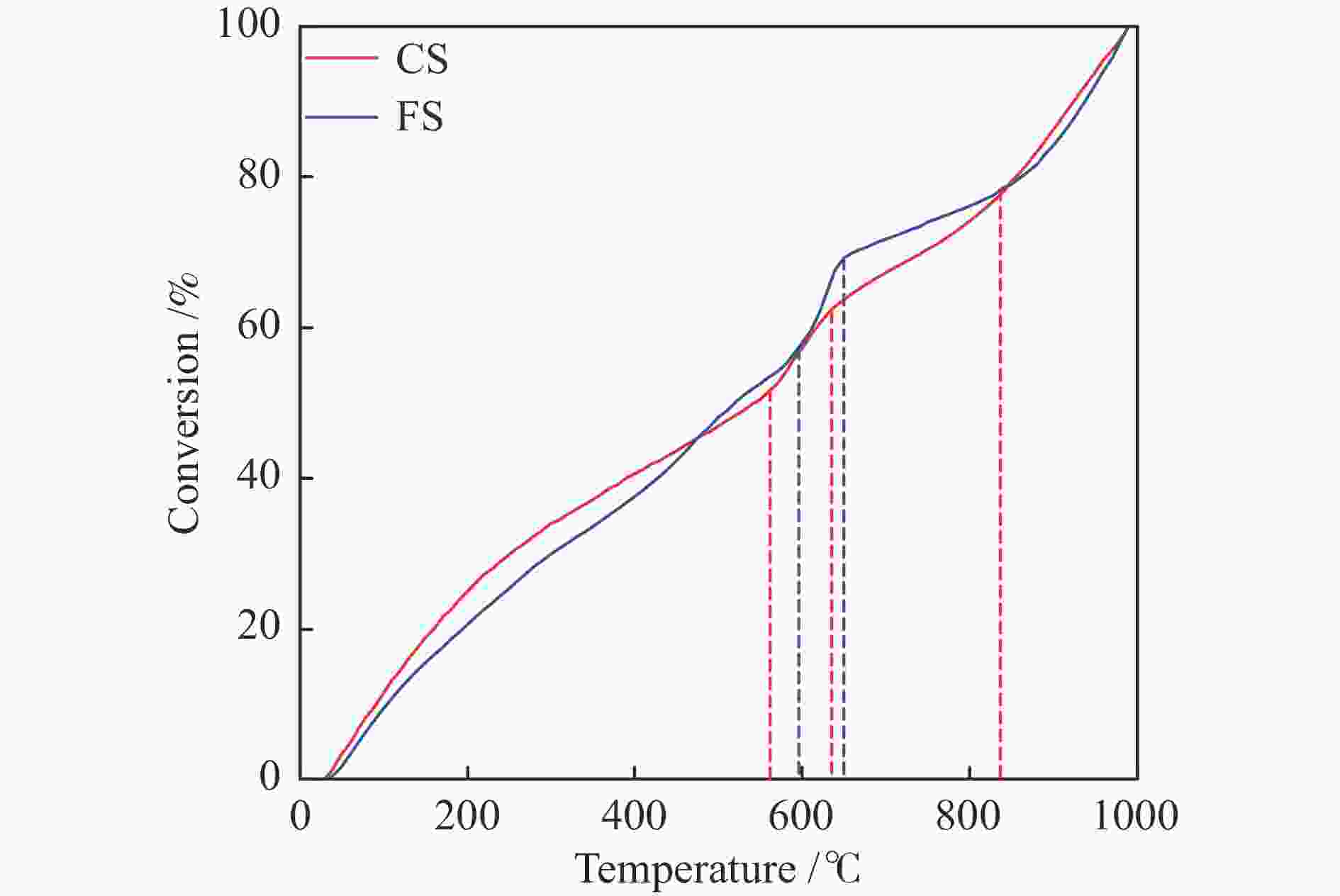
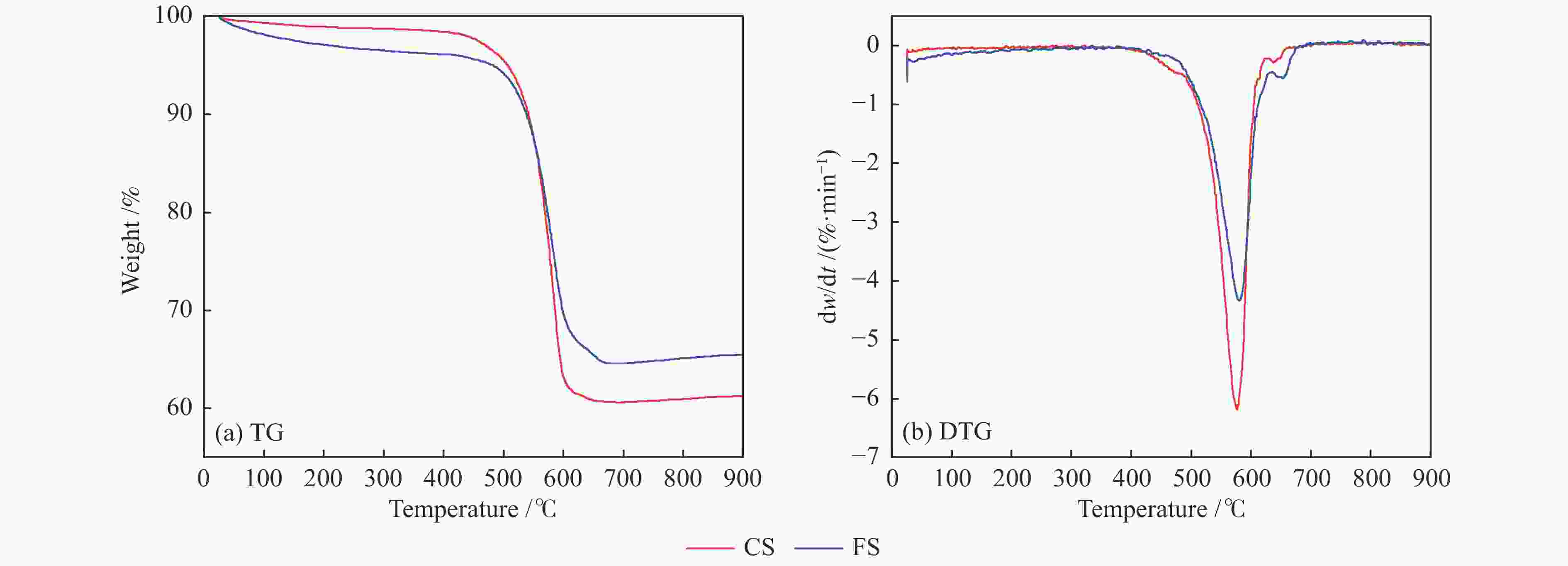
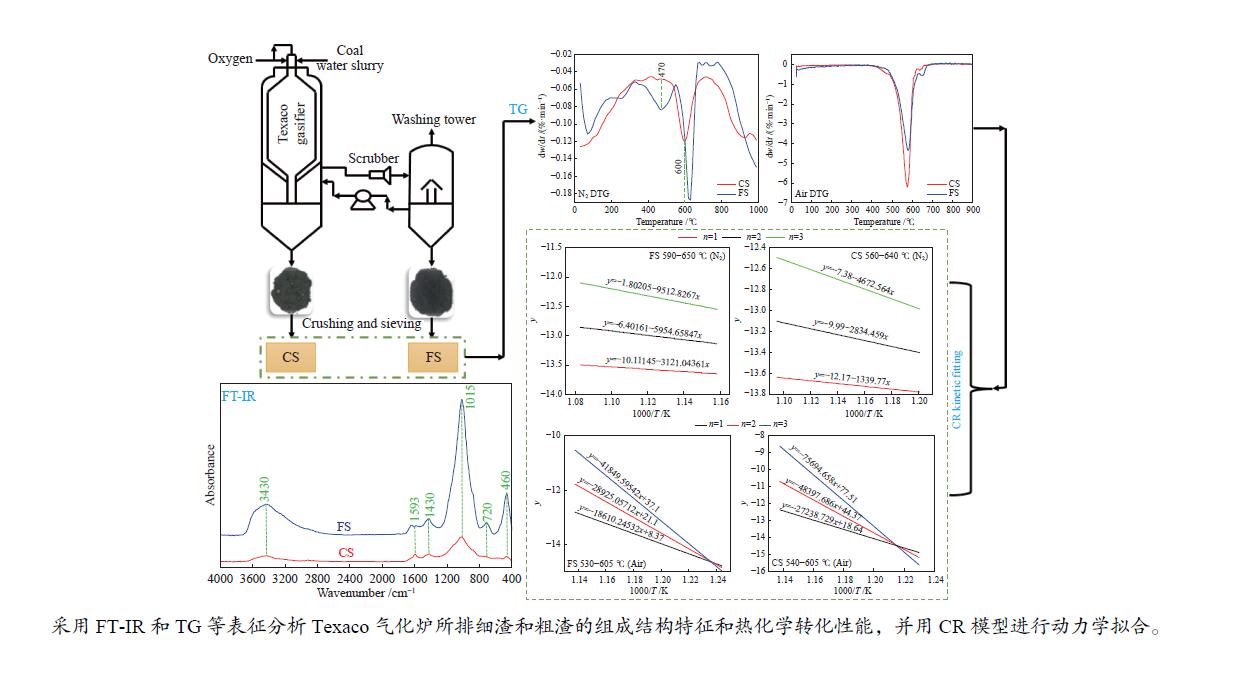
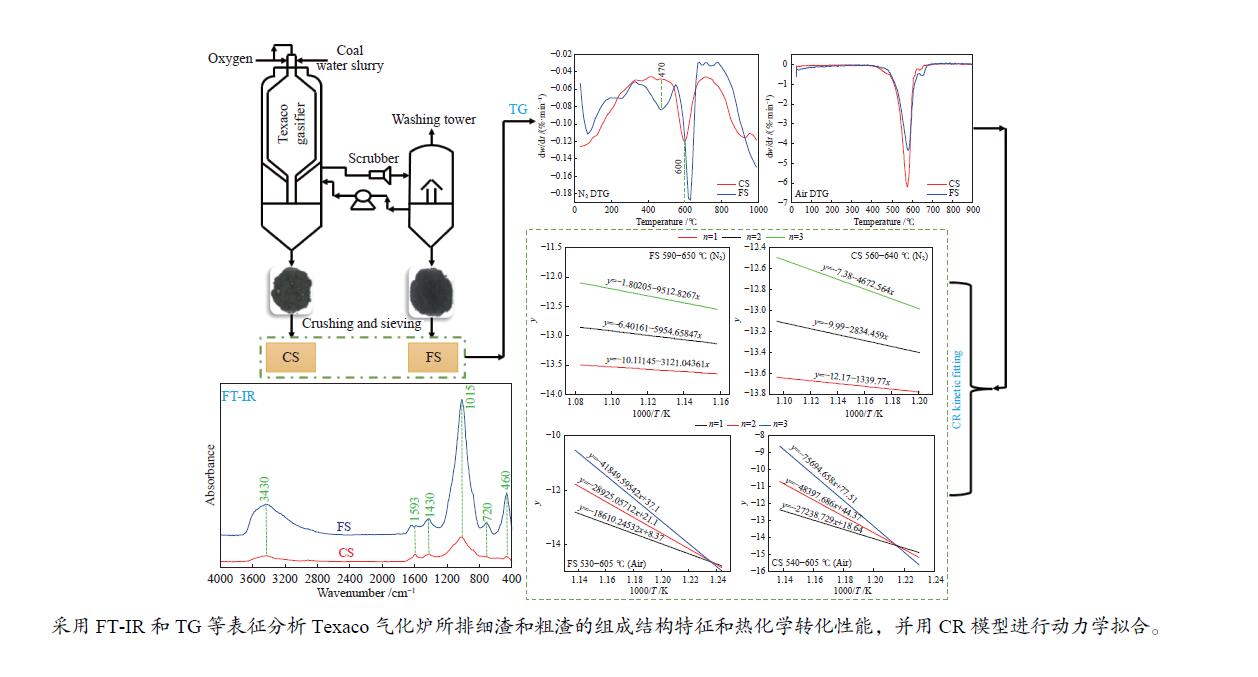
摘要: 解析以准东煤为原料的Texaco气化炉所排细渣和粗渣的组成结构特征,分析两者的热化学转化性能。结果表明,粗渣固定碳含量为42.31%,说明粗渣可以作为碳基原料实现高附加值利用。FT-IR分析显示,粗渣和细渣中Si−O的吸收峰更强,且细渣中含有少量的芳香结构。惰性气氛热分解行为发现,粗渣在600 ℃的失重速率最大,而细渣其最大失重速率峰转移到了620 ℃左右。氧化性气氛热分解结果表明,在500−700 ℃条件下,粗渣和细渣均有明显失重,且两者的失重主要源于固定碳的燃烧。采用Coats-Redfern法对惰性气氛和氧化性气氛下粗渣和细渣的失重曲线进行拟合,计算热解/燃烧活化能、相关系数等动力学参数。结果表明,在惰性气氛下,粗渣热解激烈段(560−640 ℃)反应级数设定为3时拟合效果较好,相关系数R2为0.99,活化能E为38.85 kJ/mol。细渣热解激烈段(590−650 ℃)同样在反应级数设定为3时拟合效果较好,相关系数R2为0.97,活化能E为79.09 kJ/mol。在氧化性气氛下,粗渣和细渣燃烧激烈段分别在540−605 ℃和530−605 ℃,反应级数均以n = 1时拟合效果较好,活化能E分别为226.46和154.73 kJ/mol。
-
关键词:
- 煤气化 /
- 气化渣 /
- FT-IR /
- 热重分析法 /
- Coats-Redfern模型
Abstract: Composition and structure characteristics of fine slag (FS) and coarse slag (CS) discharged from Texaco gasifier with Zhundong coal as raw material were analyzed, and their thermochemical conversion properties were analyzed. Proximate and ultimate analyses show that the contents of fixed carbon in coarse slag are 42.31%, indicating that it can be used as raw material to realize its high-added utilization. Analysis with Fourier transform infare spectrometer (FT-IR) suggest that the absorption peak of Si−O in coarse slag and fine slag is stronger, and there is a small amount of aromatic structure in fine slag. Thermal decomposition behaviors in inert atmosphere show that the maximum weight loss rate peak of coarse slag is located around 600 ℃, while that of fine slag is transferred to about 620 ℃. Results of thermal decomposition in oxidization atmosphere show that there are obvious weight loss rate peaks derived from combustion of the fixed carbon in 500−700 ℃. The weight loss profiles of fine slag and coarse slag in inert and oxidization atmosphere were fitted by Coats-Redfern method. The kinetic parameters including pyrolysis/combustion activation energy and correlation coefficient were calculated. The results show that the fitting effect is better as the reaction order selected as 3 at the intense pyrolysis section (560−640 ℃) for coarse slag in inert atmosphere, with correlation coefficient R2 of 0.99 and activation energy E of 38.85 kJ/mol. Similarly, in the intense pyrolysis stage (590−650 ℃) of fine slag, the fitting effect is better as the reaction order selected 3, with the correlation coefficient R2 of 0.97 and the activation energy E of 79.09 kJ/mol. In oxidization atmosphere, at the intense combustion stage of coarse slag (540−605 ℃) and fine slag (530−605 ℃), the fitting effect is better as n=1 for the both slags, with the activation energy E of 226.46 and 154.73 kJ/mol respectively.-
Key words:
- coal gasification /
- gasification slag /
- FT-IR /
- thermogravimetric analysis /
- Coats-Redfern model
-
表 1 FS和CS的工业和元素分析
Table 1 Proximate and ultimate analyses of FS and CS
Sample Proximate analysis wad/% Ultimate analysis w/% H/C O/C M A V FC $ {{\rm{C}}_{{\rm{daf}}}}$ $ {{\rm{H}}_{{\rm{daf}}}}$ ${\rm{O}}_{{\rm{daf}}}^*$ $ {{\rm{N}}_{{\rm{daf}}}}$ $ {{\rm{S}}_{{\rm{t, d}}}}$ FS 2.10 66.76 6.09 25.05 28.87 0. 47 > 67.63 2.28 0.75 0.23 2.05 CS 1.71 52.89 3.09 42.31 29.60 0.21 > 67.43 2.30 0.46 0.09 1.71 * by difference 表 2 FS和CS的红外光谱分峰拟合的各吸收峰面积比例
Table 2 Area ratio of each absorption peak fitted by the infrared spectrum of FS and CS
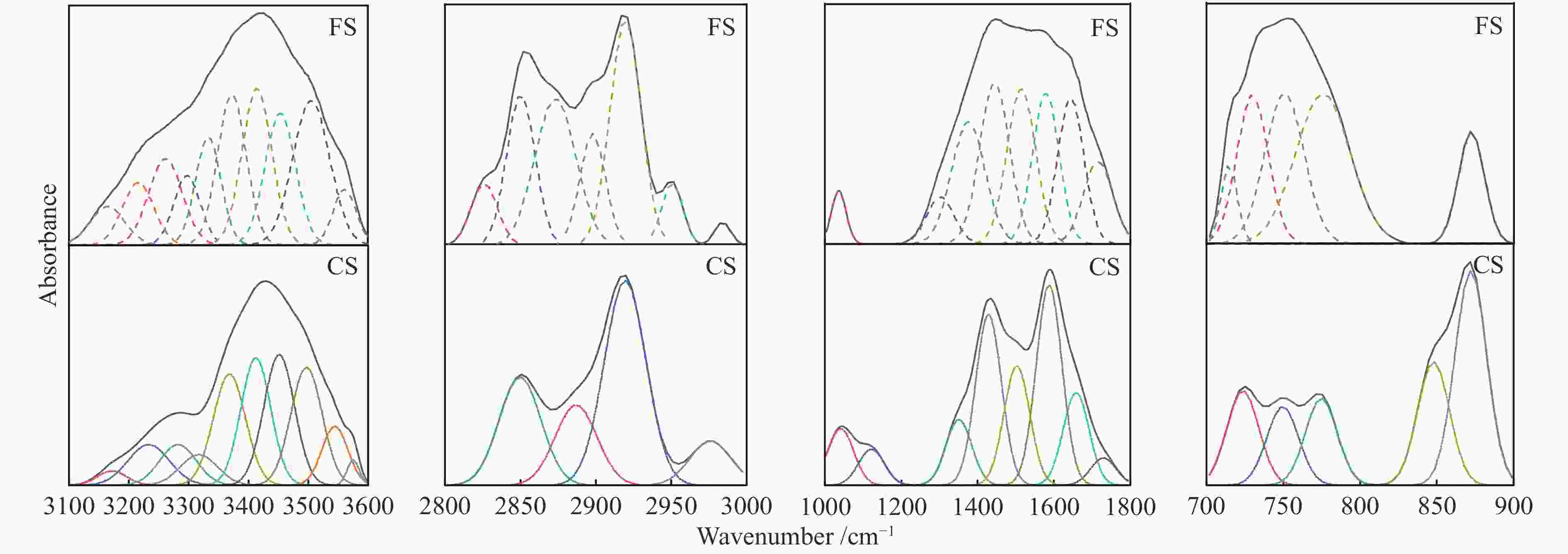
Wavenumber /cm−1 Functional group Content area/% CS FS 3600−3500 OH−π 8.43 21.74 3500−3350 self-associated OH 69.71 42.81 3350−3260 OH−ether O 12.15 24.69 3260−3170 cyclic OH 9.71 10.76 2950−2930 aliphatic −CH3 9.54 7.42 2930−2900 asymmetric aliphatic −CH2 47.09 29.54 2900−2870 aliphatic −CH 18.49 37.76 2870−2800 symmetric aliphatic −CH2 24.88 25.28 1700 carboxylic acids C = O 3.50 9.34 1650 conjugated C = O 12.18 15.00 1600−1480 C = C in ARs 42.00 33.03 1480−1400 asymmetric −CH3, −CH2 22.44 18.75 1400−1240 symmetric deformation −CH3 8.64 20.97 1240−1160 phenols C−OH 0 0 1160−1090 grease C−O 4.75 0 1090−1030 alkyl ethers 6.50 2.91 900−860 five adjacent H deformation 35.82 12.67 860−810 four adjacent H deformations 20.75 0 810−750 three adjacent H deformations 14.59 61.44 750−720 two adjacent H deformations 28.84 25.89 表 3 FS和CS热解动力学参数
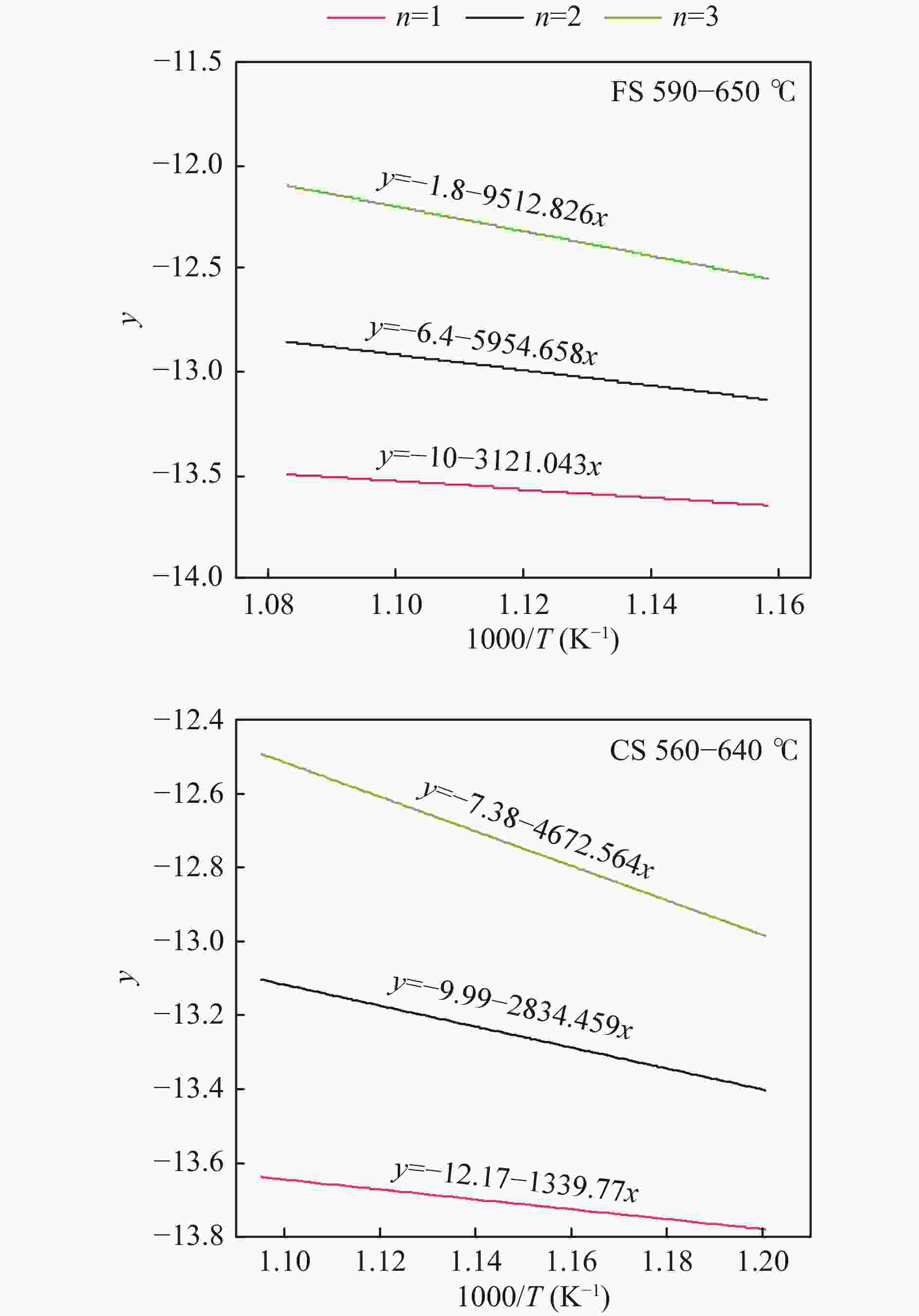
Table 3 Calculation results of pyrolysis kinetic parameters of FS and CS
Sample RO y R2 E/(kJ · mol−1) A/min−1 2RT/E CS at 560−640 ℃ 1 −12.17 −1339.77x 0.98641 11.14 6.94 × 10−2 1.30 2 −9.99 −2834.459x 0.99229 23.57 1.29 0.62 3 −7.38 −4672.564x 0.99328 38.85 20.93 0.37 CS at 640−840 ℃ 1 −13.63 −14.5552x 0.00461 0.12 1.74 × 10−4 146.06 2 −11.49 −1498.34x 0.89846 12.46 0.15 1.42 3 −8.81 −3410.567x 0.93575 28.36 5.07 0.62 FS at 590−650 ℃ 1 −10 −3121.043x 0.96494 25.95 11.27 0.57 2 −6.4 −5954.658x 0.96934 49.51 90.88 0.30 3 −1.8 −9512.826x 0.97026 79.09 1.57 × 104 0.19 FS at 650−840 ℃ 1 −13.6 −25.85916x 0.01240 0.21 3.13 × 10−4 78.73 2 −11.4 −1544.823x 0.89294 12.84 0.17 1.32 3 −8.7 −3504.8109x 0.93264 29.14 5.70 0.58 表 4 FS和CS的燃烧特性指数
Table 4 Combustion characteristic index of FS and CS
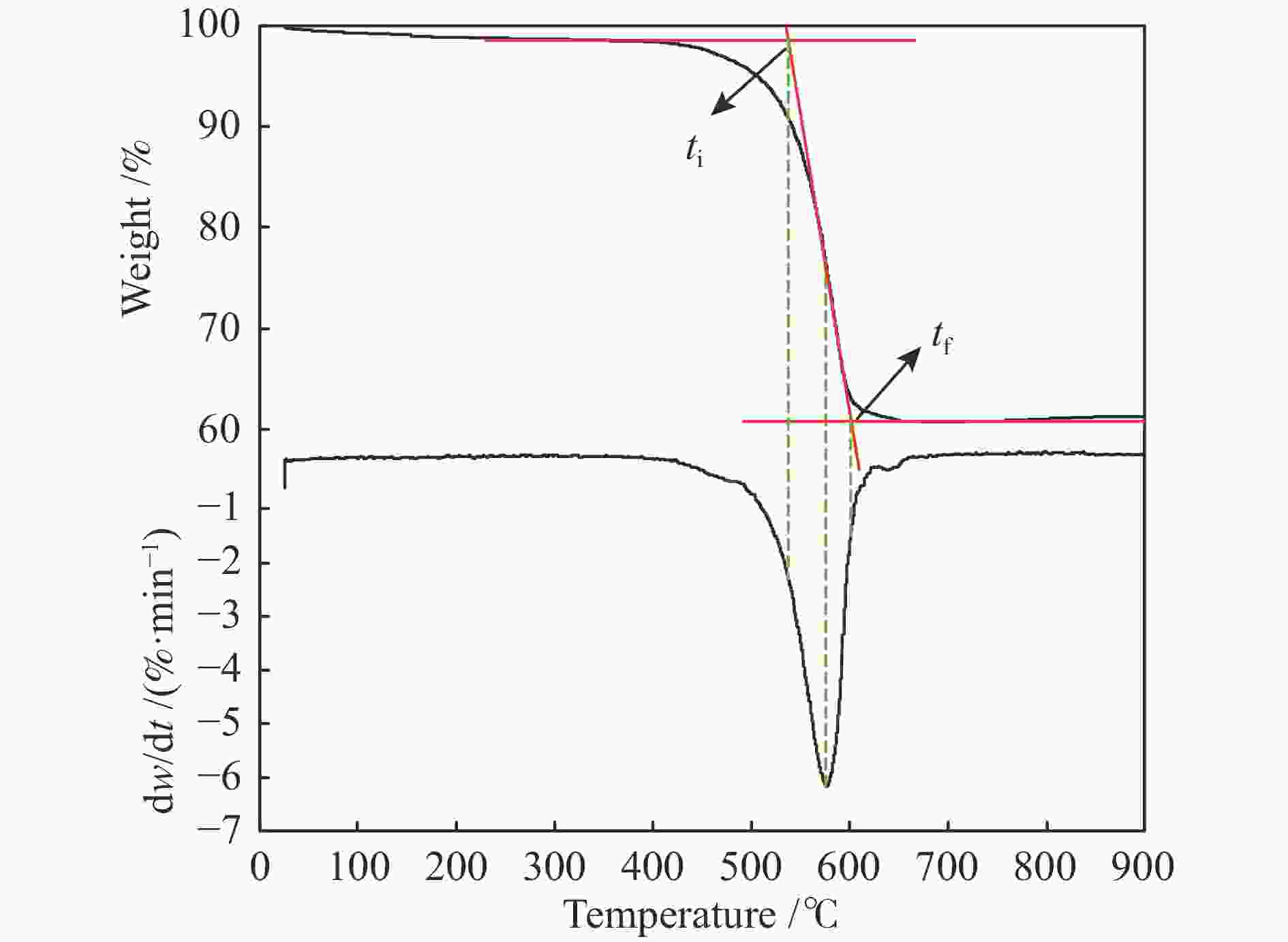
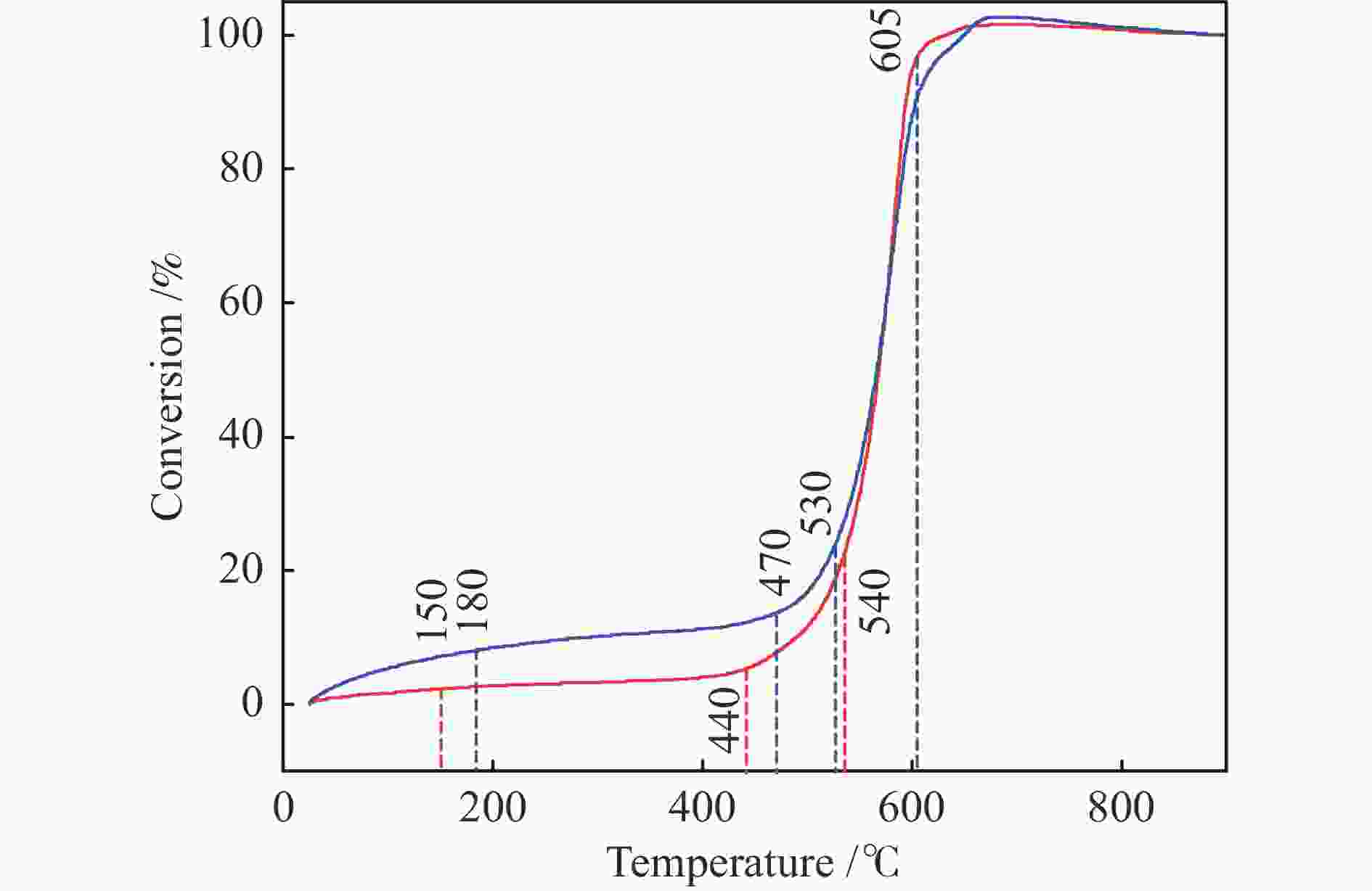
Sample Temp. /℃ DTG/(%·min−1) S ti tf tmax DTGmax DTGmean CS 530 605 575 −6.14 −3.84 1.39×10−7 FS 530 615 580 −4.33 −2.73 6.84×10−8 表 5 FS和CS燃烧动力学参数
Table 5 Calculation results of combustion kinetic parameters of FS and CS
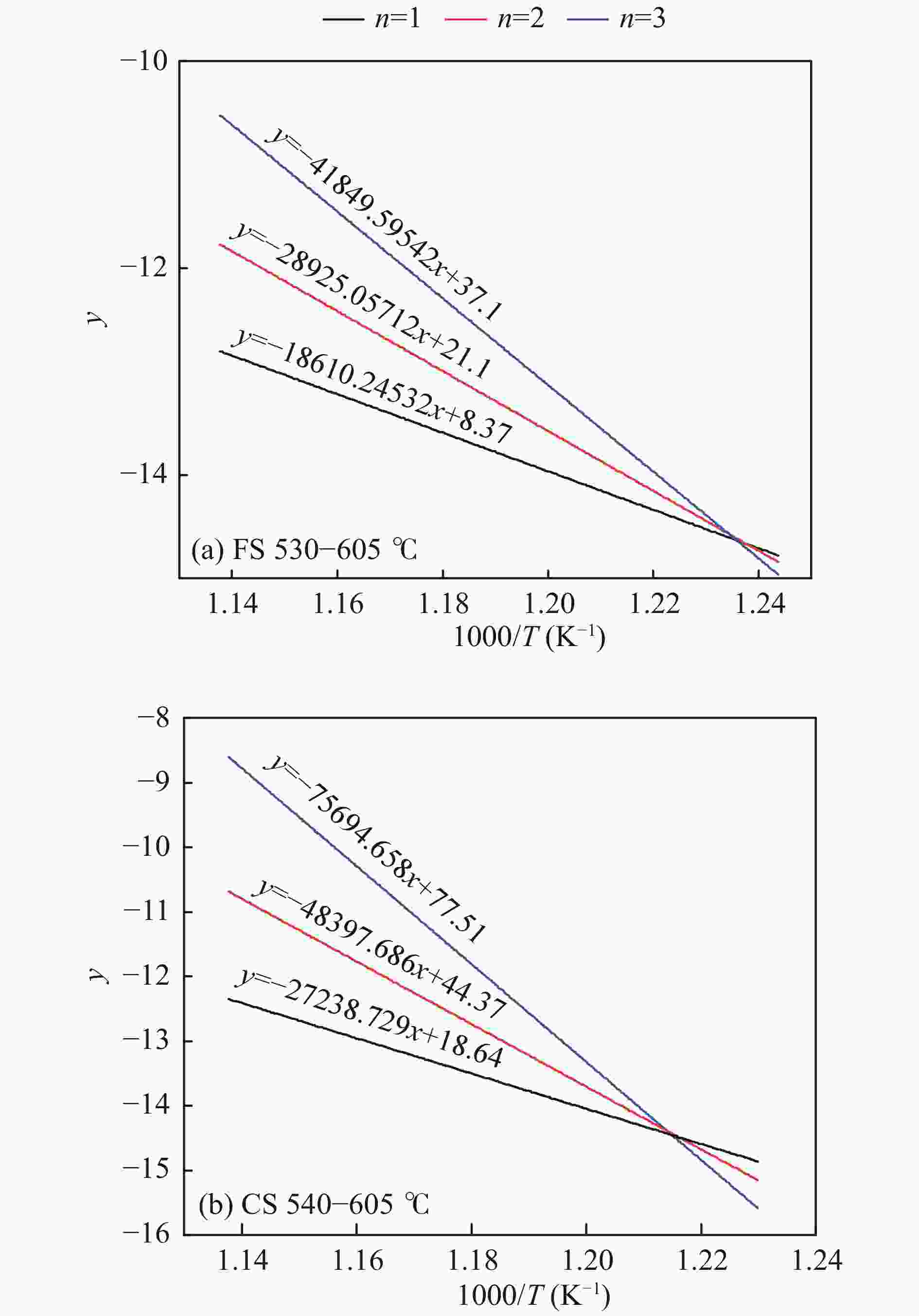
Sample RO y R2 E/(kJ·mol−1) A /min−1 2RT/E CS at 440−540 ℃ 1 −7807.17955x − 5.22 0.97621 64.91 4.21 × 102 0.20 2 −8392.50029x − 4.39 0.97159 69.78 1.04 × 103 0.18 3 −9004.53583x − 3.52 0.96685 74.86 2.65 × 103 0.17 CS at 540−605 ℃ 1 −27238.729x + 18.64 0.98599 226.46 3.41 × 1013 0.06 2 −48397.686x + 44.37 0.93520 402.38 9.06 × 1024 0.03 3 −75694.658x + 77.51 0.89456 629.33 3.50 × 1039 0.02 FS at 470−530 ℃ 1 −5307.40144x − 8.113 0.94235 44.13 12.59 0.29 2 −6001.65335x − 7.118 0.94055 49.90 40.86 0.26 3 −6740.69158x − 6.062 0.93875 56.04 1.57 × 102 0.23 FS at 530−605 ℃ 1 −18610.24532x + 8.37 0.99033 154.73 8.03 × 108 0.09 2 −28925.05712x + 21.1 0.96508 240.48 4.38 × 1014 0.06 3 −41849.59542x + 37.1 0.93957 347.94 5.37 × 1021 0.04 -
[1] XU J, YANG Y, LI Y. Recent development in converting coal to clean fuels in China[J]. Fuel,2015,152:122−130. doi: 10.1016/j.fuel.2014.11.059 [2] WANG W D, LIU D H, TU Y N, JIN L Z, WANG H. Enrichment of residual carbon in entrained-flow gasification coal fine slag by ultrasonic flotation[J]. Fuel,2020,278:118195. doi: 10.1016/j.fuel.2020.118195 [3] GUO F H, MIAO Z K, GUO Z K, LI J, ZHANG Y X, WU J J. Properties of flotation residual carbon from gasification fine slag[J]. Fuel,2020,267:117043. doi: 10.1016/j.fuel.2020.117043 [4] DAI G F, ZHENG S J, WANG X B, BAI Y H, DONG Y S, DU J, SUN X W, TAN H Z. Combustibility analysis of high-carbon fine slags from an entrained flow gasifier[J]. J Environ Manage,2020,271:111009. doi: 10.1016/j.jenvman.2020.111009 [5] MIAO Z K, WU J J, ZHANG Y X, ZHAO X, GUO F H, GUO Z K, GUO Y. Chemical characterizations of different sized mineral-rich particles in fine slag from Entrained-flow gasification[J]. Adv Powder Technol,2020,31(9):3715−3723. doi: 10.1016/j.apt.2020.07.010 [6] LI C C, QIAO X C, YU J G. Large surface area MCM-41 prepared from acid leaching residue of coal gasification slag[J]. Minerals,2016,167:246−249. [7] ZHANG Y X, DONG J X, GUO F H, SHAO Z Y, WU J J. Zeolite synthesized from coal fly ash produced by a gasification process for Ni2+ removal from water[J]. Minerals,2018,8(3):116. doi: 10.3390/min8030116 [8] GUO Y, GUO F H, ZHOU L, GUO Z K, MIAO Z K, LIU H, ZHANG X X, WU J J, ZHANG Y X. Investigation on co-combustion of coal gasification fine slag residual carbon and sawdust char blends: Physiochemical properties, combustion characteristic and kinetic behavior[J]. Fuel,2021,292:120387. doi: 10.1016/j.fuel.2021.120387 [9] DU M J, HUANG J J, LIU Z Y, ZHOU X, GOU S, WANG Z Q, FANG Y T. Reaction characteristics and evolution of constituents and structure of a gasification slag during acid treatment[J]. Fuel,2018,224:178−185. doi: 10.1016/j.fuel.2018.03.073 [10] GU Y Y, QIAO X C. A carbon silica composite prepared from water slurry coal gasification slag[J]. Microporous Mesoporous Mater,2018,276:303−307. [11] ZHOU C C, LIU G J, FANG T, PAUL KWAN SING LAM. Investigation on thermal and trace element characteristics during co-combustion biomass with coal gangue[J]. Bioresour Technol,2015,175:454−462. doi: 10.1016/j.biortech.2014.10.129 [12] MIAN I, LI X, JIAN Y M, DACRES O D, ZHONG M, LIU J M, MA F Y, RAHMAN N. Kinetic study of biomass pellet pyrolysis by using distributed activation energy model and Coats Redfern methods and their comparison[J]. Bioresour Technol,2019,294:122099. doi: 10.1016/j.biortech.2019.122099 [13] PARK S W, JANG C H. Effects of pyrolysis temperature on changes in fuel characteristics of biomass char[J]. Energy,2012,39(1):187−195. doi: 10.1016/j.energy.2012.01.031 [14] LI H, XIA S Q, MA P S. Thermogravimetric investigation of the co-combustion between the pyrolysis oil distillation residue and lignite[J]. Bioresour Technol,2016,218:615−622. doi: 10.1016/j.biortech.2016.06.104 [15] JIANG D H, SONG W J, WANG X F, ZHU Z P. Physicochemical properties of bottom ash obtained from an industrial CFB gasifier[J]. J Energy Inst,2021,95:1−7. doi: 10.1016/j.joei.2020.12.004 [16] OZVEREN U, KARTAL F, SEZER S, OZDOGAN Z S. Investigation of steam gasification in thermogravimetric analysis by means of evolved gas analysis and machine learning[J]. Energy,2021,239(C):122232. [17] MAGALHAES D, KAZANC F, RIAZA J, ERENSOY S, KABAKLI O, CHALMERS H. Combustion of Turkish lignites and olive residue: Experiments and kinetic modelling[J]. Fuel,2017,203:868−876. doi: 10.1016/j.fuel.2017.05.050 [18] WANG J, ZHANG S Y, GUO X, DONG A X, CHEN C, XIONG S W, FANG Y T, YIN W D. Thermal behaviors and kinetics of Pingshuo coal/biomass blends during copyrolysis and cocombustion[J]. Energy Fuels,2012,26(12):7120−7126. doi: 10.1021/ef301473k [19] ULLAH H, LIU G, YOUSAF B, ALI M U, ABBSA Q, ZHOU C C. Combustion characteristics and retention-emission of selenium during co-firing of torrefied biomass and its blends with high ash coal[J]. Bioresour Technol,2017,245(A):73−80. [20] LIU Z G, QUEK A, HOEKMAN S K, SRINIVASAN M P, BALASUBRAMANIAN R. Thermogravimetric investigation of hydrocarlignite co-combustion[J]. Bioresour Technol,2012,123:646−652. doi: 10.1016/j.biortech.2012.06.063 [21] WANG X B, LI S S, ADEOSUN A, CUJANOVIC M, TAN H Z, DUIC N. Effect of potassium-doping and oxygen concentration on soot oxidation in O2/CO2 atmosphere: A kinetics study by thermogravimetric analysis[J]. Energy Convers Manage,2017,149:686−697. doi: 10.1016/j.enconman.2017.01.003 [22] MO W L, WU Z F, HE X Q, QIANG W J, WEI B, WEI X Y, WU Y L, FAN X, MA F Y. Functional group characteristics and pyrolysis/combustion performance of fly ashes from Karamay oily sludge based on FT-IR and TG-DTG analyses[J]. Fuel,2021,296(5):120669. [23] WANG G W, ZHANG J L, CHANG W W, LI R P, LI Y J, WANG C. Structural features and gasification reactivity of biomass chars pyrolyzed in different atmospheres at high temperature[J]. Energy,2018,147:25−35. doi: 10.1016/j.energy.2018.01.025 [24] LIN X C, WANG C H, IDETA K, MIYAWAKI J, NISHIYAMA Y, WANG Y G, YOON S, MOCHIDA I. Insights into the functional group transformation of a Chinese brown coal during slow pyrolysis by combining various experiments[J]. Fuel,2014,118:257−264. doi: 10.1016/j.fuel.2013.10.081 [25] ZHANG S, CHEN Z D, ZHANG H Y, WANG Y G, XU X Q, CHENG L, ZHANG Y M. The catalytic reforming of tar from pyrolysis and gasification of brown coal: Effects of parental carbon materials on the performance of char catalysts[J]. Fuel Process Technol,2018,174:142−148. doi: 10.1016/j.fuproc.2018.02.022 [26] SONIBARE O O, HAEGER T, FOLEY S F. Structural characterization of Nigerian coals by X-ray diffraction, Raman and FTIR spectroscopy[J]. Energy,2010,35(12):5347−5353. doi: 10.1016/j.energy.2010.07.025 [27] IBARRA I, MUNOZ E, MOLINER R. FTIR study of the evolution of coal structure during the coalification process[J]. Org Geochem,1996,24(6/7):725−735. doi: 10.1016/0146-6380(96)00063-0 [28] DLAPA P, BODI M B, MATAIX-SOLERA J, CERDA A, DOERR S H. FT-IR spectroscopy reveals that ash water repellency is highly dependent on ash chemical composition[J]. Catena,2013,108:35−43. doi: 10.1016/j.catena.2012.02.011 [29] CHANDRASEKHAR S, PRAMADA P N, PRAVEEN L. Effect of organic acid treatment on the properties of rice husk silica[J]. J Mater Sci,2005,40(24):6535−6544. doi: 10.1007/s10853-005-1816-z [30] HE X Q, LIU X F, NIE B S, SONG D Z. FTIR and Raman spectroscopy characterization of functional groups in various rank coals[J]. Fuel,2017,206:555−563. doi: 10.1016/j.fuel.2017.05.101 [31] SONG H J, LIU G R, ZHANG J Z, WU J H. Pyrolysis characteristics and kinetics of low rank coals by TG-FTIR method[J]. Fuel Process Technol,2017,156:454−460. doi: 10.1016/j.fuproc.2016.10.008 [32] ZHAO L, NI G H, WANG H, SUN Q, WANG G, JIANG B Y, ZHANG C. Molecular structure characterization of lignite treated with ionic liquid via FTIR and XRD spectroscopy[J]. Fuel,2020,272:117705. doi: 10.1016/j.fuel.2020.117705 -




 下载:
下载: