Effect of Fe3O4 content on the CO2 selectivity of iron-based catalyst for Fischer-Tropsch synthesis
-
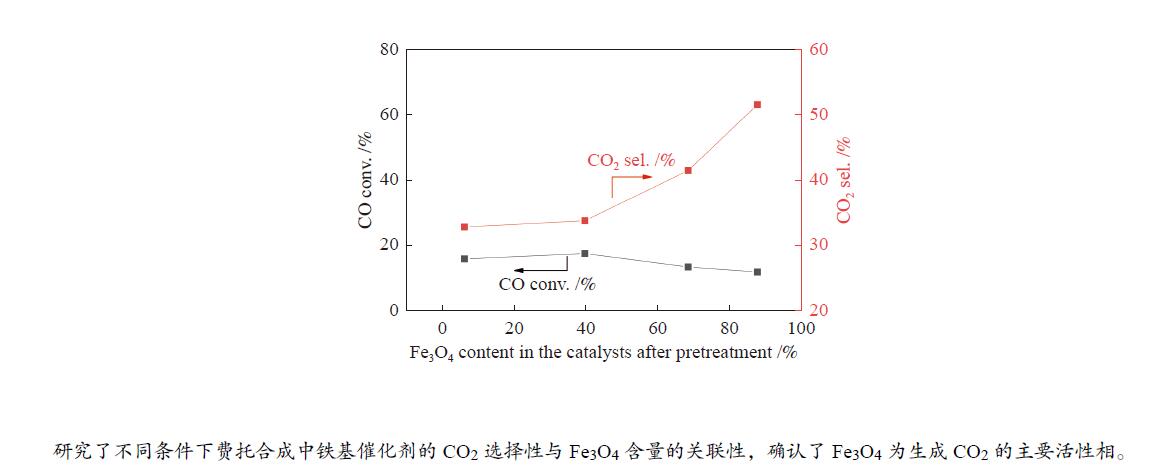
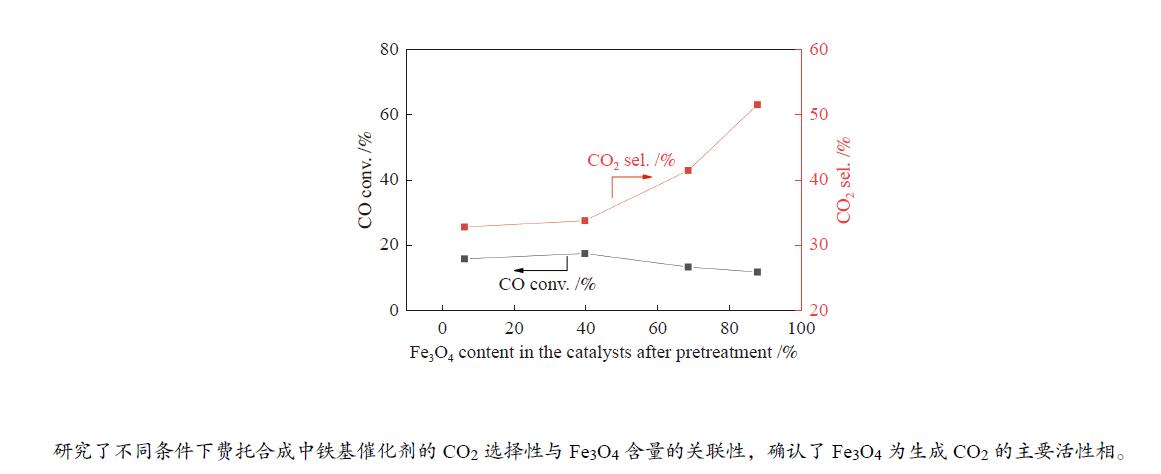
摘要: 本研究以共沉淀法制备的α-Fe2O3催化剂为前驱体,通过调变碳化温度和碳化时间制备了不同物相组成的系列催化剂,采用XRD、Mössbauer谱、XPS和Raman光谱等技术考察了催化剂体相和表面物相组成,在此基础上研究了不同条件下(不同CO转化率和H2O分压)催化剂的物相组成与催化剂性能之间的关系,重点探究了费托合成条件下CO2生成的活性相。结果表明,升高碳化温度和延长碳化时间有利于Fe3O4向碳化铁转变。在典型的费托合成条件下,催化剂的活性受到碳化铁含量和积炭程度的共同影响。当H2O分压较低时,动力学因素限制了水煤气变换(WGS)反应的进行,CO2选择性仅受CO转化率的影响,Fe3O4含量变化对CO2选择性无明显影响;而在较高的H2O分压下,随着催化剂中Fe3O4含量增加, CO2选择性也随之增加。本文初步阐明了Fe3O4是铁基费托合成催化剂中WGS反应的主要活性相,为认识Fe基费托合成催化剂CO2生成的活性相提供了新的信息,为新型低CO2选择性费托合成工业催化剂的设计奠定了基础。Abstract: In this study, a series of catalysts with different Fe3O4 to iron carbide ratios were obtained by carburizing the α-Fe2O3 precursor prepared by co-precipitation method, under various carburization conditions. XRD, Mössbauer spectroscopy, XPS, and Raman spectroscopy were used to characterize the bulk and surface phase compositions of the Fe-based catalysts. The results show that increasing the carburization temperature and prolonging the carburization time lead to higher iron carbide concentration. To explore the active phase of CO2 formation, the catalysts were tested under different reaction conditions by tuning either CO conversion or H2O partial pressure. It turns out that the catalytic performance of the Fe-based catalyst in the FTS and water-gas shift (WGS) reactions is influenced by both the content of iron carbide and the degree of carbon deposition. Under typical Fischer-Tropsch reaction condition, the CO2 selectivity is determined by the CO conversion rather than the Fe3O4 content in the catalyst, meaning that the WGS reaction is here limited by the kinetic factors. On the contrary, adding H2O to the reaction gas results in the trend that higher CO2 selectivity is promoted by higher content of Fe3O4 in the Fe-based catalyst. It seems that Fe3O4 is the main active phase for the WGS reaction in the iron-based catalyst for FTS. These results provide a new insight into the active phase of CO2 generation on the Fe-based catalysts, which could be the theoretical basis for the design of new industrial FTS catalysts with low CO2 selectivity.
-
Key words:
- Fischer-Tropsch synthesis /
- iron-based catalyst /
- Fe3O4 /
- CO2 selectivity /
- WGS reaction
-
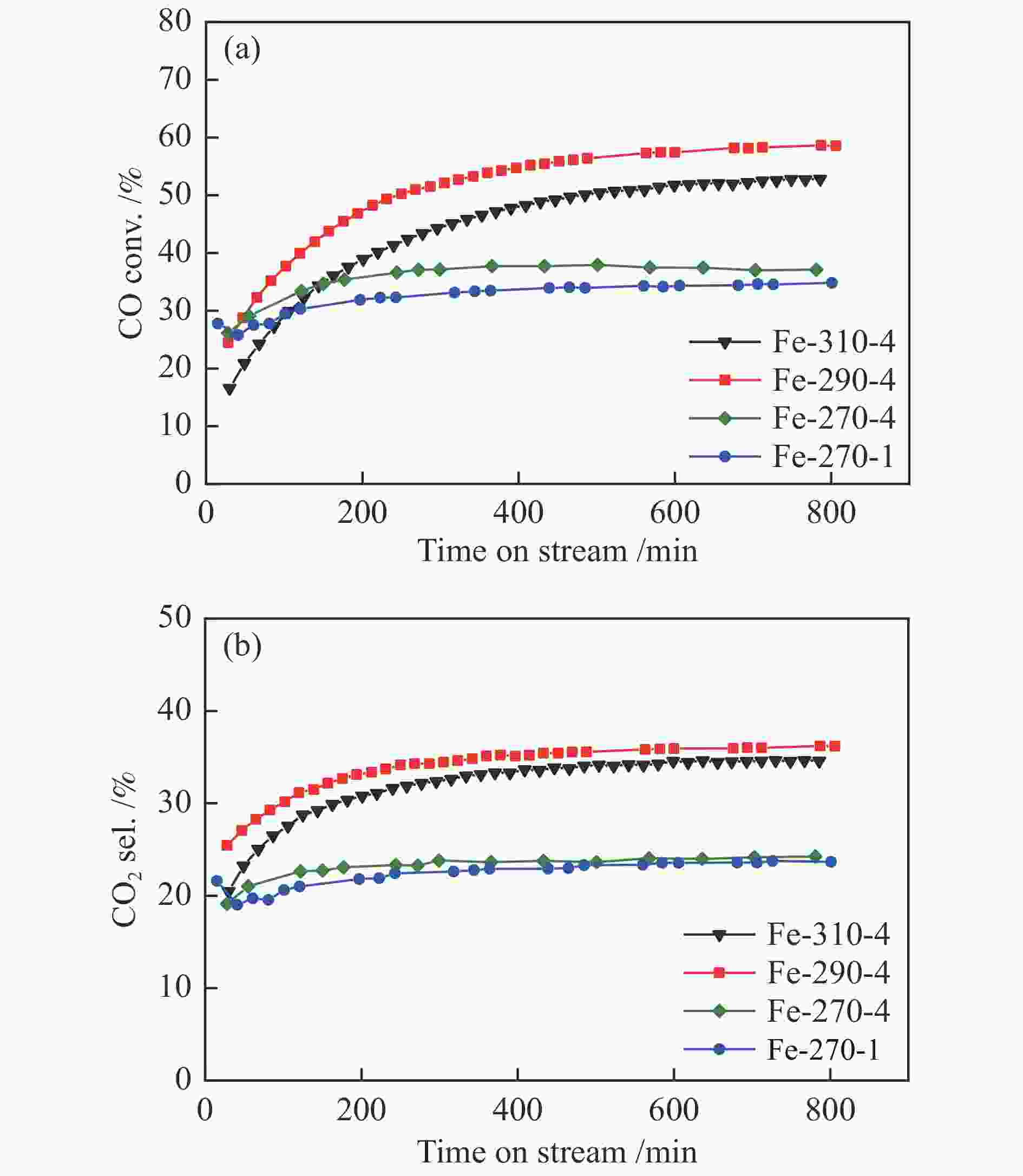
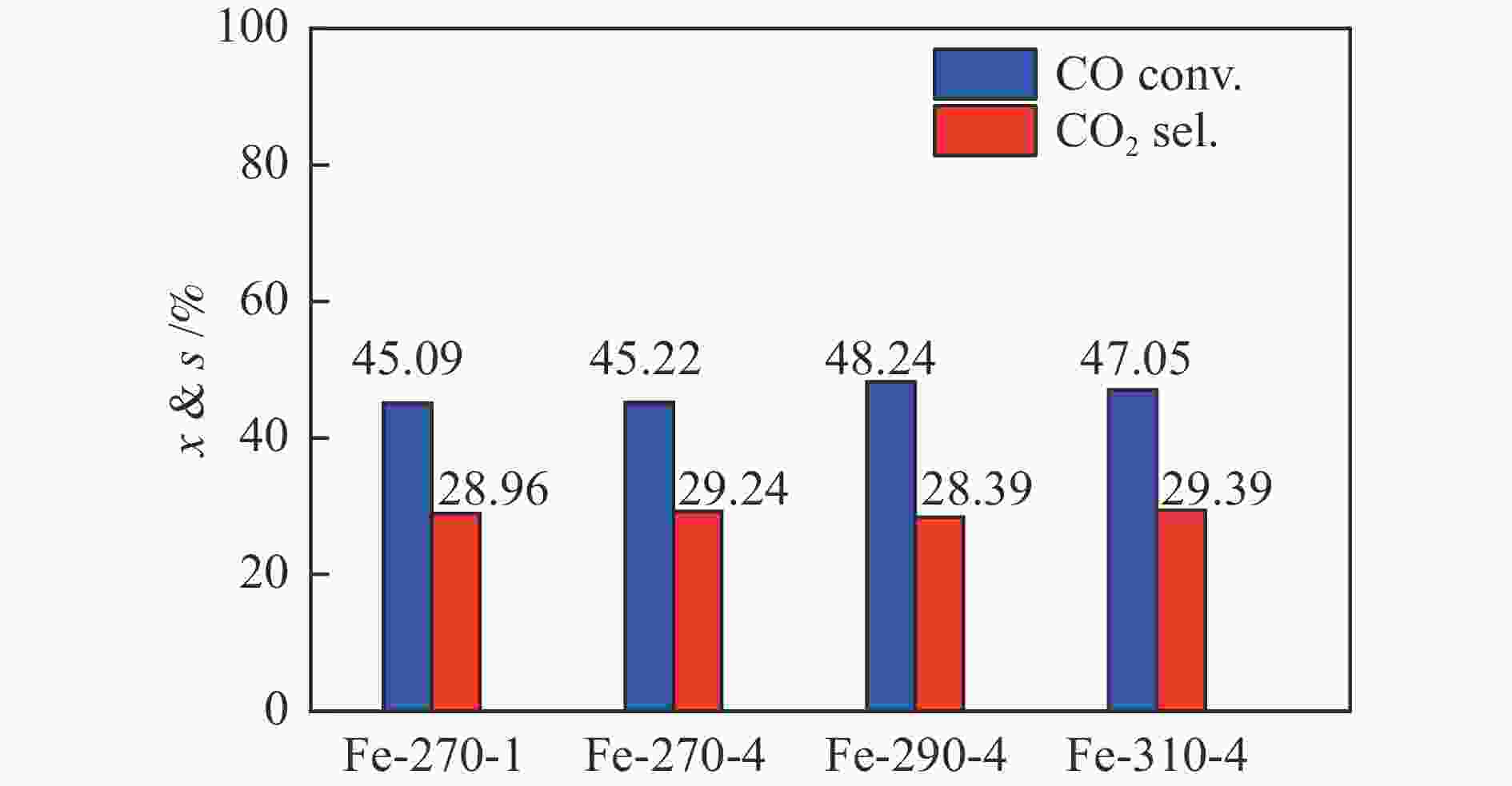
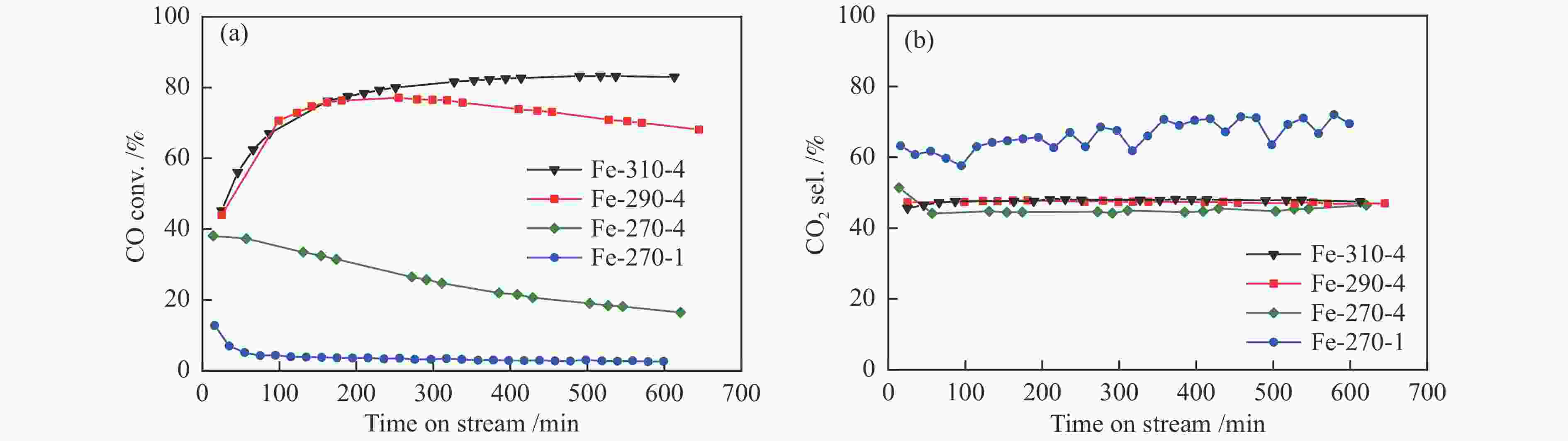
图 12 添加3%H2O条件下(调整空速后)Fe催化剂在费托合成评价中的CO转化率(a)与CO2选择性(b)
Figure 12 CO conversion (a) and CO2 selectivity (b) of various Fe-based catalysts during the FTS reaction with the addition of 3% H2O in the feed; the CO conversion over different catalysts was controlled at a similar level (15%–20%) by adjusting the space velocity
表 1 Fe催化剂的碳化条件
Table 1 Conditions for the carburization of the Fe-based catalysts
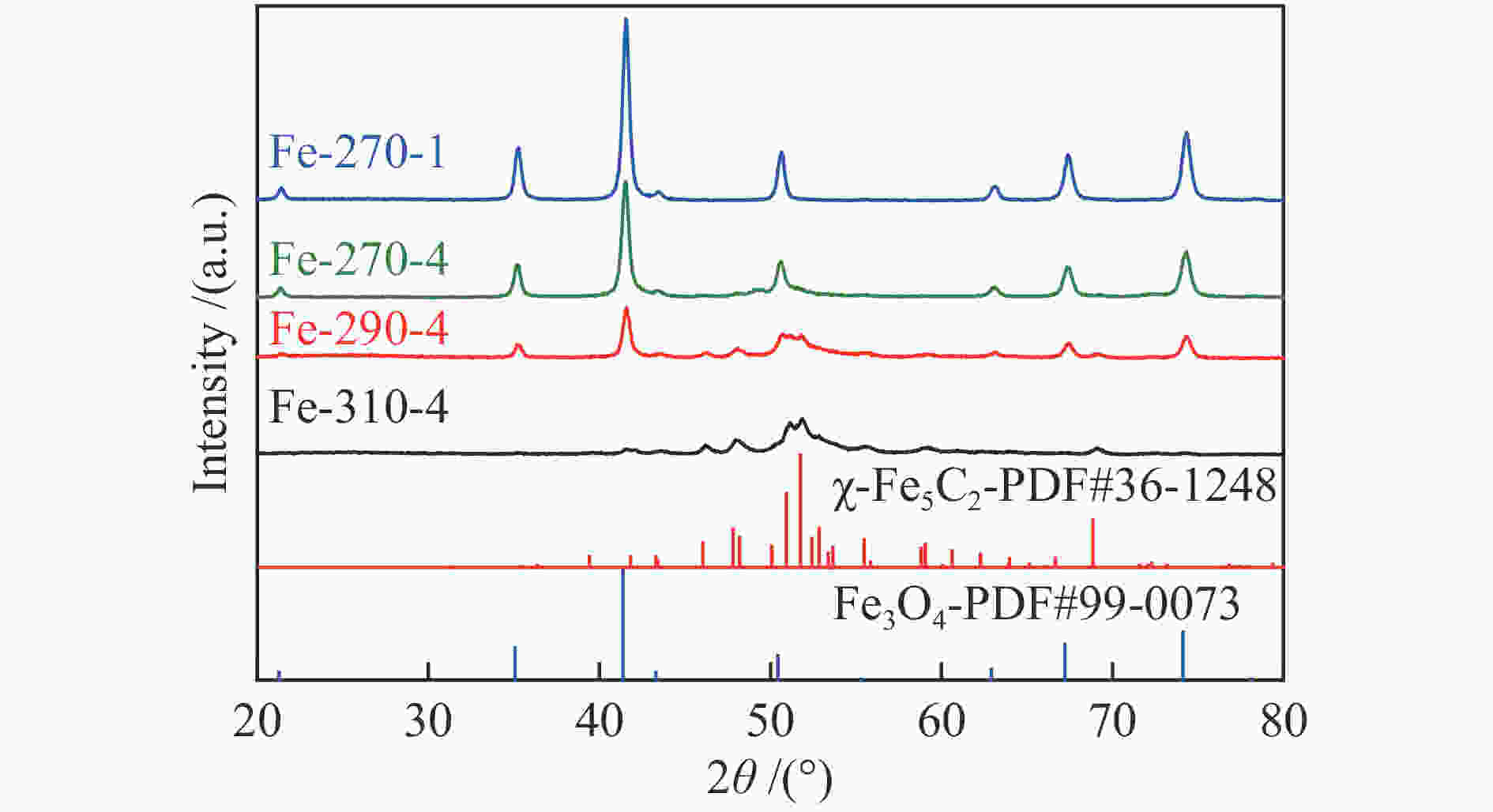
Catalyst Carburization temp. t/℃ Time t/h Fe-270a-1b 270 1 Fe-270-4 270 4 Fe-290-4 290 4 Fe-310-4 310 4 a: carburization temperature (℃); b: carburization time (h) 表 2 不同条件碳化后Fe催化剂的物相组成及其含量
Table 2 Iron phase composition and content of Fe-based catalysts pretreated under different carburization conditions
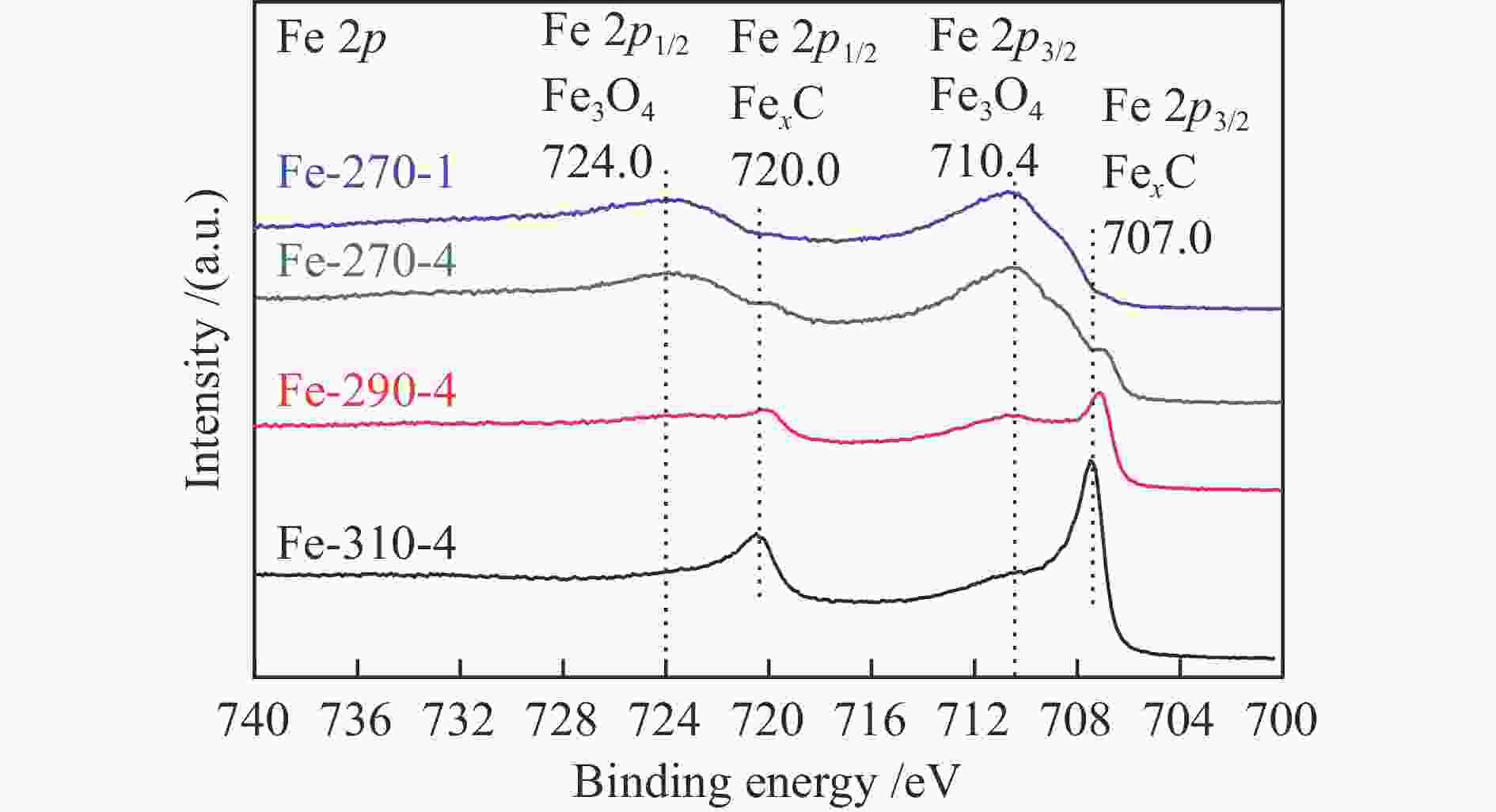
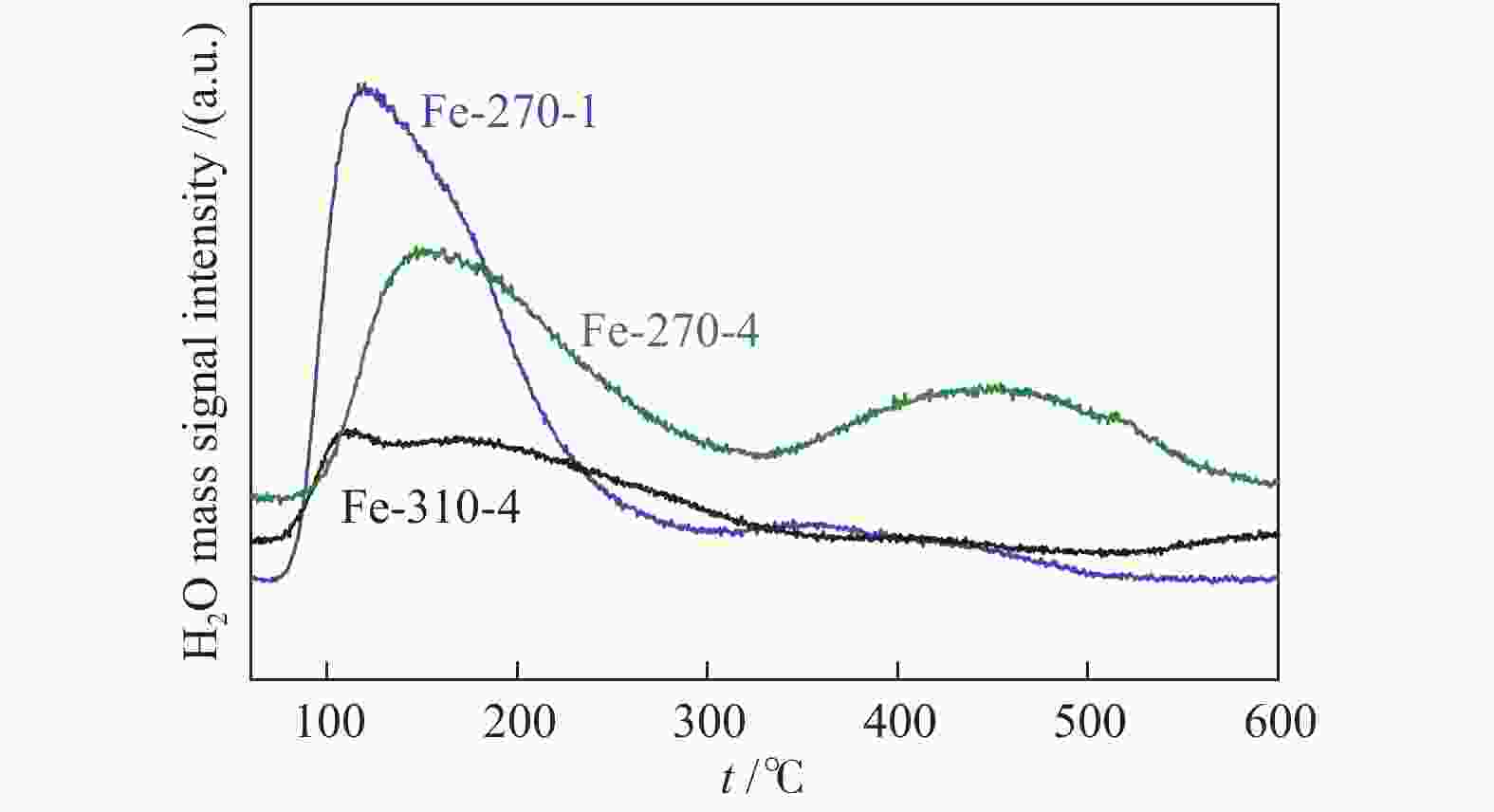
Catalyst Phase composition /% Fe3O4 FexC χ-Fe5C2 ε-Fe2C Fe-270-1 87.79 9.55 2.66 Fe-270-4 68.51 6.75 24.73 Fe-290-4 39.76 25.27 34.97 Fe-310-4 6.28 37.03 56.69 表 3 反应后催化剂的物相组成及其含量
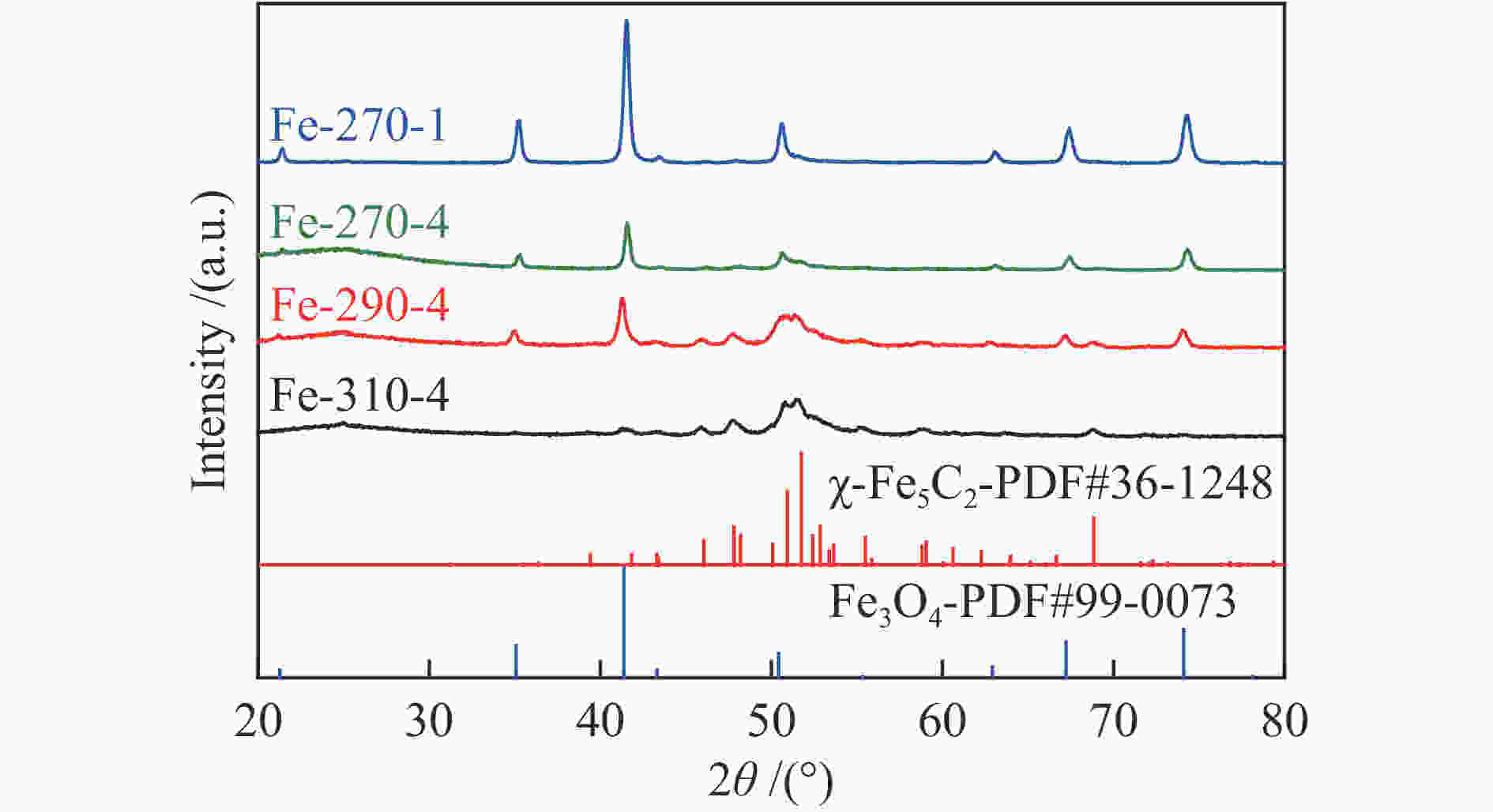
Table 3 Iron phase composition of various spent catalysts after the FTS reaction test
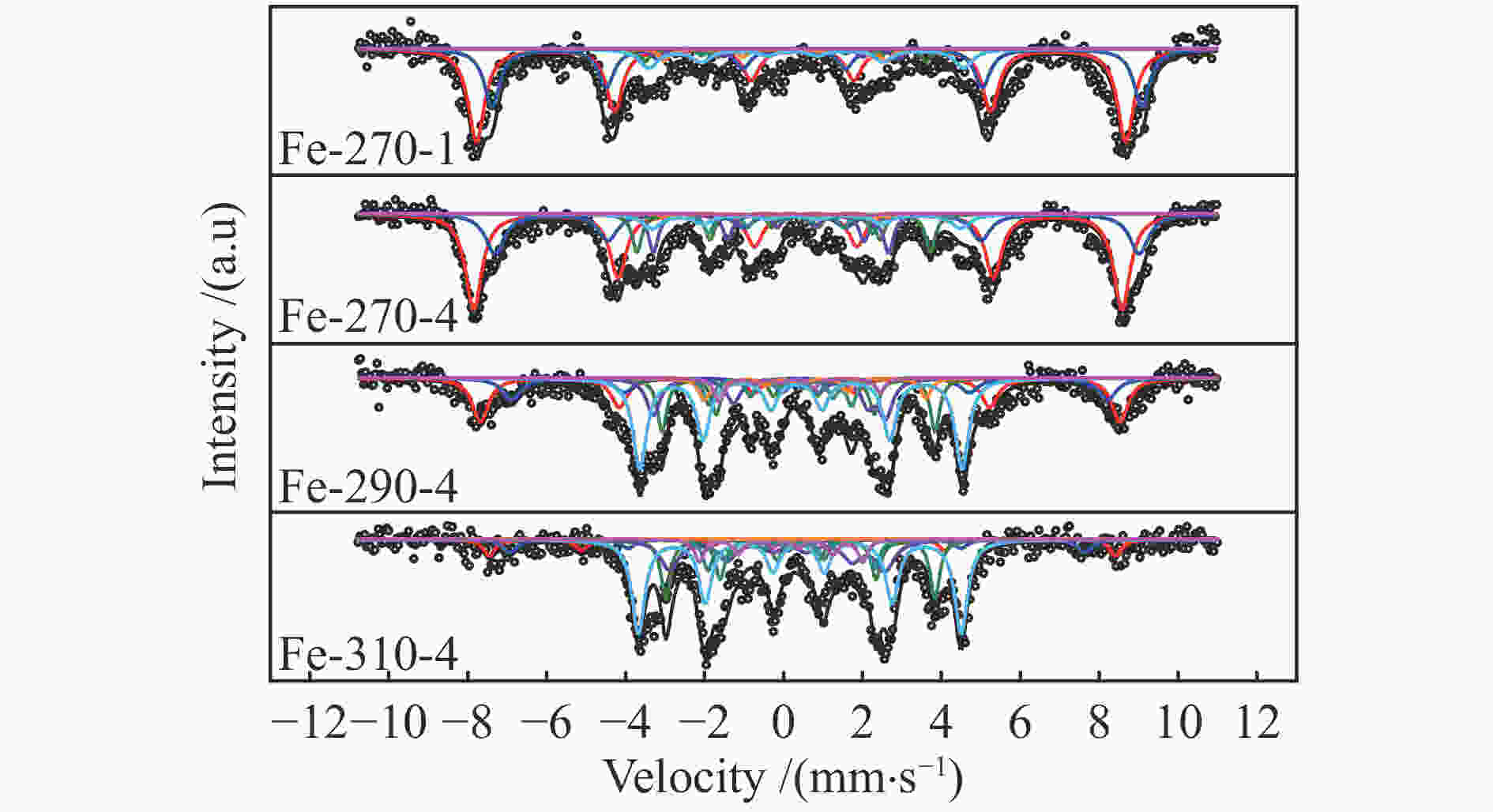
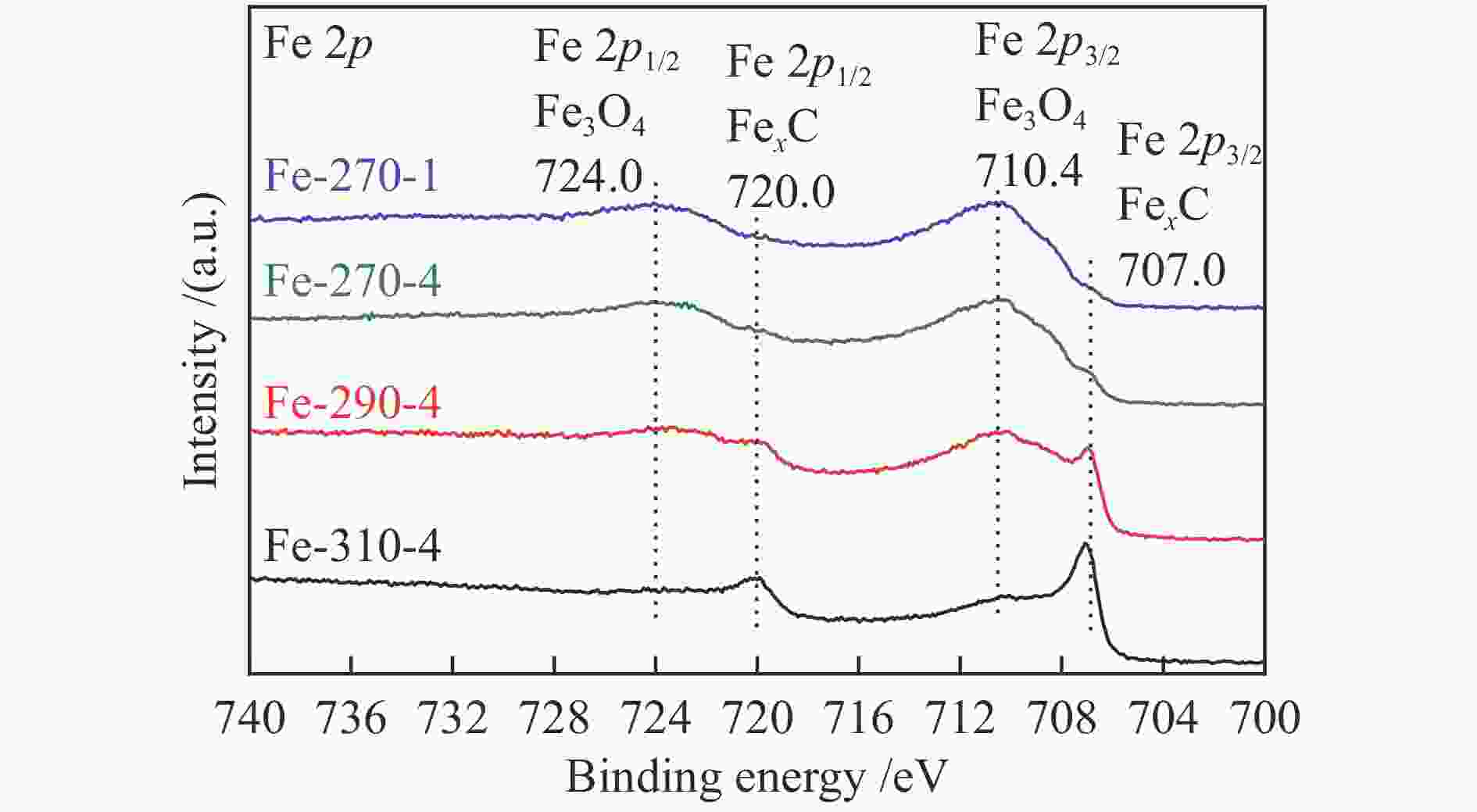
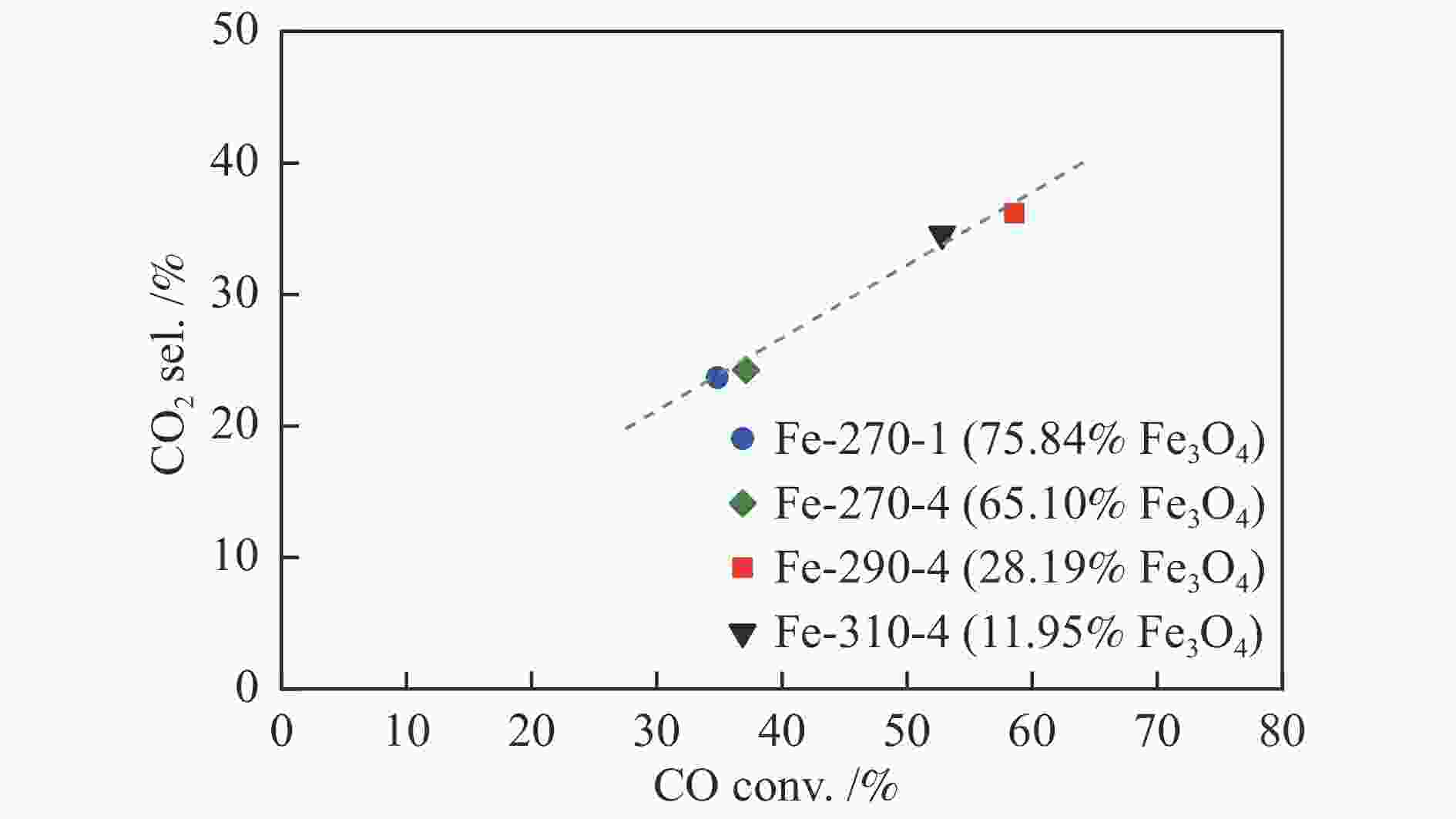
Catalyst Phase composition /% Fe3O4 FexC χ-Fe5C2 ε-Fe2C Fe-270-1 75.84 9.50 14.67 Fe-270-4 65.10 25.80 9.08 Fe-290-4 28.19 32.03 39.78 Fe-310-4 11.95 43.67 44.38 表 4 添加3%H2O反应后催化剂的物相组成及其含量
Table 4 Iron phase composition of various spent catalysts after FTS upon adding 3% H2O in the feed
Catalyst Phase composition /% Fe3O4 FexC χ-Fe5C2 ε-Fe2C Fe-270-1 90.61 1.00 8.40 Fe-270-4 88.62 6.32 5.06 Fe-290-4 52.90 35.67 11.43 Fe-310-4 7.55 70.30 22.14 -
[1] 相宏伟, 杨勇, 李永旺. 煤炭间接液化: 从基础到工业化[J]. 中国科学: 化学,2014,44(12):1876−1892.XIANG Hong-wei, YANG Yong, LI Yong-wang. Indirect coal-to-liquids technology from fundamental research to commercialization[J]. Sci China Chem,2014,44(12):1876−1892. [2] WANG P, CHEN W, CHIANG F K, DUGULAN A, SONG Y, PESTMAN R, ZHANG K, YAO J, FENG B, MIAO P, XU W, HENSEN E. Synthesis of stable and low-CO2 selective ε-iron carbide Fischer-Tropsch catalysts[J]. Sci Adv,2018,4(10):eaau2947. [3] TOPSØE H, BOUDART M. Mössbauer spectroscopy of CO shift catalysts promoted with lead[J]. J Catal,1973,31(3):346−359. doi: 10.1016/0021-9517(73)90304-7 [4] RETHWISCH D G, PHILLIPS J, CHEN Y, HAYDEN T F, DUMESIC J A. Water-gas shift over magnetite particles supported on graphite: Effects of treatments in CO/CO2 and H2/H2O gas mixtures[J]. J Catal,1985,91(1):167−180. doi: 10.1016/0021-9517(85)90298-2 [5] KETURAKIS C J, ZHU M, GIBSON E K, DATURI M, TAO F, FRENKEL A I, WACHS I E. Dynamics of CrO3-Fe2O3 catalysts during the high-temperature water-gas shift reaction: Molecular structures and reactivity[J]. ACS Catal,2016,6(7):4786−4798. doi: 10.1021/acscatal.6b01281 [6] OKI S, MEZAKI R. Identification of rate-controlling steps for the water-gas shift reaction over an iron oxide catalyst[J]. J Phys Chem,1973,77:447−452. doi: 10.1021/j100623a006 [7] LOX E S, FROMENT G F. Kinetics of the Fischer-Tropsch reaction on a precipitated promoted iron catalyst. 2. Kinetic modeling[J]. Ind Eng Chem Res,1993,32(1):71−82. doi: 10.1021/ie00013a011 [8] DING M, YANG Y, XU J, TAO Z, WANG H, XIANG H W, LI Y W. Effect of reduction pressure on precipitated potassium promoted iron-manganese catalyst for Fischer-Tropsch synthesis[J]. Appl Catal A: Gen,2008,345:176−184. doi: 10.1016/j.apcata.2008.04.036 [9] WANG H, YANG Y, WU B S, XU J, DING M, WANG H L, FAN W H, XIANG H W, LI Y W. Hydrogen reduction kinetics modeling of a precipitated iron Fischer-Tropsch catalyst[J]. J Mol Catal A: Chem,2009,308:96−107. doi: 10.1016/j.molcata.2009.03.030 [10] PAALANEN P P, VAN VREESWIJK S H, DUGULAN A I, WECKHUYSEN B M. Identification of Iron Carbides in Fe(-Na-S)/α-Al2O3 Fischer-Tropsch synthesis catalysts with X-ray powder diffractometry and Mössbauer absorption spectroscopy[J]. ChemCatChem,2020,12(20):5121−5139. doi: 10.1002/cctc.202000707 [11] YUAN X, ZHOU Y W, HUO C F, GUO W, YANG Y, LI Y, WEN X D. Crystal structure prediction approach to explore the iron carbide phases: Novel crystal structures and unexpected magnetic properties[J]. J Phys Chem C, 2020, 124(31): 17244–17254. [12] LU F, CHEN X, LEI Z, WEN L, ZHANG Y. Revealing the activity of different iron carbides for Fischer-Tropsch synthesis[J]. Appl Catal B: Environ,2021,281:119521. doi: 10.1016/j.apcatb.2020.119521 [13] 张昱. 铁基 FT 合成催化剂助剂效应(SiO2、Mn)研究[D]. 北京: 中国科学院大学, 2020.ZHANG Yu. Study on the promoter effect of Fe-based Fischer-Tropsch synthesis catalysts: the case of SiO2 and Mn[D]. Beijing: University of Chinese Academy of Sciences, 2020. [14] FU D, DAI W, XU X, MAO W, SU J, ZHANG Z, SHI B, SMITH J, LI P P, XU P J. Probing the structure evolution of iron-based Fischer-Tropsch to produce olefins by operando Raman spectroscopy[J]. ChemCatChem,2015,7(5):752−756. [15] TAN P, ZHANG S L, YUE K T, HUANG F, SHI Z, ZHOU X, GU Z. Comparative Raman study of carbon nanotubes prepared by D. C. Arc discharge and catalytic methods[J]. J Raman Spectr,1997,28(5):369−372. doi: 10.1002/(SICI)1097-4555(199705)28:5<369::AID-JRS107>3.0.CO;2-X [16] 定明月, 杨勇, 相宏伟, 李永旺. 费托合成Fe基催化剂中铁物相与活性的关系[J]. 催化学报,2010,31(9):1145−1150.DING Ming-yue, YANG Yong, XIANG Hong-wei, LI Yong-wang. Relationship between iron phase and activity of iron-based Fischer-Tropsch synthesis catalyst[J]. Chin J Catal,2010,31(9):1145−1150. [17] DING M, YANG Y, WU B, WANG T, MA L, XIANG H, LI Y. Transformation of carbonaceous species and its influence on catalytic performance for iron-based Fischer-Tropsch synthesis catalyst[J]. J Mol Catal A: Chem,2011,351:165−173. doi: 10.1016/j.molcata.2011.10.001 [18] NING W, KOIZUMI N, CHANG H, MOCHIZUKI T, ITOH T, YAMADA M. Phase transformation of unpromoted and promoted Fe catalysts and the formation of carbonaceous compounds during Fischer-Tropsch synthesis reaction[J]. Appl Catal A: Gen,2006,312:35−44. doi: 10.1016/j.apcata.2006.06.025 [19] BUTT J B. Carbide phases on iron-based Fischer-Tropsch synthesis catalysts part II: Some reaction studies[J]. Catal Lett,1990,7(1):83−105. [20] DING M, YANG Y, WU B, XU J, ZHANG C, XIANG H, LI Y. Study of phase transformation and catalytic performance on precipitated iron-based catalyst for Fischer-Tropsch synthesis[J]. J Mol Catal A: Chem,2009,303(1):65−71. [21] PÉREZ-ALONSO F J, HERRANZ T, ROJAS S, OJEDA M, GRANADOS M L, TERREROS P, FIERRO J L G, GRACIA M, GANCEDO J R. Evolution of the bulk structure and surface species on Fe-Ce catalysts during the Fischer-Tropsch synthesis[J]. Green Chem,2007,9(6):663−670. [22] SHROFF M D, KALAKKAD D S, COULTER K E, KOHLER S D, HARRINGTON M S, JACKSON N B, SAULT A G, DATYE A K. Activation of precipitated iron Fischer-Tropsch synthesis catalysts[J]. J Catal,1995,156(2):185−207. doi: 10.1006/jcat.1995.1247 [23] THÜNE P, MOODLEY P, SCHEIJEN F, FREDRIKSSON H, LANCEE R, KROPF J, MILLER J, NIEMANTSVERDRIET J W. The effect of water on the stability of iron oxide and iron carbide nanoparticles in hydrogen and syngas followed by in Situ X-ray absorption spectroscopy[J]. J Phys Chem C,2012,116(13):7367−7373. doi: 10.1021/jp210754k [24] KRISHNAMOORTHY S, LI A, IGLESIA E. Pathways for CO2 formation and conversion during Fischer-Tropsch synthesis on iron-based catalysts[J]. Catal Lett,2002,80(1):77−86. [25] OJEDA M, NABAR R, NILEKAR A U, ISHIKAWA A, MAVRIKAKIS M, IGLESIA E. CO activation pathways and the mechanism of Fischer-Tropsch synthesis[J]. J Catal,2010,272(2):287−297. doi: 10.1016/j.jcat.2010.04.012 [26] BUKUR D B, TODIC B, ELBASHIR N. Role of water-gas-shift reaction in Fischer-Tropsch synthesis on iron catalysts: A review[J]. Catal Today,2016,275:66−75. doi: 10.1016/j.cattod.2015.11.005 [27] 王珏, 杨勇, 青明, 白云坡, 王洪, 胡彩霞, 相宏伟, 岳仁亮. 助剂对铁基费托合成催化剂氧化行为的影响: H2O作用的解读[J]. 燃料化学学报,2020,48(1):63−74. doi: 10.3969/j.issn.0253-2409.2020.01.008WANG Jue, YANG Yong, QING Ming, BAI Yun-po, WANG Hong, HU Cai-xia, XIANG Hong-wei, YUE Ren-liang. Effect of the promoters on oxidation behavior of Fe-based Fischer-Tropsch catalyst: Deciphering the role of H2O[J]. J Fuel Chem Technol,2020,48(1):63−74. doi: 10.3969/j.issn.0253-2409.2020.01.008 [28] 郭天雨, 刘粟侥, 青明, 冯景丽, 吕振刚, 王洪, 杨勇. 原位XRD反应装置下H2O对Fe5C2的物相及F-T反应性能影响的研究[J]. 燃料化学学报,2020,48(1):75−82. doi: 10.3969/j.issn.0253-2409.2020.01.009GUO Tian-yu, LIU Su-yao, QING Ming, FENG Jing-li, LV Zhen-gang, WANG Hong, YANG Yong. In situ XRD study of the effect of H2O on Fe5C2 phase and Fischer-Tropsch performance[J]. J Fuel Chem Technol,2020,48(1):75−82. doi: 10.3969/j.issn.0253-2409.2020.01.009 [29] GALVIS H M T, BITTER J H, DAVIDIAN T, RUITENBEEK M, JONG K P D. Iron particle size effects for direct production of lower olefins from synthesis gas[J]. J Am Chem Soc,2012,134(39):16207−16215. doi: 10.1021/ja304958u -




 下载:
下载: