Study of the mechanism of nitrogen doping in carbon supports on promoting electrocatalytic oxygen reduction reaction over platinum nanoparticles
-
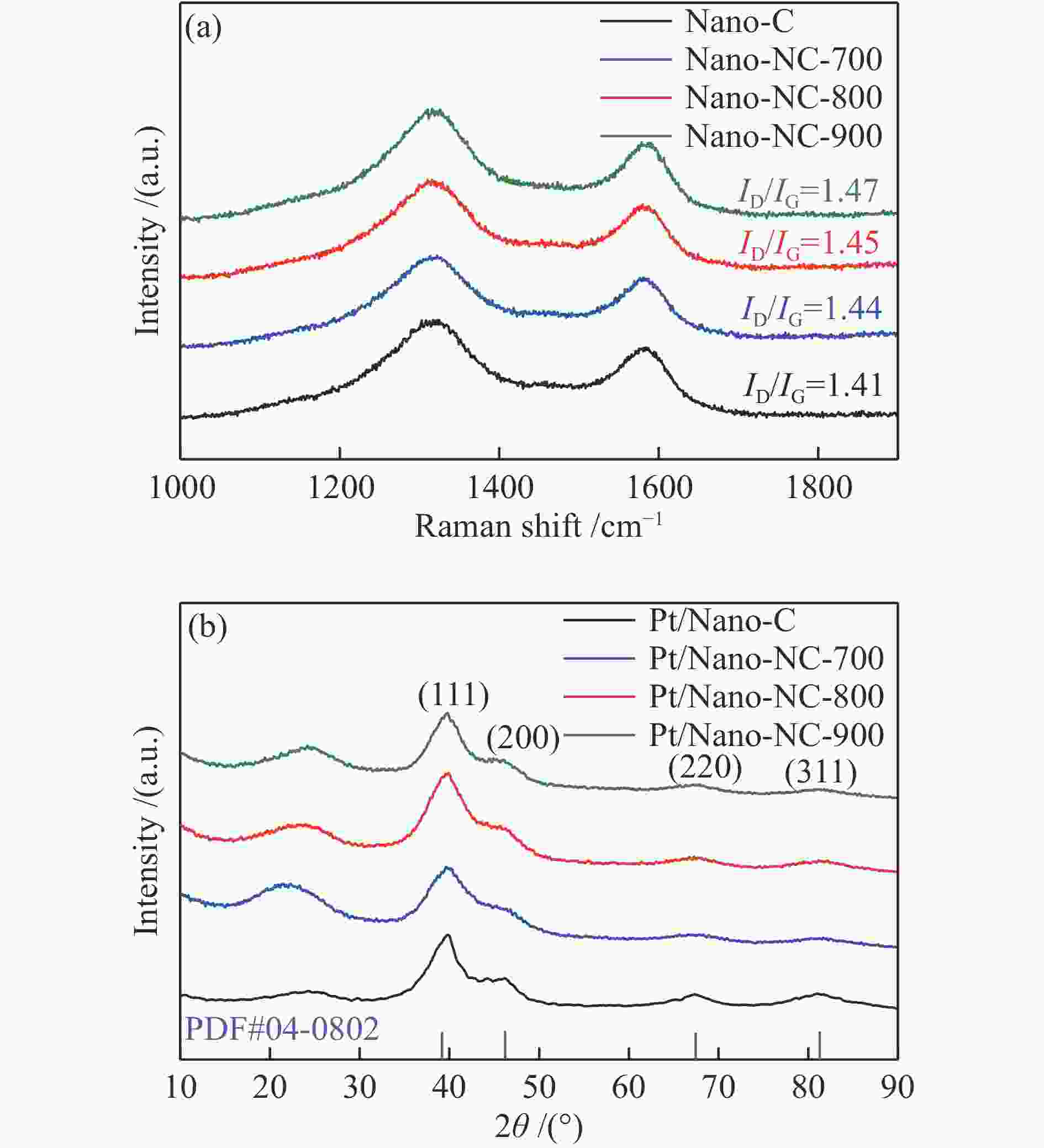
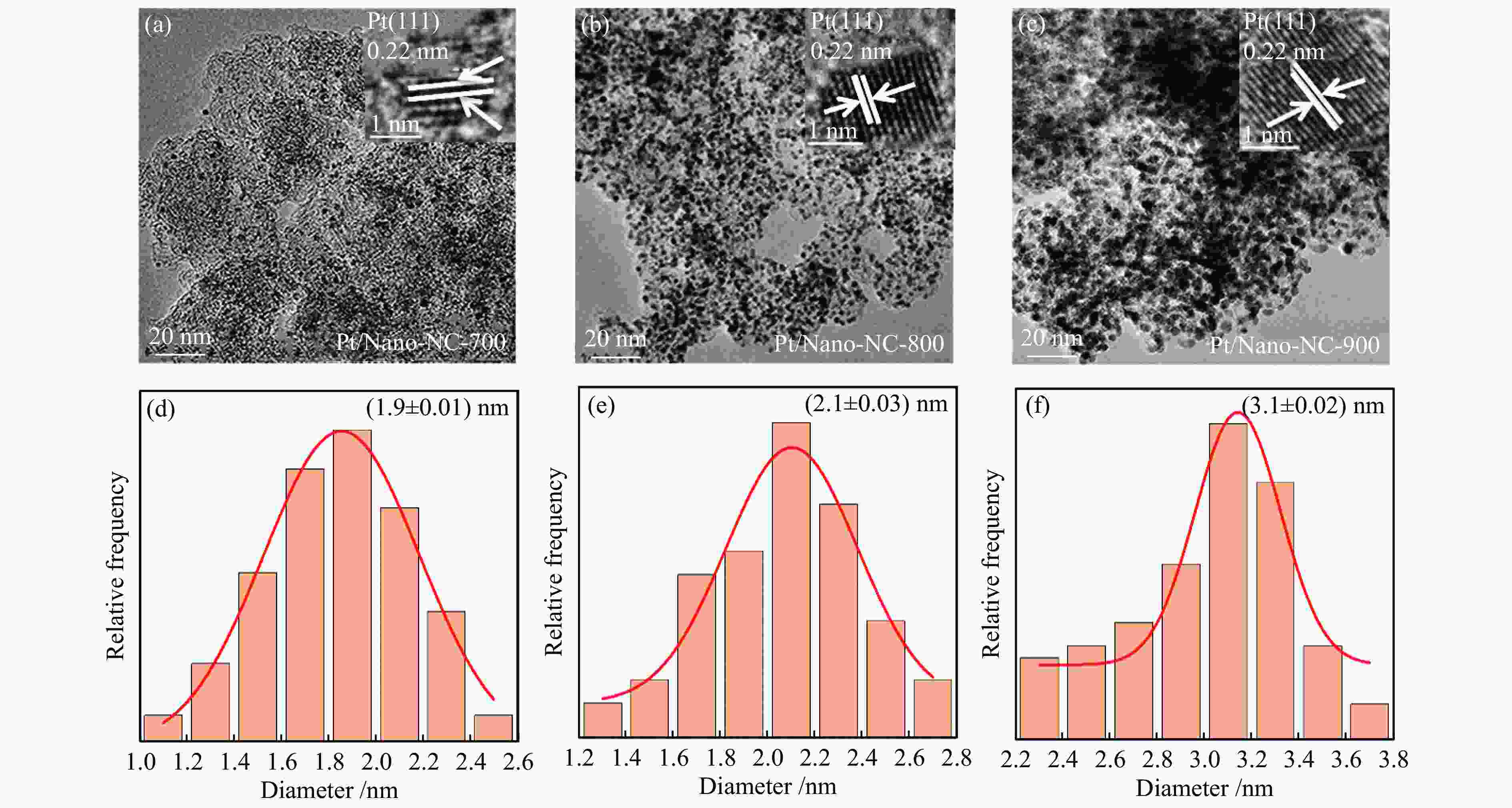
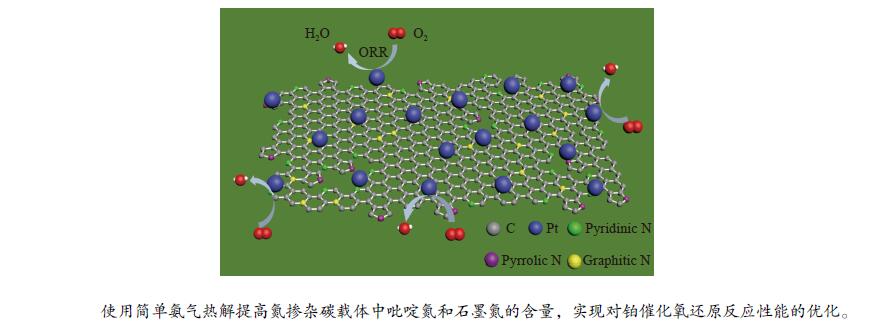
摘要: 氮掺杂碳通常被用作铂基催化剂电催化氧还原反应的功能载体,但是,掺杂的氮对分子氧在铂活性中心上的吸附和还原机理尚不清楚。本研究采用氨气热解的方法制取氮掺杂纳米碳作为载体,并采用调节氨气热解温度进而控制不同种类氮掺杂的含量,可以使铂催化剂获得较高的零价铂含量、较大的电化学活性面积、合适的铂粒径 (2.10 nm)和电子快速传输能力从而提高电催化活性。研究发现,具有最佳氮含量掺杂的Pt/Nano-NC-800催化剂显示出优异的电催化氧还原性能(例如,半波电位为0.80 V vs RHE,极限扩散电流为5.37 mA/cm2),以及强的抗甲醇和一氧化碳中毒能力。该性能优于商业铂碳催化剂(20%,JM)以及大多数沉积在碳纳米颗粒或其他载体上的铂催化剂,表现出优异的应用潜力。Abstract: Nitrogen-doped carbons (Nano-NC) are often employed as functional supports for boosting oxygen reduction reaction (ORR) over Pt-based catalysts, however, the mechanism of N doping on the adsorption and activation of molecular oxygen on Pt active sites is still not clear. Herein, Nano-NCs as the supports were prepared by a facile NH3 antipyretic method, which allowed to tune the kinds of nitrogen species in carbon matrix and their contents by adjusting the NH3 antipyretic temperatures. With such an exquisite control, the Pt nanoparticles loaded on the as-obtained Nano-NC showed an optimal Pt particle size (2.10 nm), a higher content of Pt0, a large electrochemically active surface area, and fast electron transport ability. As a consequence, the Pt/Nano-NC-800 catalyst with the optimal N-doping showed an outstanding ORR performance with half-wave potential of 0.80 V vs. RHE, limit diffusion current of 5.37 mA/cm2 and improved methanol/CO anti-poisoning, which is superior to the commercial Pt/C catalyst (20%, JM), and most of previously reported Pt-based catalysts. This work may pave a way for the design of the advanced supports for Pt-based catalysts for the ORR applications.
-
Key words:
- N-doped carbon /
- nanoparticle size /
- oxygen reduction reaction /
- mechanism
-
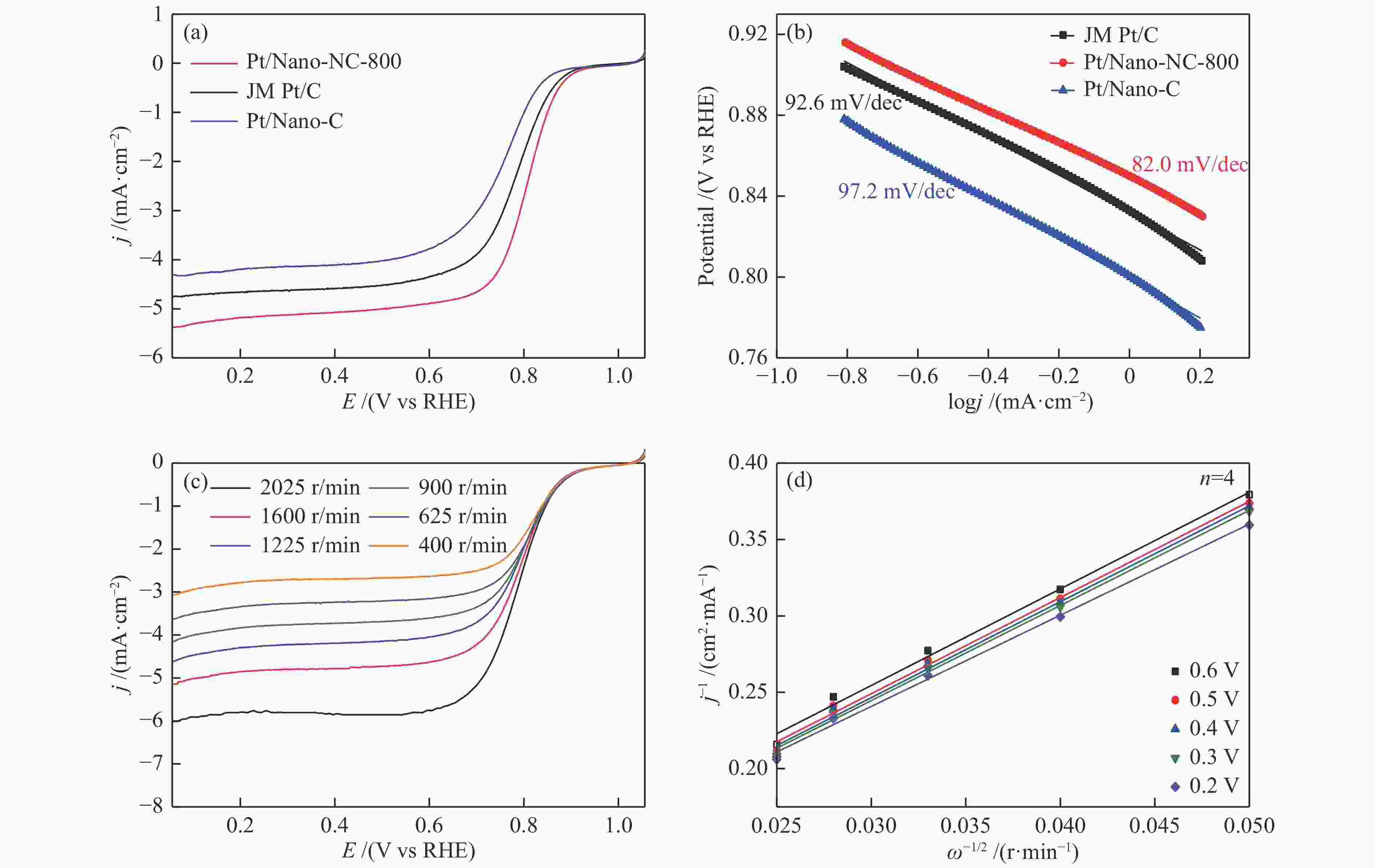
Figure 4 (a) RDE voltammogram of the Pt/Nano-NC-800, Pt/Nano-C, and JM Pt/C catalysts recorded in O2-saturated 0.1 mol/L HClO4 electrolyte at a scan rate of 5 mV/s at 1600 r/min, (b) corresponding Tafel plots, (c) LSVs in O2-saturated 0.1 mol/L HClO4 at different RDE rotation rates of Pt/Nano-NC-800, (d) The corresponding K-L plots of Pt/Nano-NC-800
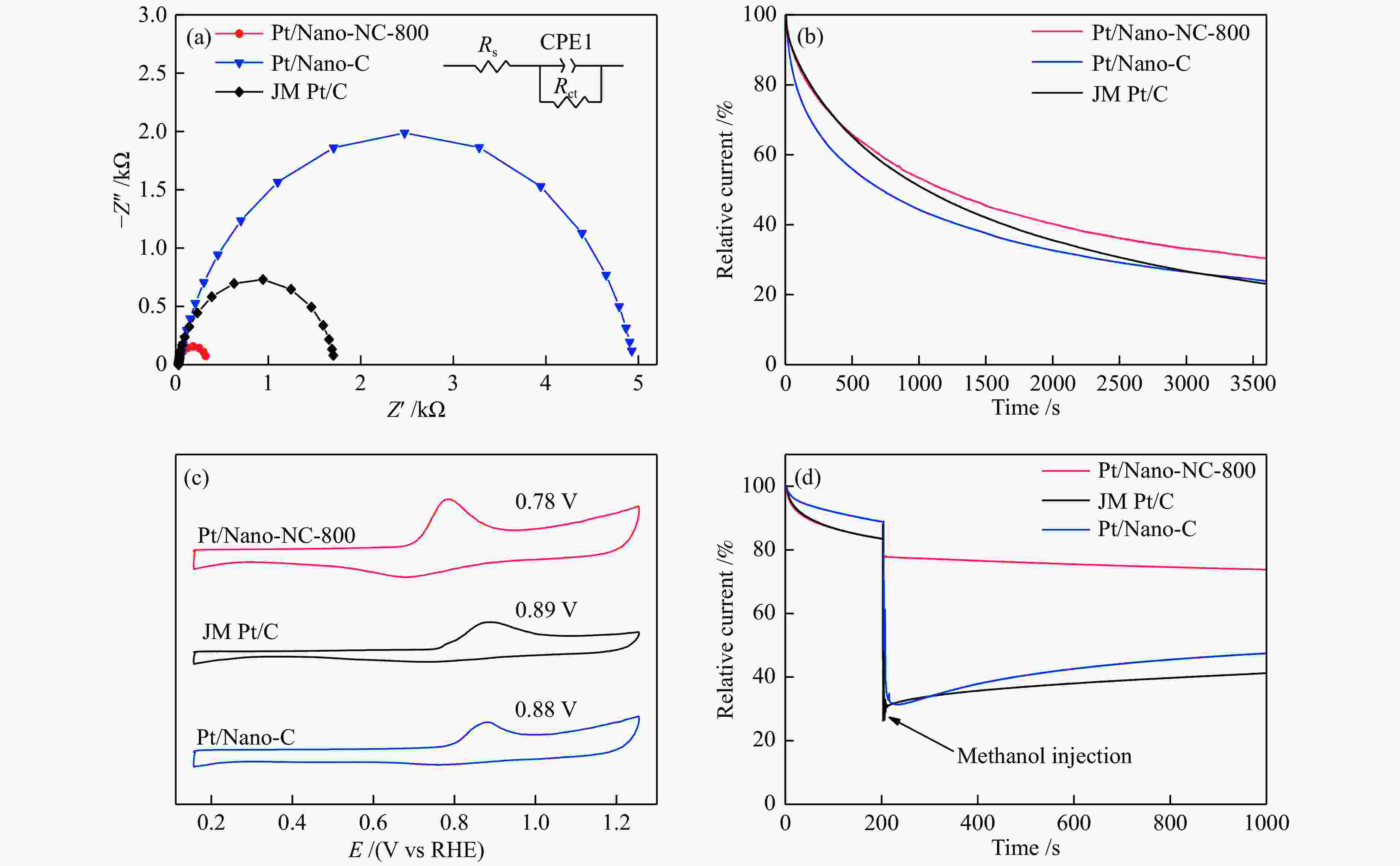
Figure 5 (a) Nyquist plots of the Pt/Nano-NC-800, Pt/Nano-C, and JM Pt/C catalysts and equivalent circuit model; (b) Endurance test of three catalysts tested at 0.756 V in O2-saturated 0.1 mol/L HClO4; (c) CO stripping experiments on three catalysts at 10 mV/s; (d) the addition of 4 mL of 3 mol/L methanol in O2-saturated solution at 1600 r/min
Table 1 Results of the fits of the N 1s XPS For each single component, the binding energy (eV) and amount (%) values are given
Sample Pyridinic-N
398.6 eVPt-N
399.4 eVPyrrolic-N
400.2 eVGraphitic-N
401.1 eVOxidized-N
402.0 eVPt/Nano-NC-700 7.4% 32.5% 19.5% 33.0% 7.6% Pt/Nano-NC-800 8.4% 31.9% 19.6% 34.6% 5.5% Pt/Nano-NC-900 17.8% 21.5% 26.4% 30.6% 3.7% -
[1] REN X F, WANG Y R, LIU A M, ZHANG Z H, LV Q Y, LIU B H. Current progress and performance improvement of Pt/C catalysts for fuel cells[J]. J Mater Chem A,2020,8:24284−24306. doi: 10.1039/D0TA08312G [2] SHEN G R, LIU J, WU H B, XU P C, LIU F, TONGSH C, JIAO K, LI J L, LIU M L, CAI M, LEMMON J P, SOLOVEICHIK G, LI H X, ZHU J, LU Y F. Multi-functional anodes boost the transient power and durability of proton exchange membrane fuel cells[J]. Nat Commun,2020,11(1):1191. doi: 10.1038/s41467-020-14822-y [3] WANG Y, DIAZ D F R D, CHRN K S, WANG Z, ADROHER X C. Materials, technological status, and fundamentals of PEM fuel cells – A review[J]. Mater Today,2020,32:1369−7021. [4] MAJLAN E H, ROHENDI D, DAUD W R W, HUSAINI T, HAQUE M A. Electrode for proton exchange membrane fuel cells: A review[J]. Renewable Sustainable Energy Rev,2018,89:117−134. doi: 10.1016/j.rser.2018.03.007 [5] TIAN X L, LU X F, XIA B Y, LOU W X. Advanced electrocatalysts for the oxygen reduction reaction in energy conversion technologies[J]. Joule,2020,4:45−68. doi: 10.1016/j.joule.2019.12.014 [6] SHAO M H, CHANG Q W, DODELET J P, CHENITZ R. Recent advances in electrocatalysts for oxygen reduction reaction[J]. Chem Rev,2016,116:3594−3657. doi: 10.1021/acs.chemrev.5b00462 [7] JING H Y, ZHU P, ZHENG X B, ZHANG Z D, WANG D S, LI Y D. Theory-oriented screening and discovery of advanced energy transformation materials in electrocatalysis[J]. Adv Powder Mater, 2021, 1(1): 100013. [8] ZAMAN S, HUANG L, DOUKA A I, YANG H, YOU B, XIA B Y. Oxygen Reduction Electrocatalysts toward Practical Fuel Cells: Progress and Perspectives[J]. Angew Chem Int Ed Eng,2021,60(33):17832−17852. doi: 10.1002/anie.202016977 [9] ZHANG X W, LI H, YANG J, LEI Y J, WANG C, WANG J L, TANF Y P, MAO Z G. Recent advances in Pt-based electrocatalysts for PEMFCs[J]. RSC Adv,2021,11(22):13316−13328. doi: 10.1039/D0RA05468B [10] IOROI T, SIROMA Z, YAMAZAKI S I, YASUDA K. Electrocatalysts for PEM Fuel Cells[J]. Adv Energy Mater, 2018, 9(23): 1801284. [11] CHEN Y L, CHENG T, GODDARD W A. Atomistic explanation of the dramatically improved oxygen reduction reaction of jagged platinum nanowires, 50 times better than Pt[J]. J Am Chem Soc,2020,142(19):8625−8632. doi: 10.1021/jacs.9b13218 [12] LIN R, CAI X, ZENG H, YU Z P. Stability of high-performance Pt-based catalysts for oxygen reduction reactions[J]. Adv Mater,2018,30(17):e1705332. doi: 10.1002/adma.201705332 [13] YU S C, LI F Q, YANG H S, LI G P, ZHU G H, ZHANG L H, LI Y P. Pt-nanoflower as high performance electrocatalyst for fuel cell vehicle[J]. Int J Hydrog Energy,2017,42(50):29971−29976. doi: 10.1016/j.ijhydene.2017.06.228 [14] JIANG K Z, ZHAO D D, GUO S J, ZHANG X, ZHU X, GUO J, LU G, HUANG X Q. Efficient oxygen reduction catalysis by subnanometer Pt alloy nanowires[J]. Sci Adv,2017,3:e1601705. doi: 10.1126/sciadv.1601705 [15] LUO M C, SUN Y J, ZHANG X, QIN Y N, LI M Q, LI Y J, LI C J, YANG Y, WANG L, GAO P, LU G, GUO S J. Stable high-index faceted Pt skin on Zigzag-like PtFe nanowires enhances oxygen reduction catalysis[J]. Adv Mater, 2018, 30(10). [16] LIU J, LAN J Z, YANG L Y, WANG F, YIN J. PtM (M = Fe, Co, Ni) bimetallic nanoclusters as active, methanol-tolerant, and stable catalysts toward the oxygen reduction reaction[J]. ACS Sustainable Chem Eng,2019,7(7):6541−6549. doi: 10.1021/acssuschemeng.8b04929 [17] CHOI J, CHO J, ROH C W, KIM B S, CHOI M S, JEONG H J, HAM H C, LEE H. Au-doped PtCo/C catalyst preventing Co leaching for proton exchange membrane fuel cells[J]. Appl Catal B: Environ,2019,247:142−149. doi: 10.1016/j.apcatb.2019.02.002 [18] LI X, WANG H J, YU H, LIU Z W, PENG F. An opposite change rule in carbon nanotubes supported platinum catalyst for methanol oxidation and oxygen reduction reactions[J]. J Power Sources,2014,260:1−5. doi: 10.1016/j.jpowsour.2014.03.001 [19] TONG X, ZHANG J M, ZHANG G X, WEI Q L, CHENITZ R, CLAVERIE J P, SUN S H. Ultrathin carbon-coated Pt/carbon nanotubes: A highly durable electrocatalyst for oxygen reduction[J]. Chem Mater,2017,29(21):9579−9587. doi: 10.1021/acs.chemmater.7b04221 [20] KOU R, SHAO Y Y, WANG D H, ENGELHARD M H, KWAK J H, VISWANATHAN V V, WANG C M, LIN Y H, WANG Y, AKSAY I A, LIU J. Enhanced activity and stability of Pt catalysts on functionalized graphene sheets for electrocatalytic oxygen reduction[J]. Electrochem Commun,2009,11(5):954−957. doi: 10.1016/j.elecom.2009.02.033 [21] CHENG K, HE D P, PENG T, LV H F, PAN M, MU S C. Porous graphene supported Pt catalysts for proton exchange membrane fuel cells[J]. Electrochim Acta,2014,132:356−363. doi: 10.1016/j.electacta.2014.03.181 [22] LIU J W, MA Q L, HUANG Z Q, LIU G G, ZHANG H. Recent progress in graphene-based noble-metal nanocomposites for electrocatalytic applications[J]. Adv Mater,2019,31(9):e1800696. doi: 10.1002/adma.201800696 [23] WANG Y, LI G, JIN J H, YANG S L. Hollow porous carbon nanofibers as novel support for platinum-based oxygen reduction reaction electrocatalysts[J]. Int J Hydrog Energy,2017,42(9):5938−5947. doi: 10.1016/j.ijhydene.2017.02.012 [24] YING J, LI J, JIANG G P, GANO Z, MA Z, ZHONG C, SU D, CHEN Z W. Metal-organic frameworks derived platinum-cobalt bimetallic nanoparticles in nitrogen-doped hollow porous carbon capsules as a highly active and durable catalyst for oxygen reduction reaction[J]. Appl Catal B: Environ,2018,225:496−503. doi: 10.1016/j.apcatb.2017.11.077 [25] SAKTHIVELl M, DRILLET J F. An extensive study about influence of the carbon support morphology on Pt activity and stability for oxygen reduction reaction[J]. Appl Catal B: Environ,2018,231:62−72. doi: 10.1016/j.apcatb.2018.02.050 [26] LIU J, JIAO M G, MEI B B, TONG Y X, RUAN M B, SONG P, SUN G Q, JIANG L H, WANG Y, JIANG Z, GU L, ZHOU Z, XU W L. Carbon-supported divacancy-anchored platinum single-atom electrocatalysts with superhigh Pt utilization for the oxygen reduction reaction[J]. Angew Chem Int Ed Eng,2019,58(4):1163−1167. doi: 10.1002/anie.201812423 [27] STAMENKOVIC V, GRGUR B N, ROSS P N, MARKOVIC N M. Oxygen reduction reaction on Pt and Pt-bimetallic electrodes covered by CO[J]. J Electrochem Soc, 2005, 152(2): A277−A282. [28] DAVIES J C, BONDE J, LOGADÓTTIR Á, NØRSKOV J K, CHORKENDORFF I. The Ligand Effect: CO Desorption from Pt/Ru Catalysts[J]. Fuel Cells,2005,5(4):429−435. doi: 10.1002/fuce.200400076 [29] BASCHUK J J, LI X G. Carbon monoxide poisoning of proton exchange membrane fuel cells[J]. Int J Energy Res,2001,25(8):695−713. doi: 10.1002/er.713 [30] MAILLARD F, SILVA W O, CASTANHERIRA L, DUBAU L. Carbon corrosion in proton-exchange membrane fuel cells: Spectrometric evidence for Pt-catalysed decarboxylation at anode-relevant potentials[J]. Chem Phys Chem,2019,20(22):3106−3111. [31] ZHANG Q, YU X X, LING Y L, CAI W W, YANG Z H. Ultrathin nitrogen doped carbon layer stabilized Pt electrocatalyst supported on N-doped carbon nanotubes[J]. Int J Hydrog Energy,2017,42(15):10354−10362. doi: 10.1016/j.ijhydene.2017.02.156 [32] CHEN L M, PENG Y, LU J E, WANG N, HU P G, LU B Z, CHEN S W. Platinum nanoparticles encapsulated in nitrogen-doped graphene quantum dots: Enhanced electrocatalytic reduction of oxygen by nitrogen dopants[J]. Int J Hydrog Energy,2017,42(49):29192−29200. doi: 10.1016/j.ijhydene.2017.10.078 [33] MONESTEL H G R, AMUIINU I S, GONZALEZ A A, PU Z H, MOUSAVI B, MU S C. Robust MOF-253-derived N-doped carbon confinement of Pt single nanocrystal electrocatalysts for oxygen evolution reaction[J]. Chin J Catal,2020,41(5):839−846. [34] YANG H, KO Y, LEE W, ZUTTEL A, KIM W. Nitrogen-doped carbon black supported Pt–M (M = Pd, Fe, Ni) alloy catalysts for oxygen reduction reaction in proton exchange membrane fuel cell[J]. Mater Today Energy,2019,13:374−381. doi: 10.1016/j.mtener.2019.06.007 [35] LI Y F, WANG D R, XIE H Y, ZHANG C W. Electrocatalytic activity and stability of 3D ordered N-doped hierarchically porous carbon supported Pt catalyst for methanol oxidation and oxygen reduction reactions[J]. ChemistrySelect,2019,4(43):12601−12607. doi: 10.1002/slct.201903610 [36] VARATHAN P, AKULA S, MONI P, SAHU A K. Natural aloe vera derived Pt supported N-doped porous carbon: A highly durable cathode catalyst of PEM fuel cell[J]. Int J Hydrog Energy,2020,45(38):19267−19279. doi: 10.1016/j.ijhydene.2020.05.056 [37] ZHU J B, XIAO M L, ZHAO X, LIU C P, GE J J, XING W, Strongly coupled Pt nanotubes/N-doped graphene as highly active and durable electrocatalysts for oxygen reduction reaction[J]. Nano Energy, 2015, 13: 318−326. [38] MENG F L, LI L, WU Z, ZHONG H X, LI J C. YAN J M. Facile preparation of N-doped carbon nanofiber aerogels from bacterial cellulose as an efficient oxygen reduction reaction electrocatalyst[J]. Chin J Catal,2014,35(6):877−883. doi: 10.1016/S1872-2067(14)60126-1 [39] TONG J H, LI W Y, BO L L, MA J P, LI T, LI Y L, ZHANG Q, FAN H Y. Composite of hierarchically porous N-doped carbon/carbon nanotube with greatly improved catalytic performance for oxygen reduction reaction[J]. ACS Sustainable Chem Eng,2018,6(7):8383−8391. doi: 10.1021/acssuschemeng.8b00463 [40] QIN Y X, YANG X B, LI R Y, CHEN S, WANG Y W, YU Z M, WANG Y Y, LIU X C, TONG X L. Carbon nanoparticle coated by silicon dioxide supported platinum nanoparticles towards oxygen reduction reaction[J]. Mater Res Bull,2021,139:111268. doi: 10.1016/j.materresbull.2021.111268 [41] YUAN K, ZHUANG X D, FU H Y, BRUNKLAUS G, FORSTER M, CHEN Y W, FENG X L, SCHERF U. Two-Dimensional core-shelled porous hybrids as highly efficient catalysts for the oxygen reduction reaction[J]. Angew Chem Int Ed Eng,2016,55(24):6858−6863. doi: 10.1002/anie.201600850 [42] YIN P Q, YAO T, WU Y E, ZHENG L R, LIN Y, LIU W, JU H X, ZHU J F, HONG X, DENG Z X, ZHOU G, WEI S Q, LI Y D. Single cobalt atoms with precise N-coordination as superior oxygen reduction reaction catalysts[J]. Angew Chem Int Ed Eng,2016,55(36):10800−10805. doi: 10.1002/anie.201604802 [43] RUDI S, CUI C H, GAN L, STRASSER P. Comparative study of the electrocatalytically active surface areas (ECSAs) of Pt alloy nanoparticles evaluated by Hupd and CO-stripping voltammetry[J]. Electrocatalysis,2014,5(4):408−418. doi: 10.1007/s12678-014-0205-2 [44] LI W, LANE A M. Resolving the HUPD and HOPD by DEMS to determine the ECSA of Pt electrodes in PEM fuel cells[J]. Electrochem Commun,2011,13(9):913−916. doi: 10.1016/j.elecom.2011.05.028 [45] ZHAN D P, VELMURUGAN J, MIRKIN M V. Adsorption/desorption of hydrogen on Pt nanoelectrodes: Evidence of surface diffusion and spillover[J]. JACS,2009,131:14756−14760. doi: 10.1021/ja902876v [46] ZHAO L, SUI X L, LI J Z, ZHANG J J, ZHANG L M, HUANG G S, WANG Z B. Supramolecular assembly promoted synthesis of three-dimensional nitrogen doped graphene frameworks as efficient electrocatalyst for oxygen reduction reaction and methanol electrooxidation[J]. Appl Catal B: Environ,2018,231:224−233. doi: 10.1016/j.apcatb.2018.03.020 [47] YI J D, ZHANG M D, HOU Y, HUANG Y B, CAO R. N-doped carbon aerogel derived from a metal-organic framework foam as an efficient electrocatalyst for oxygen reduction[J]. Chem Asian J,2019,14(20):3642−3647. doi: 10.1002/asia.201900727 [48] HOLZWARTH U, GIBSON N. The Scherrer equation versus the 'Debye-Scherrer equation'[J]. Nat Nanotechnol,2011,6(9):534. doi: 10.1038/nnano.2011.145 [49] MELKE J, PETER B, HABERDER A, ZIEGLER J, FASEL C, NEFEDOVV A, SEZEN H, WÖLL C, EHRENBERG H, ROTH C. Metal-support interactions of platinum nanoparticles decorated N-doped carbon nanofibers for the oxygen reduction reaction[J]. ACS Appl Mater Interfaces,2016,8(1):82−90. doi: 10.1021/acsami.5b06225 [50] SHAO M H, PELES A, SHOEMAKER K. Electrocatalysis on platinum nanoparticles: Particle size effect on oxygen reduction reaction activity[J]. Nano Lett,2011,11(9):3714−3719. doi: 10.1021/nl2017459 [51] ZHANG Y L, ROBINSON D A, MCKELVEY K, REN H, WHITE H S, EDWARDS M A. A high-pressure system for studying oxygen reduction during Pt nanoparticle collisions[J]. J Electrochem Soc,2020,167(16):166507. [52] WANG C, WANG X D, LAI F Y, LIU Z, DONG R H, LI W, SUN H X, GENG B Y. Pt nanoparticles supported on N-doped porous carbon derived from metal-organic frameworks for oxygen reduction[J]. ACS Appl Nano Mater,2020,3(6):5698−5705. doi: 10.1021/acsanm.0c00906 [53] WANG Q C, JI Y J, LEI Y P, WANG Y B, WNAG Y D, LI Y Y, WANG S Y. Pyridinic-N-dominated doped defective graphene as a superior oxygen electrocatalyst for ultrahigh-energy-density Zn-air batteries[J]. ACS Energy Lett,2018,3(5):1183−1191. doi: 10.1021/acsenergylett.8b00303 [54] VEITH G M, LUPINI A R, BAGGETTO L, BROWNING J F, KEUM J K, XILLA A, PRATI L, PAPANDREW A B, GOENAGA G A, MULLINS A R, BULLOCK S E, DUDNEY N J. Evidence for the formation of nitrogen-rich platinum and palladium nitride nanoparticles[J]. Chem Mater,2013,25(24):4936−4945. doi: 10.1021/cm403224m [55] LIU J, LI W Q, CHENG R L, WU Q, ZHAO J H, HE D P, MU S C. Stabilizing Pt nanocrystals encapsulated in N-doped carbon as double-active sites for catalyzing oxygen reduction reaction[J]. Langmuir,2019,35(7):2580−2586. doi: 10.1021/acs.langmuir.8b03947 [56] XIANG Z W, LI W, LIU F, TAN F, HAN F X, WANG X Z, SHAO C W, XU M L, LIU W P, YANG X K. Catalyst with a low load of platinum and high activity for oxygen reduction derived from strong adsorption of Pt-N4 moieties on a carbon surface[J]. Electrochem Commun,2021,127:107039. doi: 10.1016/j.elecom.2021.107039 [57] XIONG Y J, MA Y N, ZOU L L, HAN S B, CHEN H, WANG S, GU M, SHEN Y, ZHANG L P, XIA Z H, LI J, YANG H. N-doping induced tensile-strained Pt nanoparticles ensuring an excellent durability of the oxygen reduction reaction[J]. J Catal,2020,382:247−255. doi: 10.1016/j.jcat.2019.12.025 [58] ZHANG L L, WEI M, WANG S Q, LI Z, DING L X, WANG H H. Highly stable PtP alloy nanotube arrays as a catalyst for the oxygen reduction reaction in acidic medium[J]. Chem Sci,2015,6(5):3211−3216. doi: 10.1039/C5SC00124B [59] MA J, HABRIOUX A, LUO Y, SANCHEZ G R, CALVILLO L, GRANOZZI G, BALBUENA P B, NANTE N A. Electronic interaction between platinum nanoparticles and nitrogen-doped reduced graphene oxide: effect on the oxygen reduction reaction[J]. J Mater Chem A,2015,3(22):11891−11904. doi: 10.1039/C5TA01285F [60] JO H G, KIM K H, AHN H J. Nitrogen-doped carbon quantum dots decorated on platinum catalysts for improved oxygen reduction reaction[J]. Appl Surf Sci,2021,554:149594. doi: 10.1016/j.apsusc.2021.149594 [61] LIU J, JIAO M G, LU L L, BARKHOLTZ H M, LI Y P, WANG Y, JIANG L H, WU Z J, LIU D J, ZHUANG L, MA C, ZENG J, ZHANG B S, SU D S, SONG P, XING W, XU W L, WANG Y, JING Z, SUN G Q. High performance platinum single atom electrocatalyst for oxygen reduction reaction[J]. Nat Commun,2017,8:15938. doi: 10.1038/ncomms15938 [62] LIU J, Wu X X, Yang L P, WANG F, YIN J. Unprotected Pt nanoclusters anchored on ordered mesoporous carbon as an efficient and stable catalyst for oxygen reduction reaction[J]. Electrochim Acta,2019,297:539−544. doi: 10.1016/j.electacta.2018.12.017 [63] WANG J Y, XU M, ZHAO J Q, FANG H F, HUANG Q Z, XIAO W P, LI T, WANG D L. Anchoring ultrafine Pt electrocatalysts on TiO2-C via photochemical strategy to enhance the stability and efficiency for oxygen reduction reaction[J]. Appl Catal B: Environ,2018,237:228−236. doi: 10.1016/j.apcatb.2018.05.085 [64] HAM K, SHIN D, LEE J. The role of lone-pair electrons in Pt-N interactions for the oxygen reduction reaction in polymer exchange membrane fuel cells[J]. ChemSusChem,2020,13(7):1751−1758. doi: 10.1002/cssc.201903403 [65] WIGGINS-CAMACHO J D, STEVENSON K J. Effect of Nitrogen concentration on capacitance, density of states, electronic conductivity, and morphology of N-doped carbon nanotube electrodes[J]. J Phys Chem C,2009,113,:19082−19090. doi: 10.1021/jp907160v [66] JI S G, KIM H, PARK C, KIM W, CHOI C H. Underestimation of platinum electrocatalysis induced by carbon monoxide evolved from graphite counter electrodes[J]. ACS Catal,2020,10(18):10773−10783. doi: 10.1021/acscatal.0c01783 [67] DEAK D V, SINGH D, KING J C, OZKAN U S. Use of carbon monoxide and cyanide to probe the active sites on nitrogen-doped carbon catalysts for oxygen reduction[J]. Appl Catal B: Environ,2012,113−114:126−133. doi: 10.1016/j.apcatb.2011.11.029 -
 2022-S013_R+revised+supporting+information_燃料化学学报.docx
2022-S013_R+revised+supporting+information_燃料化学学报.docx
-



 下载:
下载: