Preparation and electrochemical stability of Co-doped La1.5Sr0.5Ni1−xCoxO4+δ cathode materials
-
摘要:
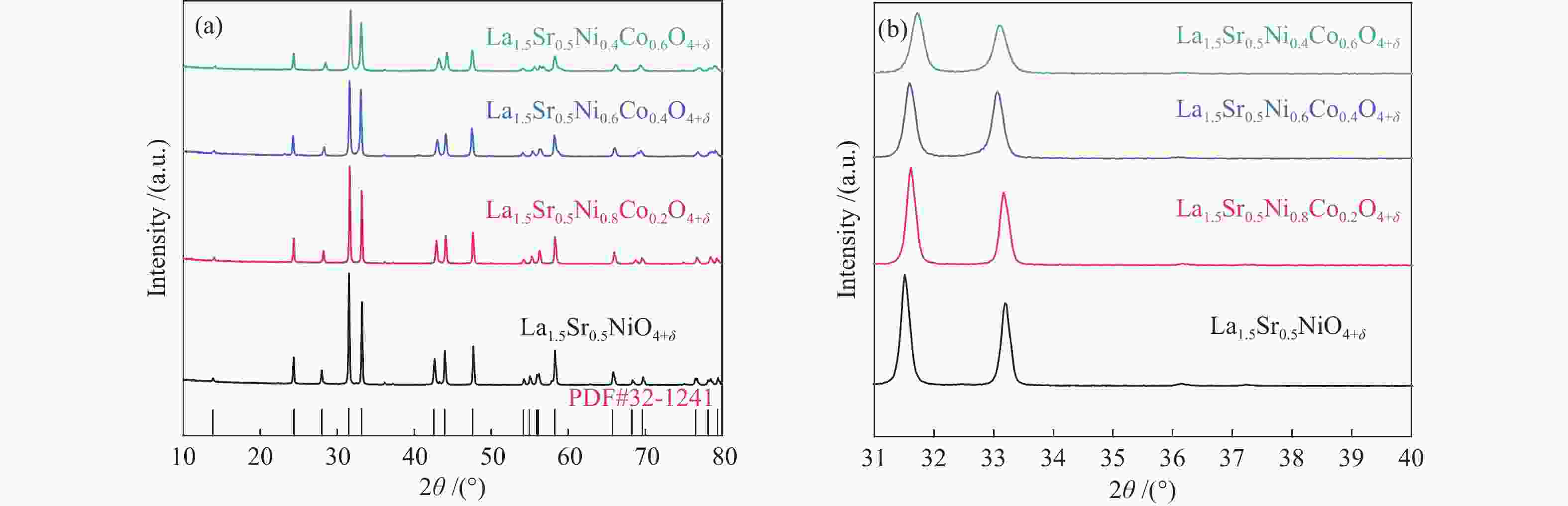
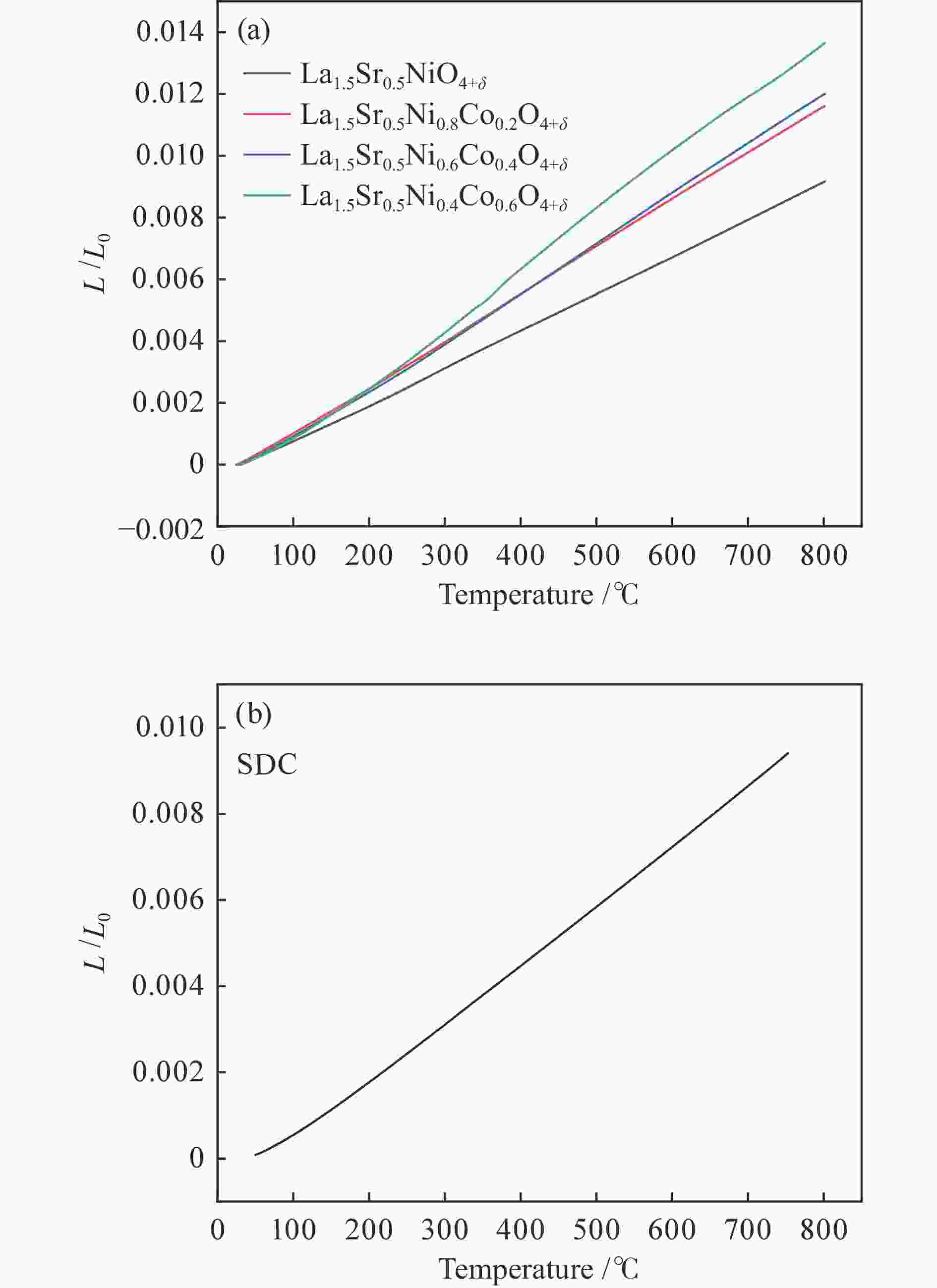
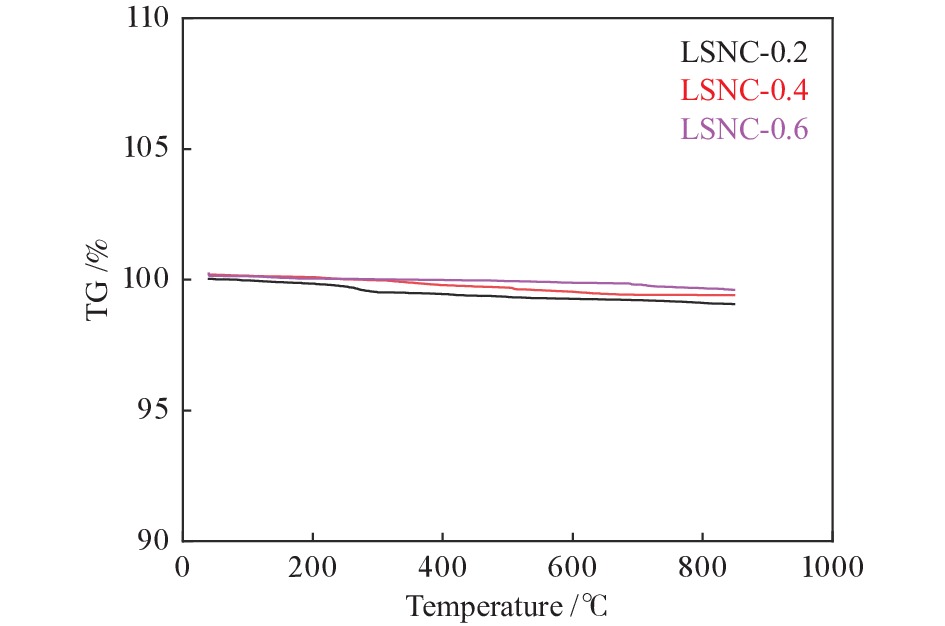
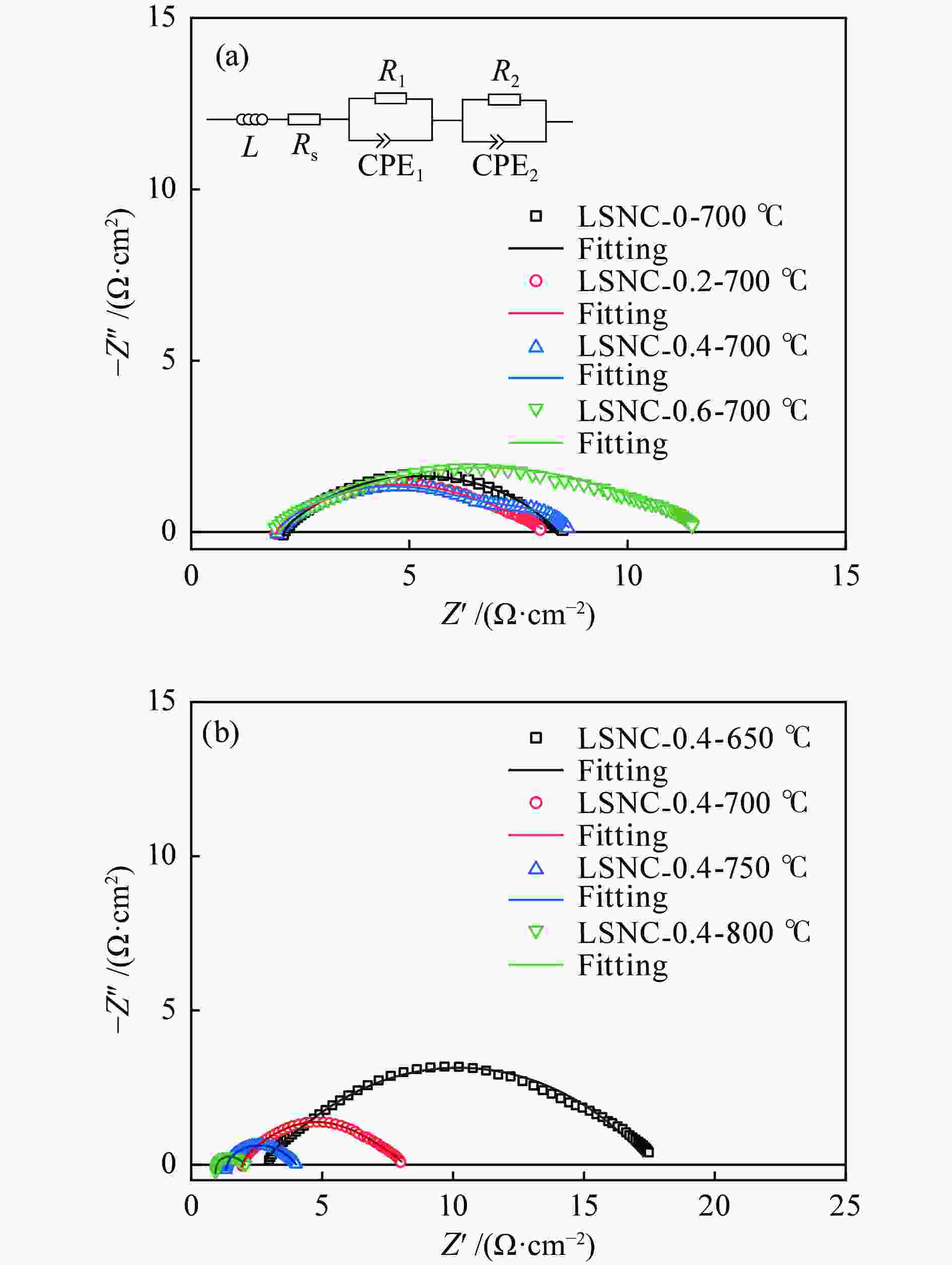
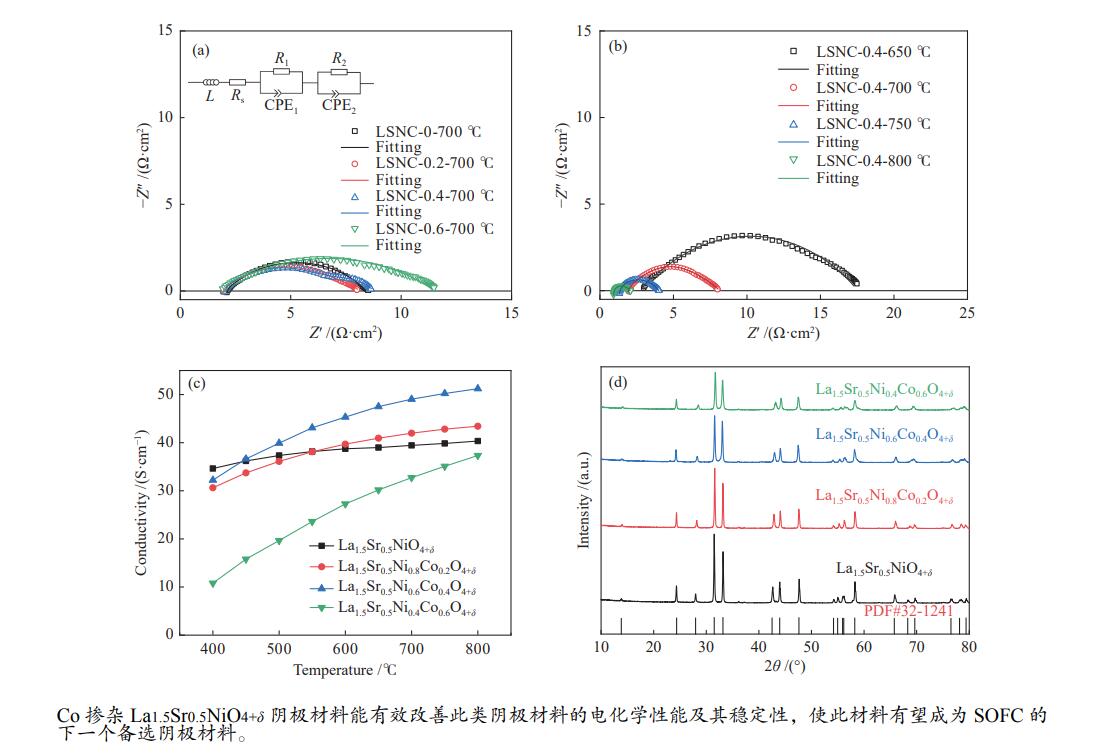
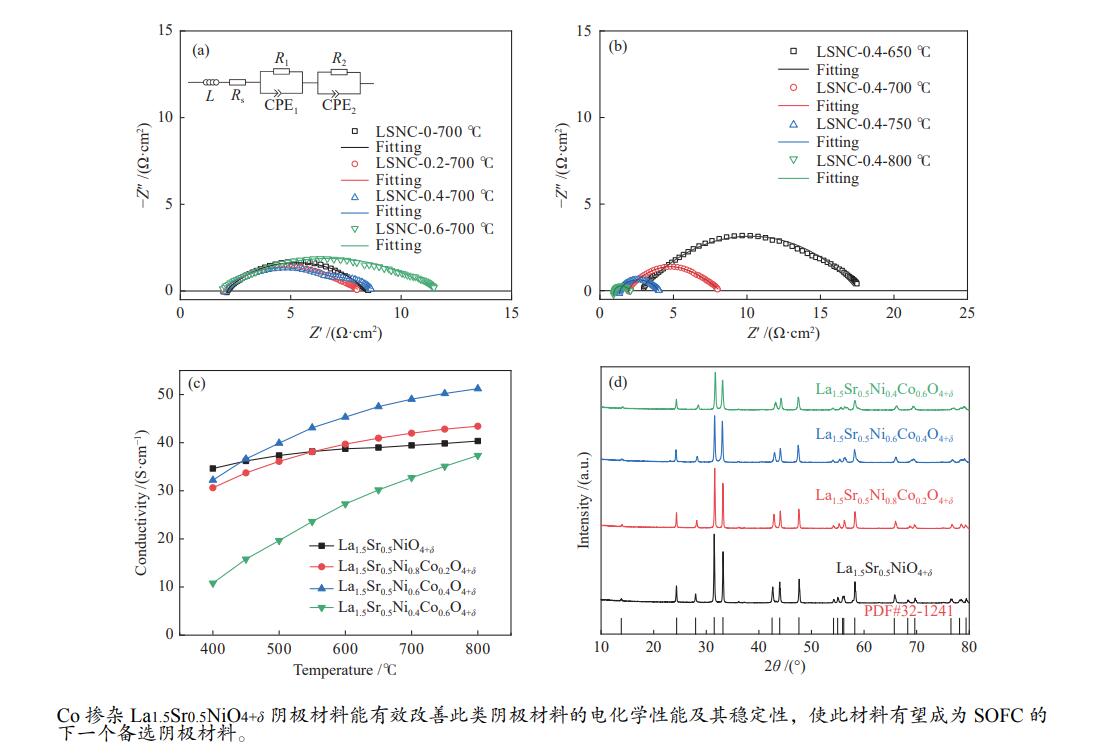
利用溶胶凝胶法合成了La1.5Sr0.5NiO4+δ掺杂Co的阴极材料La1.5Sr0.5Ni1−xCoxO4+δ (x=0、0.2、0.4、0.6)。通过X射线衍射、X射线光电子能谱、热重、热膨胀系数的测定和扫描电镜等技术探究了材料的相结构、元素组成、热力学性能和表面形貌。结果表明,所合成的样品为具有类钙钛矿型结构的单一纯相,掺杂Co元素使材料的热膨胀系数有所提高。将该材料应用于固体氧化物燃料电池(SOFC)阴极,进行了电导率及电化学阻抗谱的测定。结果发现,La1.5Sr0.5Ni1−xCoxO4+δ的电导率随着Co元素掺杂量的提高而升高,当 x = 0.4 时La1.5Sr0.5Ni0.6Co0.4O4+δ的电导率最高,达51.21 S/cm(800 ℃);当 x 值大于0.4时,其电导率明显下降。此外,La1.5Sr0.5Ni0.6Co0.4O4+δ在电化学阻抗谱测试中也呈现出了最低的极化电阻(4.180 Ω·cm2,700 ℃),表现出较好的电化学性能。
Abstract:A series of Co-doped La1.5Sr0.5Ni1−xCoxO4+δ cathode materials ( x =0, 0.2, 0.4 and 0.6) were synthesized by sol-gel method and characterized by X-ray diffraction (XRD), X-ray photoelectron spectroscopy (XPS), thermogravimetric analysis (TGA), coefficient of thermal expansion (CTE) measurement and scanning electron microscope (SEM). The results suggest that all La1.5Sr0.5Ni1−xCoxO4+δ samples have a single pure phase with the perovskite-like structure and the doping with the Co element can increase the CTE value. Using La1.5Sr0.5Ni1−xCoxO4+δ as the cathode materials in the solid oxide fuel cell (SOFC), their electrical conductivity and electrochemical impedance spectroscopy were measured. The results indicate that the conductivity increases with the increase of Co doping amount and the La1.5Sr0.5Ni0.6Co0.4O4+δ sample with x = 0.4 displays the highest conductivity of 51.21 S/cm at 800 ℃; however, a higher content of Co (x > 0.4) leads to a decrease of the conductivity. In addition, La1.5Sr0.5N0.6Co0.4O4+δ exhibits the lowest polarization resistance of 4.180 Ω·cm2 in electrochemical impedance spectrum at 700 ℃, displaying its excellent electrochemical properties as the cathode materials.
-
Key words:
- solid oxide fuel cell /
- perovskite-like structure /
- cathode material
-
表 1 LSNC-x晶体结构的晶胞参数
Table 1 Cell parameters of crystal structure of various LSNC-x materials
Sample a/Å b/Å c/Å V/Å La1.5Sr0.5NiO4+δ 3.81254 3.81254 12.74016 185.184 La1.5Sr0.5Co0.2Ni0.8O4+δ 3.81936 3.81936 12.70324 185.30 La1.5Sr0.5Co0.4Ni0.6O4+δ 3.82437 3.82437 12.68348 185.506 La1.5Sr0.5Co0.6Ni0.4O4+δ 3.82768 3.82768 12.66534 185.590 表 2 四组样品和SDC的热膨胀系数值
Table 2 CTE values of SDC and four La1.5Sr0.5Ni1−xCoxO4+δ samples
Sample x Thermal expansion
coefficient (10−6 K−1)La1.5Sr0.5NiO4+δ 0 11.82 La1.5Sr0.5Ni0.8Co0.2O4+δ 0.2 14.95 La1.5Sr0.5Ni0.6Co0.4O4+δ 0.4 15.53 La1.5Sr0.5Ni0.4Co0.6O4+δ 0.6 17.66 Sm0.2Ce0.8O1.9 SDC 13.43 表 3 LSNC-x (x=0、0.2、0.4、0.6)的XPS谱图O 1s峰拟合
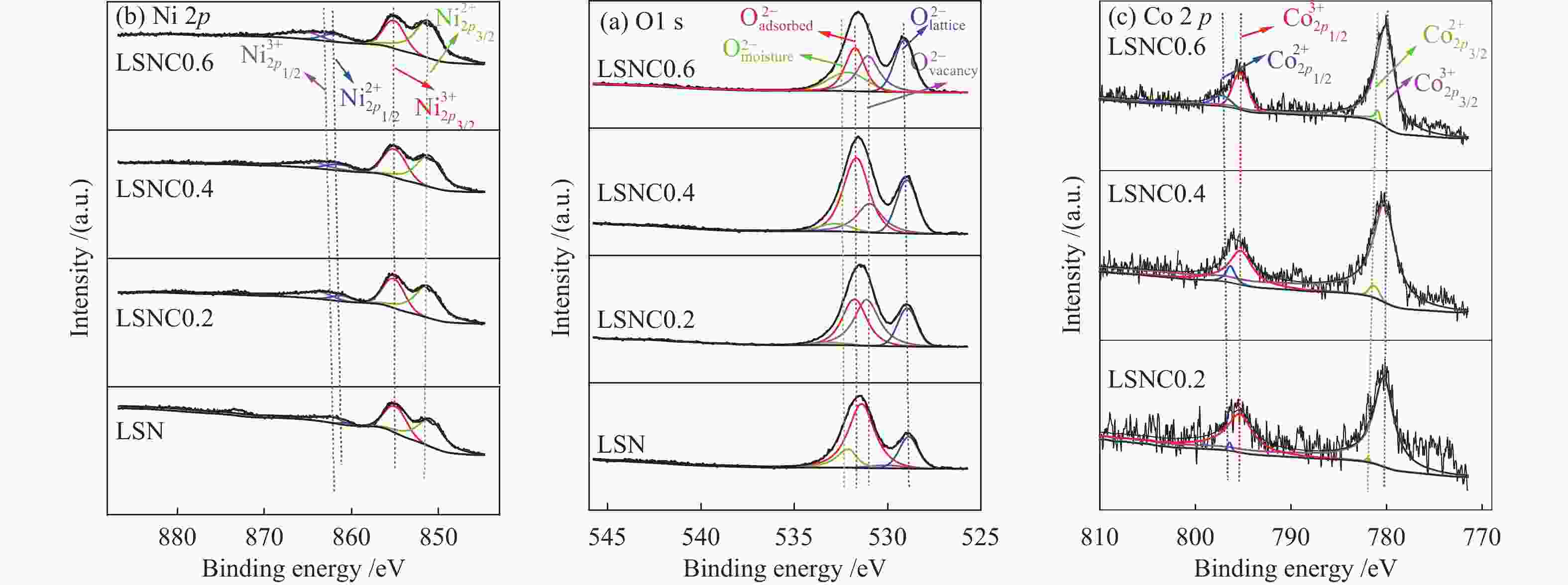
Table 3 Fitting results of O 1s XPS spectra of various LSNC-x samples
Sample Omoisture /eV Oadsorbed /eV Ovacancy /eV Olattice /eV (Oadsorbed + Ovacancy)/Olattice LSN 532.46 531.48 530.60 528.90 2.78 LSNC0.2 532.28 531.28 530.94 528.73 3.17 LSNC0.4 532.12 531.13 530.97 528.35 3.24 LSNC0.6 532.10 531.45 530.88 529.12 3.26 表 4 LSNC-x (x=0、0.2、0.4、0.6)的XPS谱图Ni 2p峰拟合
Table 4 Fitting results of Ni 2p XPS spectra of various LSNC-x samples
Sample Ni3+ 2p1/2 /eV Ni2+ 2p1/2 /eV Ni3+ 2p3/2 /eV Ni2+ 2p3/2 /eV Ni3+ /% Ni2+ /% LSN 862.24 860.70 855.38 851.60 56.25 43.75 LSNC0.2 862.20 860.72 855.49 851.61 49.53 50.48 LSNC0.4 862.25 860.74 855.21 851.54 49.47 50.53 LSNC0.6 862.26 861.01 855.39 851.68 40.77 59.23 表 5 LSNC-x (x=0、0.2、0.4、0.6)的XPS谱图Co 2p峰拟合
Table 5 Fitting results of Co 2p XPS spectra of various LSNC-x samples
Sample $ {\text{Co}}^{3 + }\;{2p_{{1/2}}} $ /eV $ {\text{Co}}^{2 + }\;{2p_{{1/2}}} $ /eV $ {\text{Co}}^{3 + }\;{2p_{{3/2}}} $ $ {\text{Co}}^{2 + }\;{2p_{{3/2}}} $ /eV ${\text{Co} }^{3 + }\text{}$ /% ${\text{Co} }^{2 + }\text{}$ /% LSNC0.2 795.47 796.82 780.47 782.01 94.40 5.610 LSNC0.4 795.22 796.89 780.38 782.03 85.06 14.94 LSNC0.6 795.52 796.71 780.41 782.13 67.23 32.77 表 6 LSNC-x (x=0、0.2、0.4、0.6)在700 ℃的极化电阻值
Table 6 Polarization resistance values of various LSNC-x samples at 700 ℃
Sample Polarization resistance value /
(Ω·cm2)La1.5Sr0.5NiO4 + δ 4.426 La1.5Sr0.5Ni0.8Co0.2O4 + δ 4.355 La1.5Sr0.5Ni0.6Co0.4O4 + δ 4.180 La1.5Sr0.5Ni0.4Co0.6O4 + δ 6.238 -
[1] BOREAVE A, TAN H, ROCHE V. Oxygen mobility in lanthanum nickelate catalysts for deep oxidation of propane[J]. Solid State Ionics,2008,179(21/26):1071−1075. doi: 10.1016/j.ssi.2008.01.052 [2] LI Y H, GEMMEN R, LIU X B. Oxygen reduction and transportation mechanisms in solid oxide fuel cell cathodes[J]. J Power Sources,2010,195(11):3345−3358. doi: 10.1016/j.jpowsour.2009.12.062 [3] JIANG S. Development and optimization of electrode materials in solid oxide fuel cells[J]. Battery,2002,32(3):133−137. [4] KHARTON V V, TSIPIS E V, YAREMCHENKO A A. Surface-limited oxygen transport and electrode properties of La2Ni0.8Cu0.2O4 + δ[J]. Solid State Ionics,2004,166(3-4):327−337. doi: 10.1016/j.ssi.2003.11.020 [5] CHEN Y, LIAO Q, WEI Y Y A. CO2-stable K2NiF4-type oxide (Nd0.9La0.1)(2)(Ni0.74Cu0.21Al0.05)O4 + δ for oxygen separation[J]. Ind Eng Chem Res,2013,52(25):8571−8578. [6] ZHAO F, WANG X F, WANG Z Y. K2NiF4 type La2−xSrxCo0.8Ni0.2O4 + δ as the cathodes for solid oxide fuel cells[J]. Solid State Ionics,2008,179(27/32):1450−1453. doi: 10.1016/j.ssi.2007.11.033 [7] 郝举红, 李强, 孙丽萍, Christophe Pijolat. 中温固体氧化物燃料电池阴极材料Nd2−xSrxCuO4的制备与性能研究[J]. 无机化学学报,2009,25(10):1818−1822.HAO Ju-hong, LI Qiang, SUN Li-ping, Christophe Pijolat. Synthesis and Performance of Nd2−xSrxCuO4 Cathode Materials for IT-SOFC[J]. Chin J Inorg Chem,2009,25(10):1818−1822. [8] WANG Y S, NIE H W, WANG S R. A2−αAα' BO4-type oxides as cathode materials for IT-SOFCs (A = Pr, Sm; A ' = Sr; B = Fe, Co)[J]. Mater Lett,2006,60(9/10):1174−1178. doi: 10.1016/j.matlet.2005.10.104 [9] NIE H W, WEN T L, WANG S R. Preparation, thermal expansion, chemical compatibility, electrical conductivity and polarization of A2−αAα' MO4 (A = Pr, Sm; A ' = Sr; M = Mn, Ni; α= 0.3, 0.6) as a new cathode for SOFC[J]. Solid State Ionics,2006,177(19/25):1929−1932. doi: 10.1016/j.ssi.2006.01.003 [10] 黄端平, 徐庆. 新型中温固体氧化物燃料电池阴极材料的研究[C]//中国硅酸盐学会陶瓷分会2006学术年会论文专辑(上). 杭州: 武汉理工大学出版社, 2006: 37−42.HUANG Duan-ping, XU Qing. Study on novel medium temperature solid oxide fuel cell cathode material[C]//China Ceramics Society Ceramic Branch 2006 Annual Conference Paper Album (I). Hangzhou: Wuhan University of Technology Press, 2006: 37−42. [11] KHARTON V V, YAREMCHENKO A A, TSIPIS E V. Oxygen transport and electrochemical activity of La2NiO4-based cathode materials[C]. 8th International Symposium on Solid Oxide Fuel Cells, 2003: 561−570. [12] AMOW G, WHITFIELD P S, DAVIDSON I J. Structural and sintering characteristics of the La2Ni1−xCoxO4 + δ series[J]. Ceram Int,2004,30(7):1635−1639. doi: 10.1016/j.ceramint.2003.12.164 [13] 华瑛. 材料的热膨胀性能及其影响因素[J]. 上海钢研,2005,26(2):60−63.HUA Ying. Thermal expansion properties of materials and its influencing factors[J]. Shanghai Steel Iron Res,2005,26(2):60−63. [14] FU D, JIN F, HE T. A-site calcium-doped Pr1−xCaxBaCo2O5 + δ double perovskites as cathodes for intermediate-temperature solid oxide fuel cells[J]. J Power Sources,2016,313(5):134−141. [15] 王悦. B位有序的Co基双钙钛矿中温固体氧化物燃料电池阴极材料Sr2Co1−xNbxFeO5 + δ的性能[D]. 吉林: 吉林大学, 2017.WANG Yue. Properties of Sr2Co1−xNbxFeO5 + δ as cathode material of Co-based double perovskite intermediate-temperature solid oxide fuel cell with Ordered B-position[D]. Jilin: Jilin University, 2017. [16] 冯晓霞, 谢志翔, 王竹梅. 中温固体氧化物燃料电池阴极材料LaFe1−xCuxO3−δ的制备与性能研究[J]. 中国陶瓷,2020,56(3):27−32.FENG Xiao-xia, XIE Zhi-xiang, WANG Zhu-mei. Preparation and properties of LaFe1−xCuxO3−δ cathode material for medium-temperature solid oxide fuel cell[J]. Chin Ceram,2020,56(3):27−32. [17] 范宇航, 孙丽萍, 霍丽华, 赵辉, Jean-Marc Bassat, Aline Rougier, Sebastien Fourcade, Jean-Claude Grenier. 钴掺杂Sr1.5La0.5MnO4的合成及高温电化学性能研究[J]. 无机化学学报,2016,32(10):1730−1738.FAN Yu-hang, SUN Li-ping, HUO Li-hua, ZHAO Hui, Jean-Marc Bassat, Aline Rougier, Sebastien Fourcade, Jean-Claude Grenier. Synthesis and high temperature electrochemical properties of Cobalt doped Sr1.5La0.5MnO4[J]. Chin J Inorg Chem,2016,32(10):1730−1738. [18] JIA W, HUANG Z, SUN W. Flexible A-site doping La0.6−xMxSr0.4Co0.2Fe0.8O3 (M=Ca, Ba, Bi; x=0, 0.1, 0.2) as novel cathode material for intermediate-temperature solid oxide fuel cells: A first-principles study and experimental exploration[J]. J Power Sources,2021,490(8):229564. [19] 李婷婷, 王振华, 孙旺, 白羽, 孙克宁, 乔金硕. 质子导体固体氧化物燃料电池Pr1.8Ba0.2Ni0.5Cu0.4Co0.1O4 + δ阴极材料制备与性能[J]. 硅酸盐学报,2021,49(12):2636−2643.LI Ting-ting, WANG Zhen-hua, SUN Wang, BAI Yu, SUN Ke-ning, QIAO Jin-shuo. Proton conductor solid oxide fuel cell Pr1.8Ba0.2Ni0.5Cu0.4Co0.1O4 + δ cathode material preparation and performance[J]. J Chin Ceram Soc,2021,49(12):2636−2643. [20] KIM S J, AKBAY T, MATSUDA J. Strain effects on oxygen reduction activity of Pr2NiO4 caused by gold bulk dispersion for low temperature solid oxide fuel cells[J]. ACS Appl Energy Mater,2019,2(2):1210−1220. doi: 10.1021/acsaem.8b01776 [21] PRABU M, KETPANG K, SHANMUGAM S. Hierarchical nanostructured NiCo2O4 as an efficient bifunctional non-precious metal catalyst for rechargeable zinc–air batteries[J]. Nanoscale,2014,6(6):3173−3181. doi: 10.1039/c3nr05835b [22] JIN F J, LIU X L, CHU X Y. Effect of nonequivalent substitution of Pr3 + /4 + with Ca2 + in PrBaCoFeO5 + δ as cathodes for IT-SOFC[J]. J Mater Sci,2021,56(2):1147−1161. doi: 10.1007/s10853-020-05375-y [23] LIM C, YANG Y, SIN Y-W. Ca- and Ni-doped Pr0.5Ba0.5FeO3−δ as a highly active and robust cathode for high-temperature solid oxide fuel cell[J]. Energy Fuels,2020,34(9):11458−11463. [24] 孟祥伟. 中温固体氧化物燃料电池阴极材料Pr1−xSrxCo0.8Fe0.2O3−δ的性能研究[D]. 吉林: 吉林大学, 2009.MENG Xiang-wei. Properties of Pr1−xSrxCo0.8Fe0.2O3−δ cathode material for Medium-temperature solid oxide fuel cell[D]. Jilin: Jilin university, 2009. [25] 封常乾, 隋静, 杨鹏. 疏松多孔Ce0.9Gd0.1O1.95电解质骨架对GdBa0.7Sr0.3Co2O(5 + δ)阴极材料性能的影响[J]. 青岛科技大学学报(自然科学版),2020,41(4):6−81 + 87.FENG Chang-qian, SUI Jing, YANG Peng. Effect of Loose porous Ce0.9Gd0.1O1.95 electrolyte skeleton on the properties of GdBa0.7Sr0.3Co2O(5 + δ) cathode materials[J]. J Qingdao Univ Sci Technol (Nat Sci),2020,41(4):6−81 + 87. [26] REN R Z, WANG Z H, XU C M. Tuning the defect of triple conducting oxide BaCo0.4Fe0.4Zr0.1Y0.1O3–δ perovskite toward activity enhanced cathode of protonic ceramic fuel cells[J]. J Mater Chem A,2019,7(31):18365−18372. doi: 10.1039/C9TA04335G [27] VIBHU V, ROUGIER A, NICOLLET C. La2−xPrxNiO4 + δ as suitable cathodes for metal supported SOFCs[J]. Solid State Ionics,2015,278(21/23):32−37. [28] LIU J J, QIU W H, YU L Y. Synthesis and electrochemical characterization of layered Li(Ni1/3Co1/3Mn1/3)O2 cathode materials by low-temperature solid-state reaction[J]. J Alloys Compounds,2008,449(1/2):326−330. doi: 10.1016/j.jallcom.2006.01.149 [29] BOEHM E, BASSAT J M, DORDOR P. Oxygen diffusion and transport properties in non-stoichiometric Ln2−xNiO4 + δ oxides[J]. Solid State Ionics,2005,176(37/38):2717−2725. doi: 10.1016/j.ssi.2005.06.033 -




 下载:
下载: