Insight into the catalytic oxidation of toluene over M/ZSM-5 (M=Cu, Mn, Fe, Ce, Ti) catalysts
-
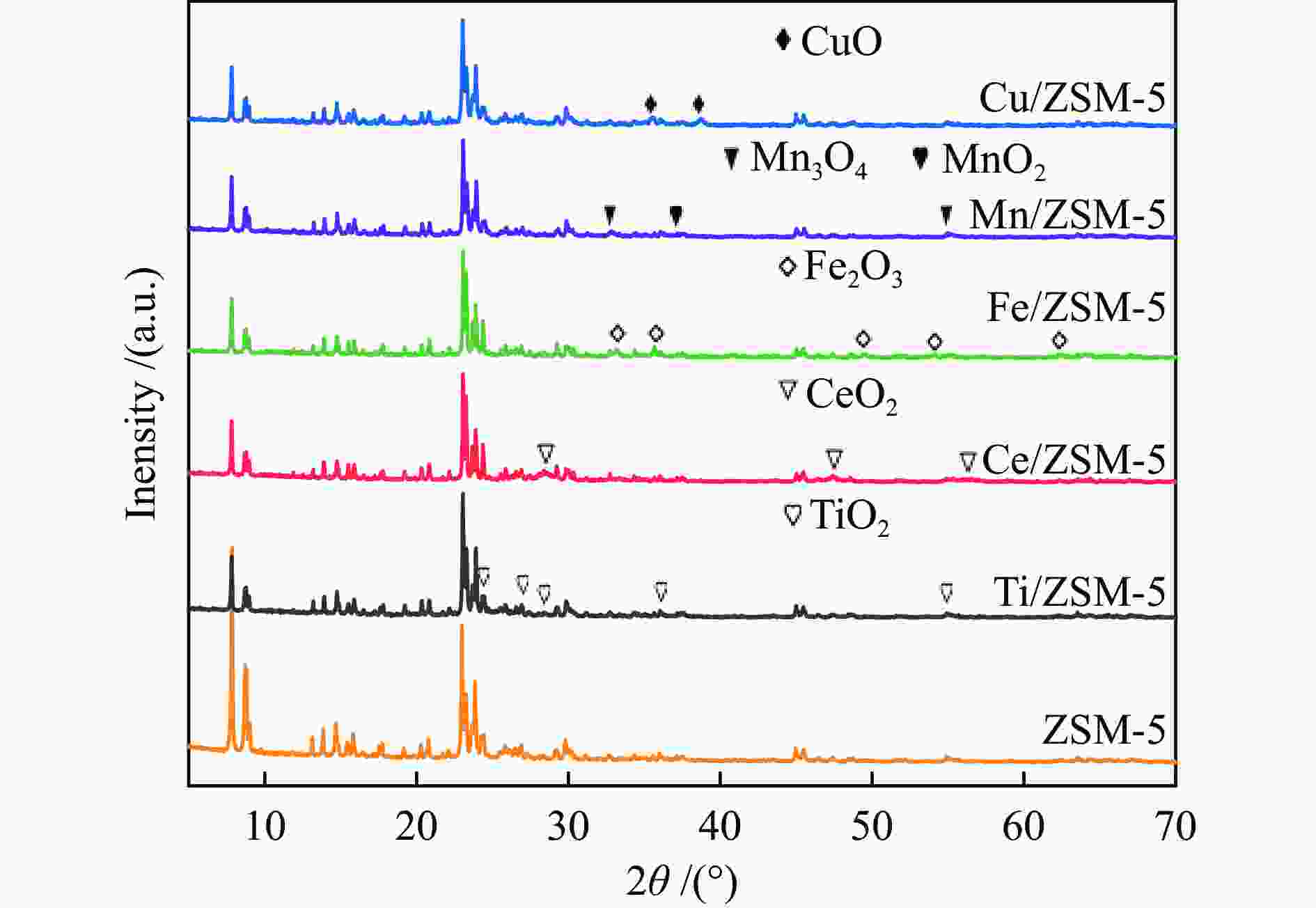
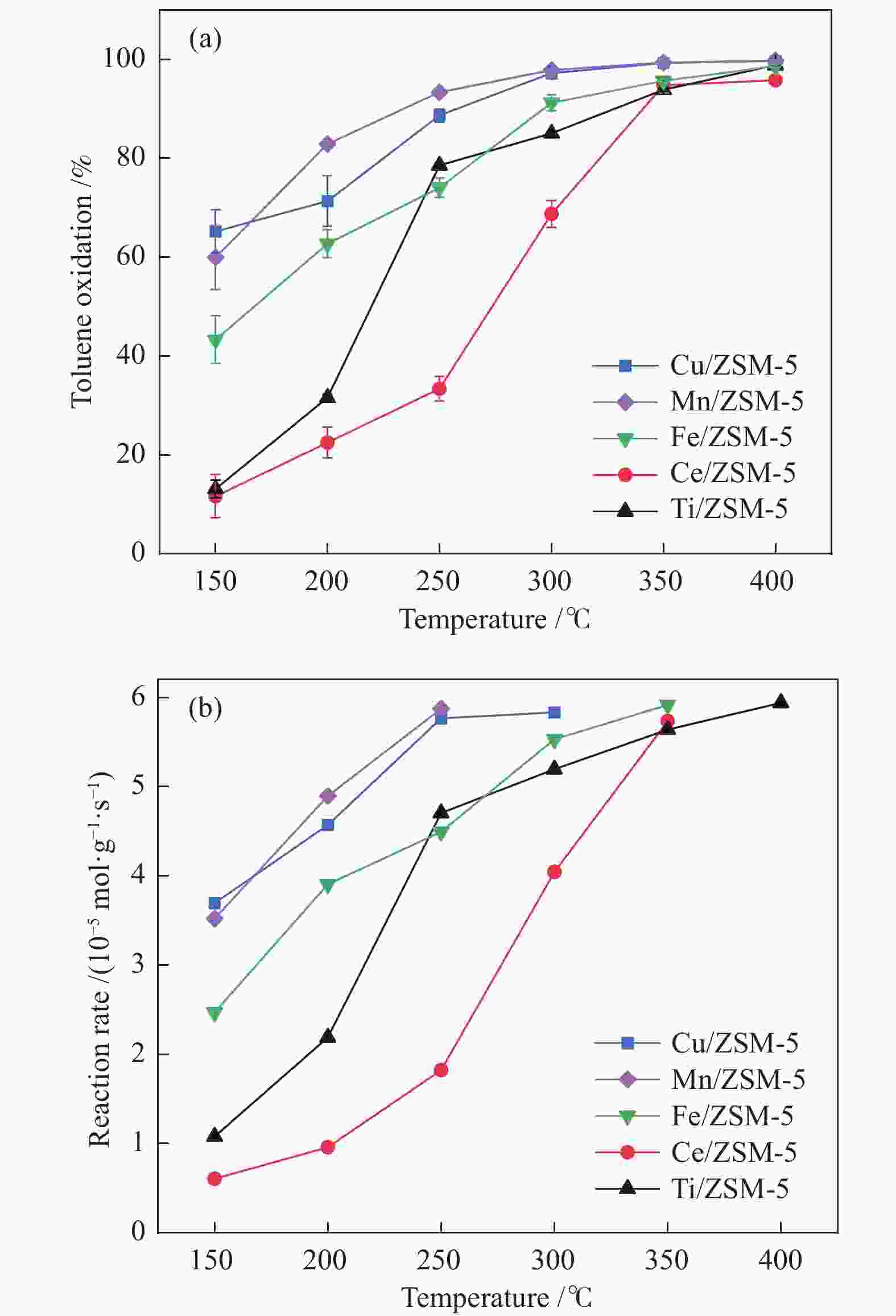
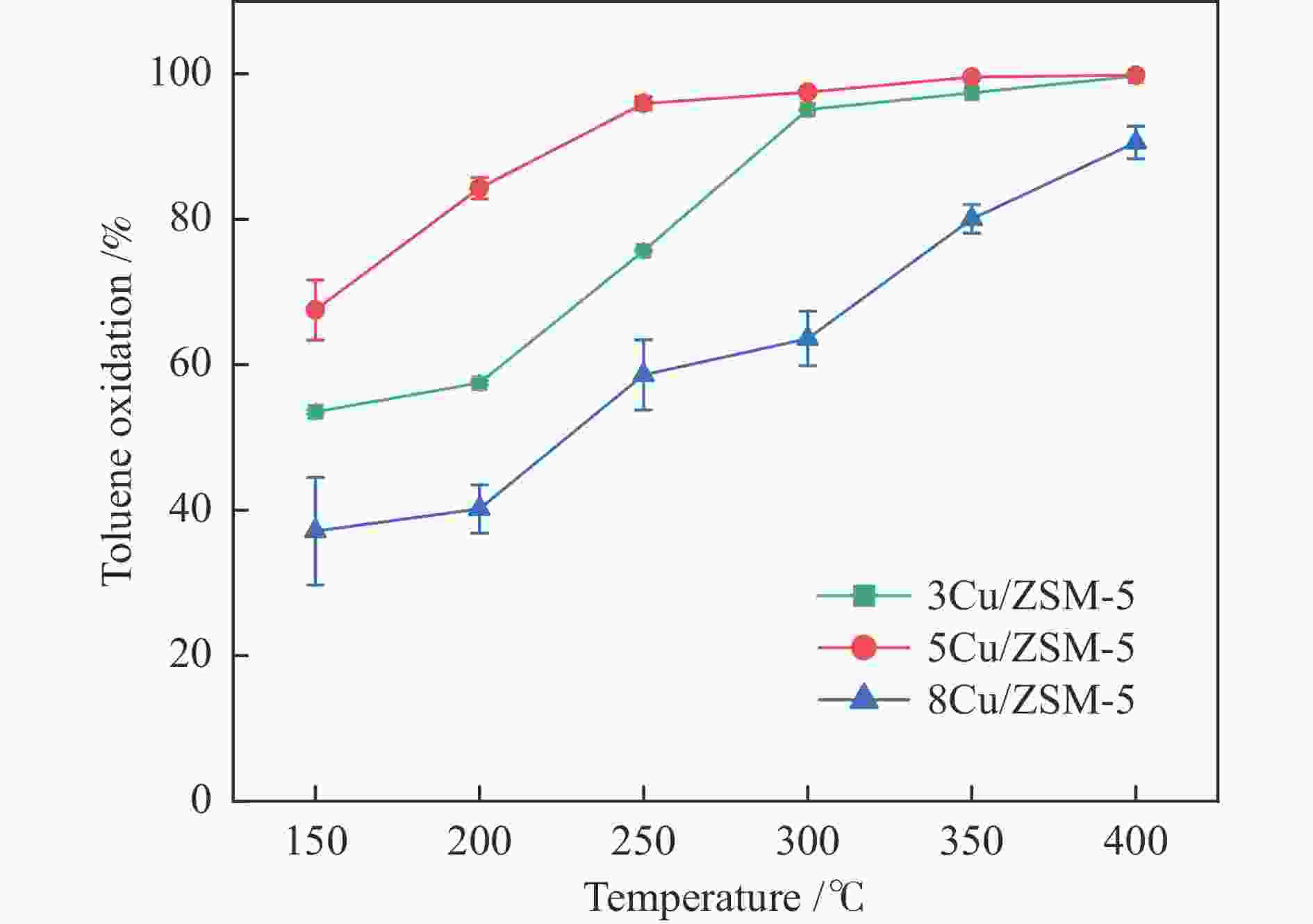
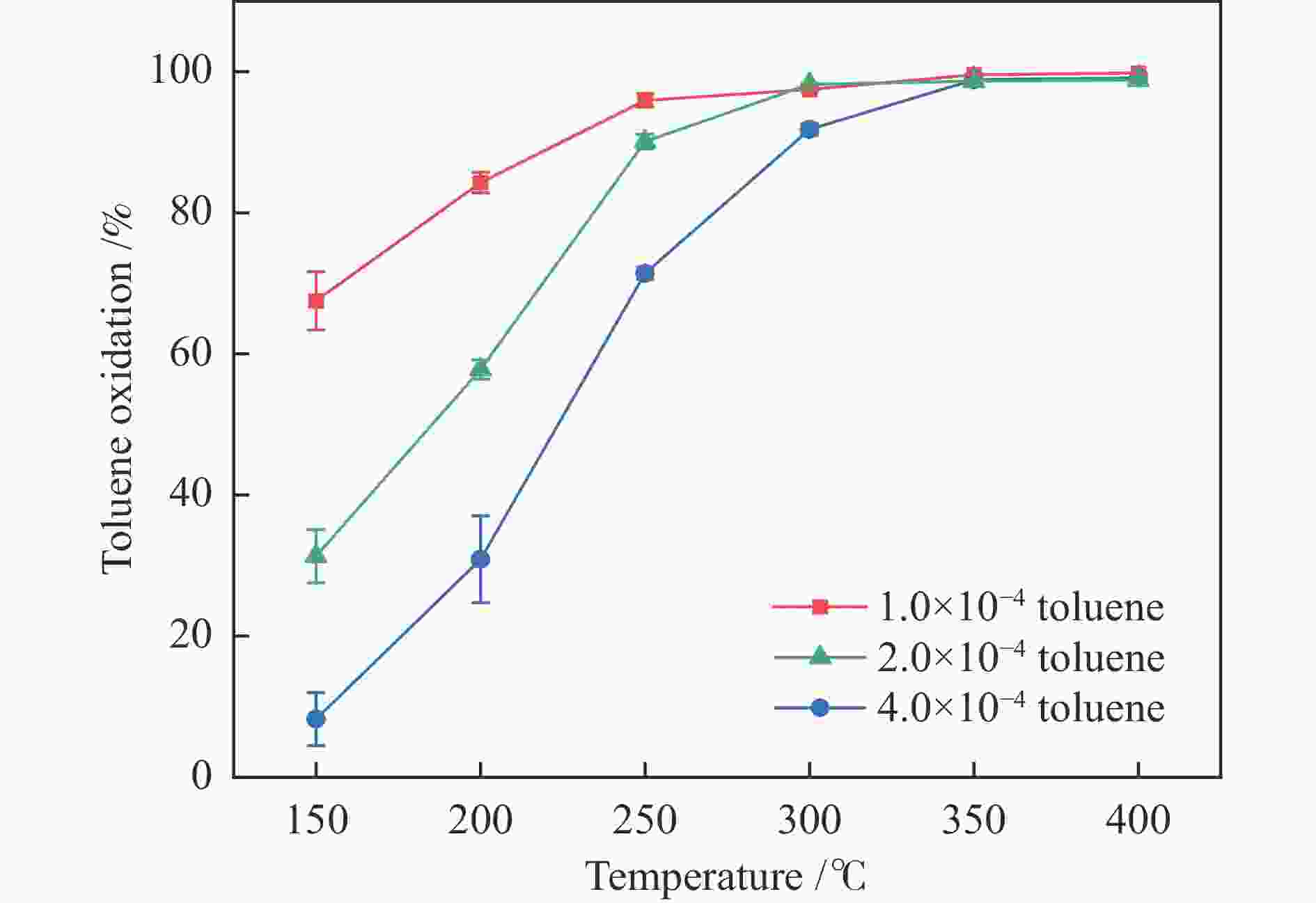
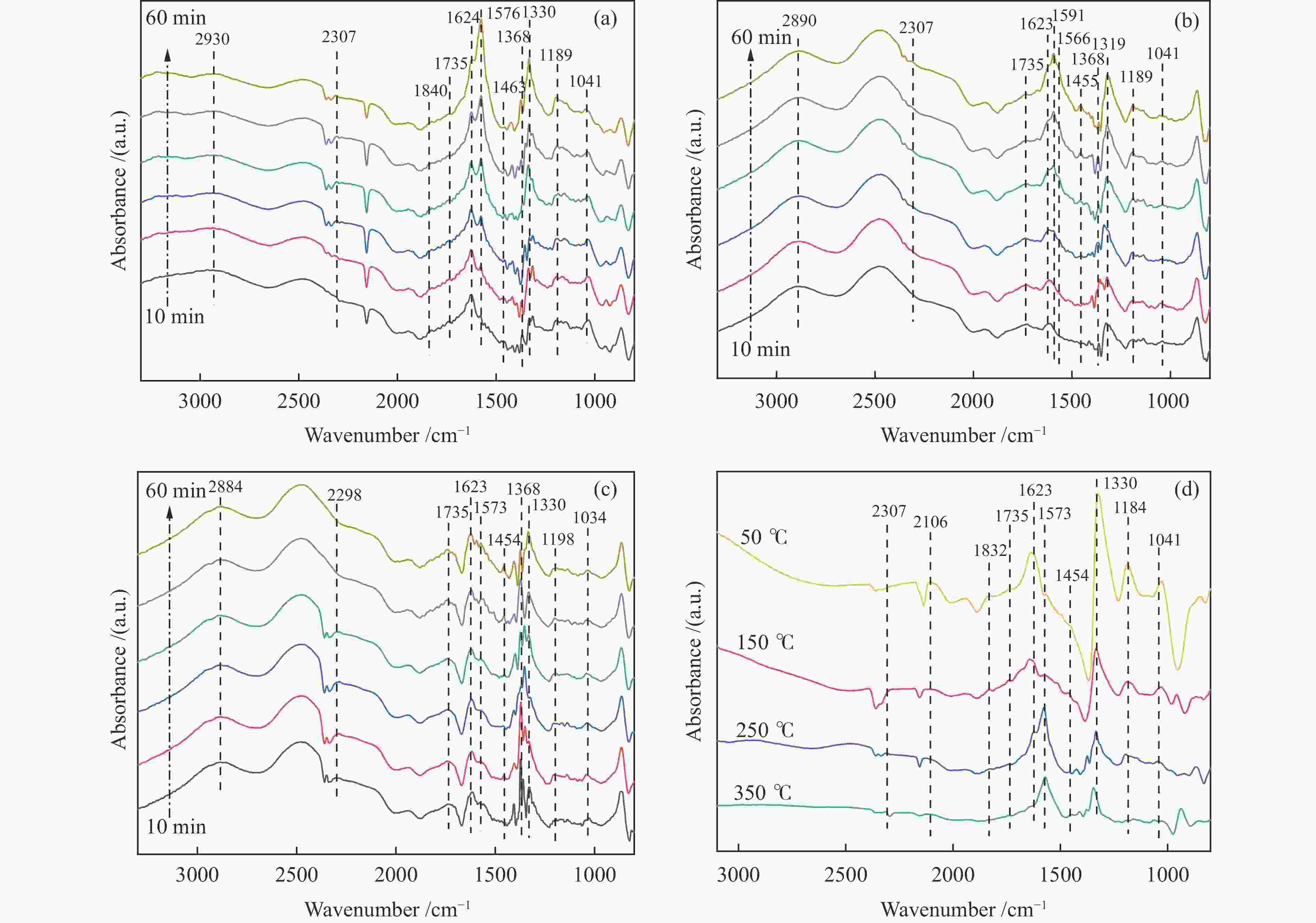
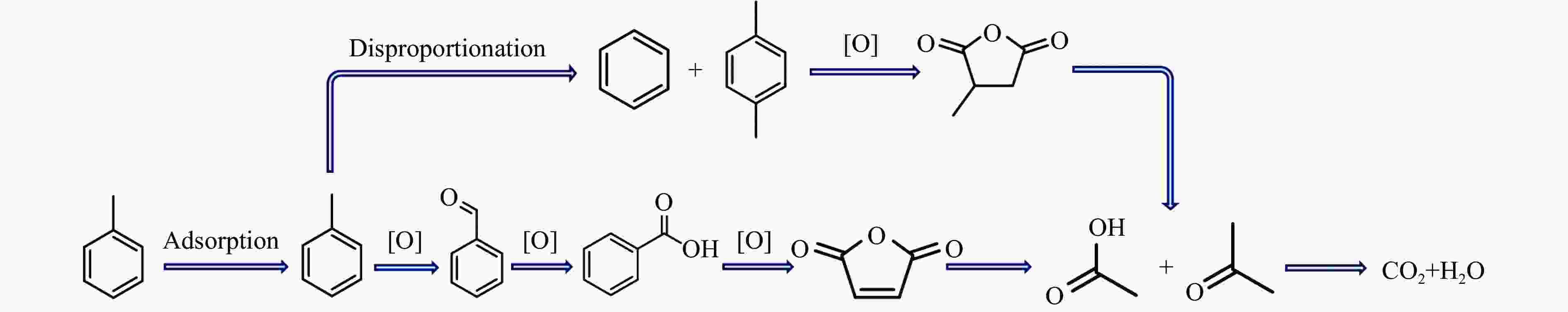
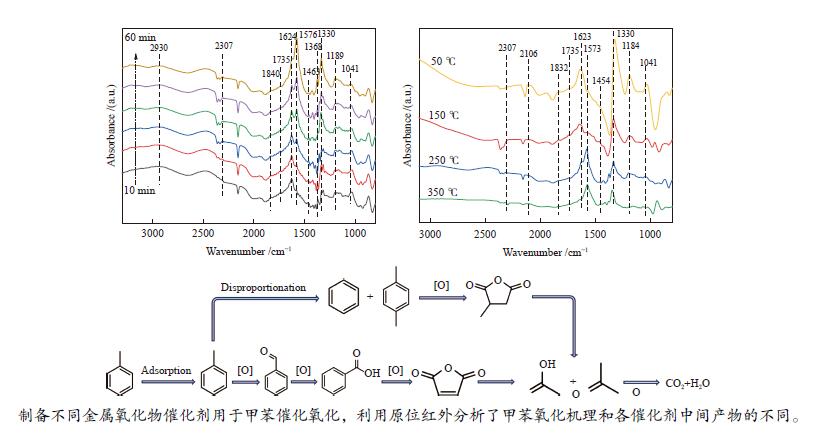
摘要: 以ZSM-5分子筛为载体,采用浸渍法负载Cu、Mn、Fe、Ce、Ti制备一系列金属氧化物催化剂,利用SEM、XRD、N2吸附-脱附、XPS、H2-TPR对催化剂的理化性质进行了表征,并考察了催化剂的催化氧化甲苯性能。结果表明,Cu/ZSM-5表面粗糙,金属元素分布均匀,具有较好的孔径结构、良好的低温还原性和丰富的吸附氧物种,且负载量为5%的Cu/ZSM-5表现出优异的甲苯催化活性和最佳的抗硫性,在SO2环境下t90为224 ℃ (GHSV=24000 h−1)。原位红外测试结果表明,甲苯的降解遵循以下途径,甲苯首先被吸附在催化剂表面形成吸附态甲苯,随后在催化剂作用下依次被转化为苯甲醛和苯甲酸,再经过开环反应形成马来酸、羧酸等小分子有机物,最终被氧化为CO2和H2O。Abstract: A series of metal oxide catalysts were prepared by impregnating Cu, Mn, Fe, Ce and Ti on ZSM-5 molecular sieve. The physicochemical properties of the catalysts were characterized by SEM, XRD, N2 adsorption/desorption, XPS, H2-TPR, and the catalytic oxidation of toluene was investigated. The results showed that Cu/ZSM-5 had rough surface, uniform distribution of metal, good pore structure, superior low-temperature reducibility and abundant adsorbed oxygen species. Cu/ZSM-5 with 5% loading exhibited excellent catalytic activity for toluene oxidation and the best sulfur resistance performance, with t90 (GHSV=24000 h−1) being 224 ℃ in SO2 environment. In-situ DRIFTS experiments revealed that the degradation path of toluene was as follows: toluene was first adsorbed on the surface of the catalyst to form adsorbed toluene, then it was converted into benzaldehyde and benzoic acid successively on the catalyst. And small molecule organics such as maleic acid and carboxylic acid were formed through ring opening reaction, and finally was oxidized to CO2 and H2O.
-
Key words:
- toluene /
- catalytic oxidation /
- Cu/ZSM-5
-
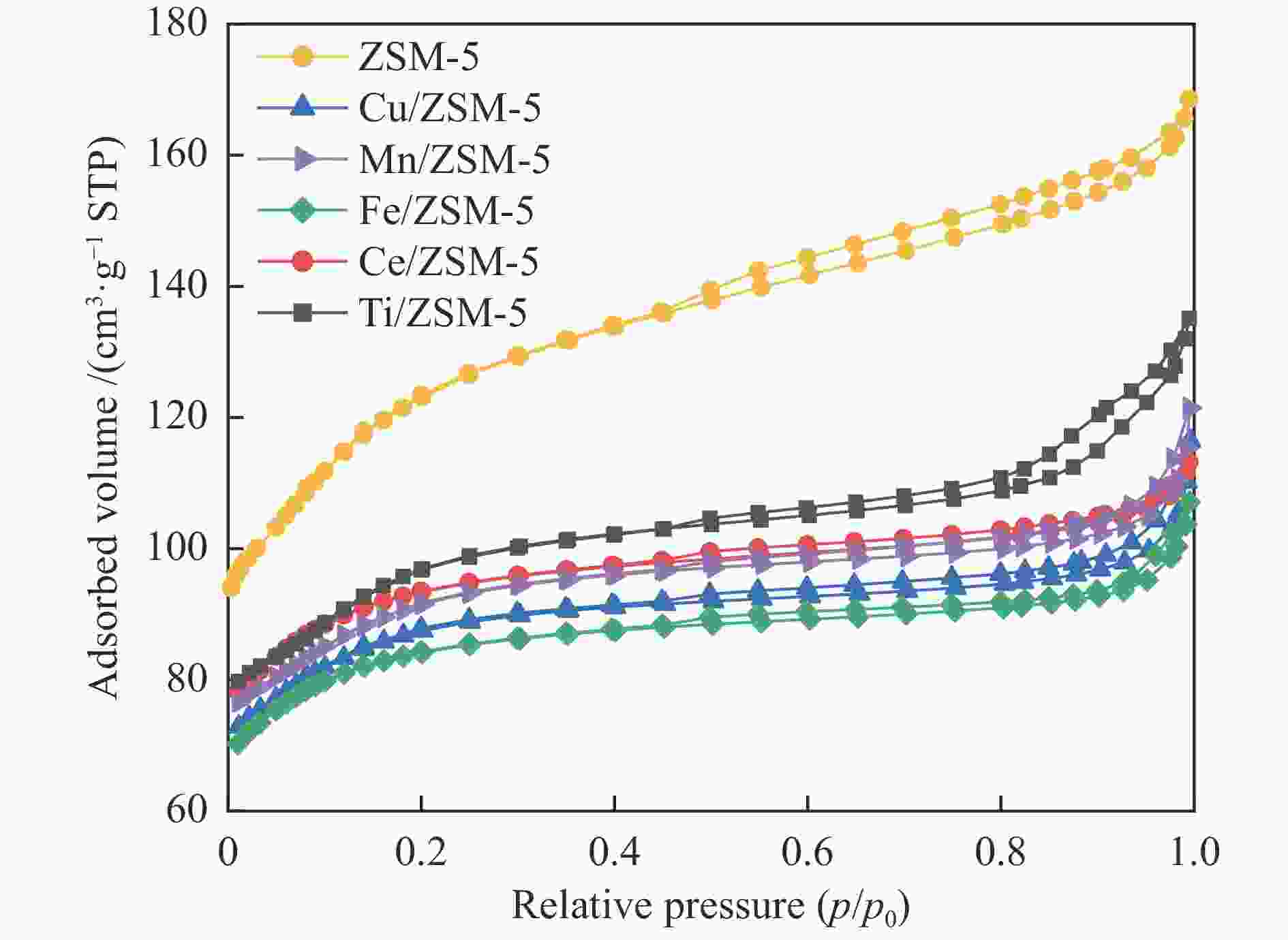
表 1 不同催化剂的孔结构参数
Table 1 Structure properties of different catalysts.
Catalyst BET surface area /
(m2·g−1)Micropore volume /
(cm3·g−1)Mesopore volume /
(cm3·g−1)Pore volume /
(cm3·g−1)Average pore diameter /
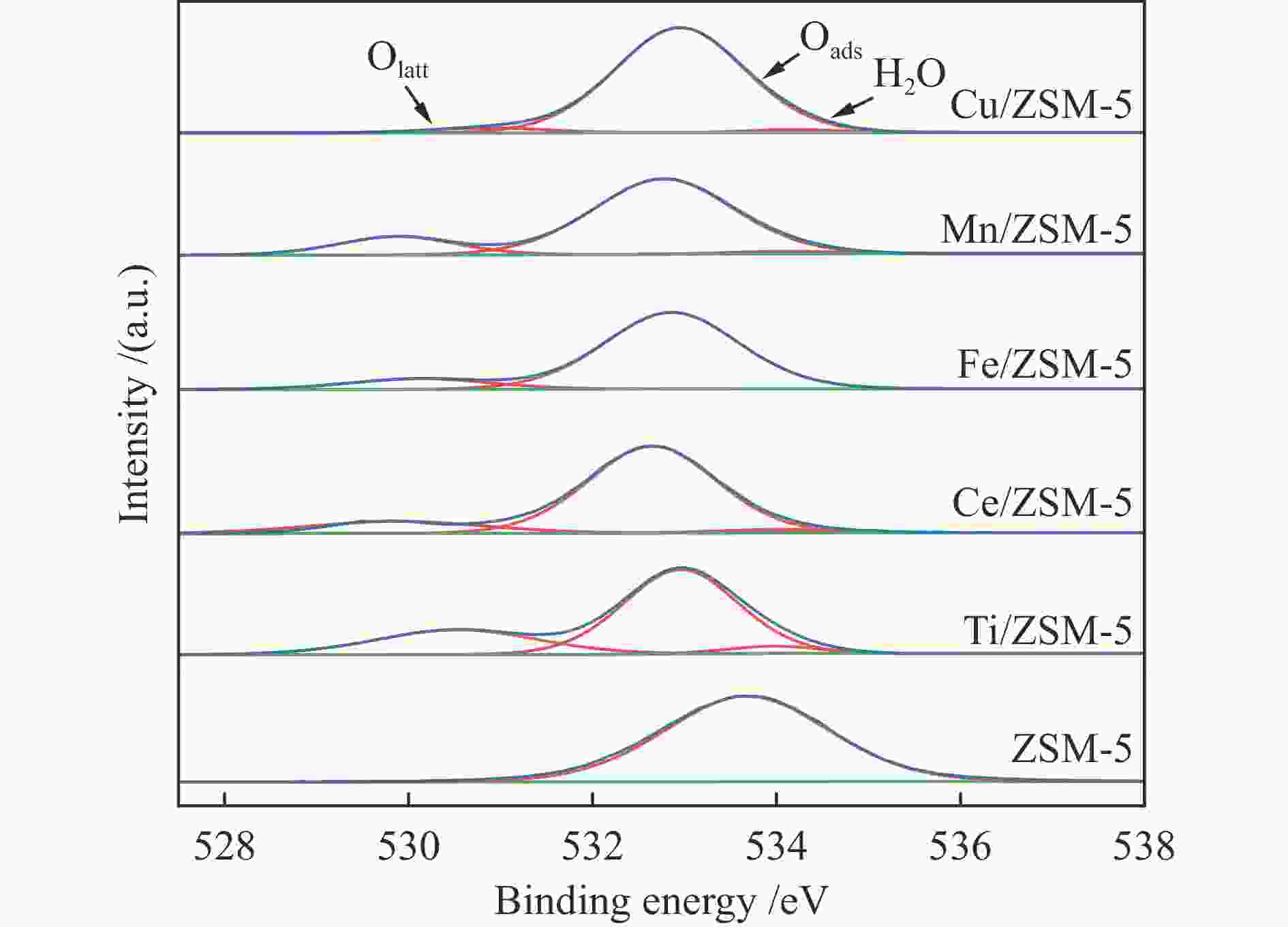
nmZSM-5 400.7 0.085 0.175 0.261 2.60 Cu/ZSM-5 276.8 0.083 0.097 0.180 2.61 Mn/ZSM-5 291.1 0.082 0.106 0.188 2.58 Fe/ZSM-5 264.9 0.089 0.077 0.166 2.50 Ce/ZSM-5 294.6 0.098 0.078 0.175 2.38 Ti/ZSM-5 310.4 0.075 0.134 0.209 2.69 表 2 不同催化剂中氧物种物质的量比和耗氢量
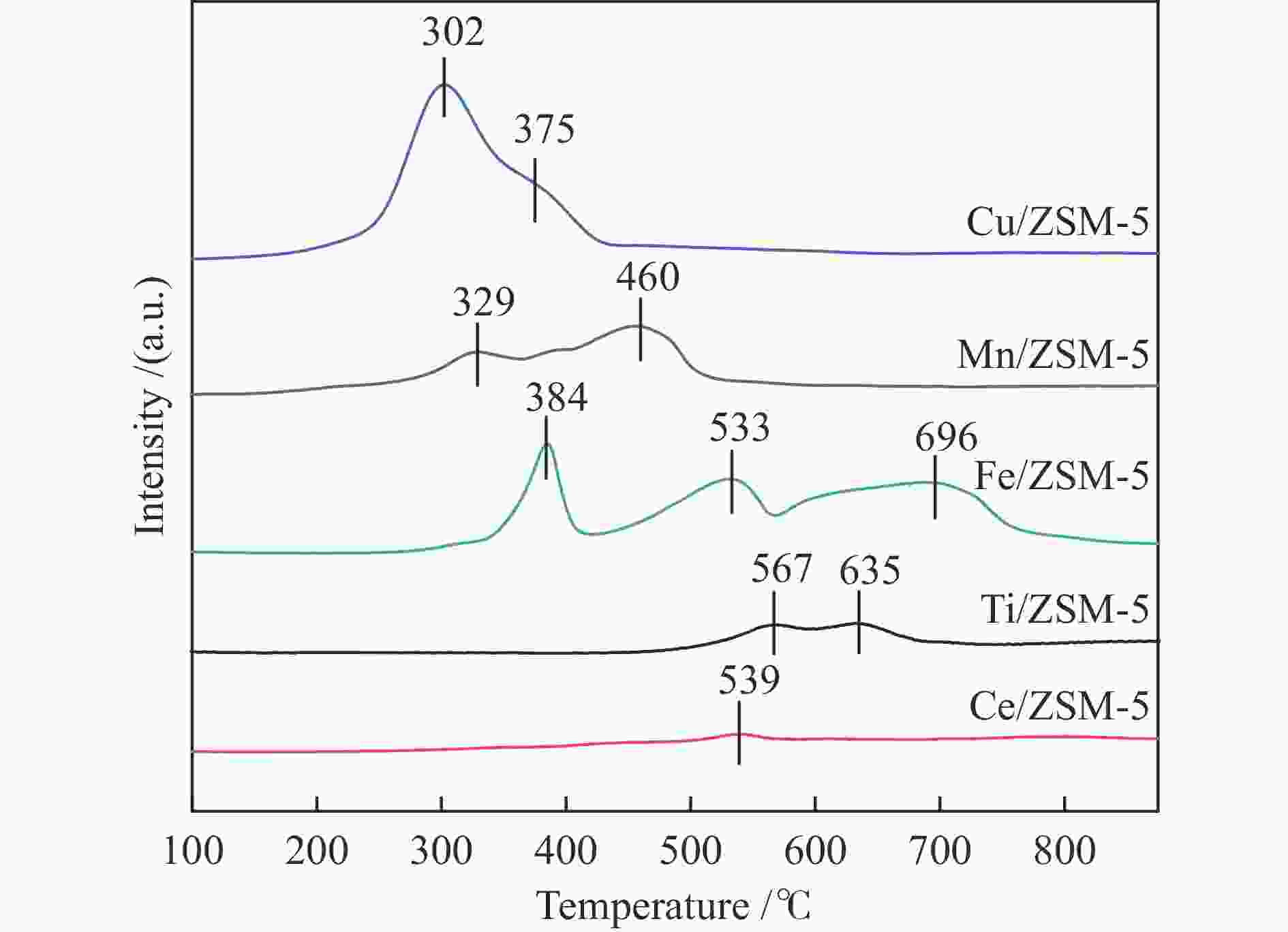
Table 2 Molar ratio of oxygen species of different catalysts and H2 consumption
Catalyst Cu/ZSM-5 Mn/ZSM-5 Fe/ZSM-5 Ti/ZSM-5 Ce/ZSM-5 Olatt /% 3.98 16.75 12.81 27.98 15.81 Oads /% 94.27 81.31 87.19 67.17 80.87 Oads/Olatt 23.69 4.85 6.81 2.40 5.12 H2 consumption
/(mmol·g−1)2.10 1.44 2.19 0.54 0.15 -
[1] LI W B, WANG J X, GONG H. Catalytic combustion of VOCs on non-noble metal catalysts [J]. Catal Today, 2009, 148(1–2): 81–87. [2] WANG H, NIE L, LI J, WANG Y, WANG G, WANG J, HAO Z. Characterization and assessment of volatile organic compounds (VOCs) emissions from typical industries[J]. Chin Sci Bull,2013,58(7):724−730. doi: 10.1007/s11434-012-5345-2 [3] GUO Y L, WEN M C, LI G Y, AN T C. Recent advances in VOC elimination by catalytic oxidation technology onto various nanoparticles catalysts: a critical review[J]. Appl Catal B: Environ,2021,281:119447. doi: 10.1016/j.apcatb.2020.119447 [4] ZHAI X, JING F, LI L, JIANG X, ZHANG J, MA J, CHU W. Toluene catalytic oxidation over the layered MOx-δ-MnO2 (M = Pt, Ir, Ag) composites originated from the facile self-driving combustion method[J]. Fuel,2021,283:118888. doi: 10.1016/j.fuel.2020.118888 [5] XIE Y, ZHANG L, JIANG Y, HAN S, WANG L, MENG X, XIAO F-S. Enhanced catalytic performance of methane combustion over zeolite-supported Pd catalysts with the lanthanum[J]. Catal Today,2021,364:16−20. doi: 10.1016/j.cattod.2019.11.030 [6] LI J H, XIAO G F, GUO Z Y, LIN B L, HU Y, FU M L, YE D Q. ZSM-5-supported V-Cu bimetallic oxide catalyst for remarkable catalytic oxidation of toluene in coal-fired flue gas[J]. Chem Eng J,2021,419:129675. doi: 10.1016/j.cej.2021.129675 [7] HU J, LI W B, LIU R F. Highly efficient copper-doped manganese oxide nanorod catalysts derived from CuMnO hierarchical nanowire for catalytic combustion of VOCs[J]. Catal Today,2018,314:147−153. doi: 10.1016/j.cattod.2018.02.009 [8] LIN L-Y, BAI H. Salt-templated synthesis of Ce/Al catalysts supported on mesoporous silica for acetone oxidation[J]. Appl Catal B: Environ,2014,148-149:366−376. doi: 10.1016/j.apcatb.2013.11.026 [9] ZHU L, ZHANG L, QU H X, ZHONG Q. A study on chemisorbed oxygen and reaction process of Fe-CuOx/ZSM-5 via ultrasonic impregnation method for low-temperature NH3-SCR[J]. J Mol Catal A: Chem,2015,409:207−215. doi: 10.1016/j.molcata.2015.08.029 [10] LI J R, ZHANG W P, LI C, XIAO H, HE C. Insight into the catalytic performance and reaction routes for toluene total oxidation over facilely prepared Mn-Cu bimetallic oxide catalysts[J]. Appl Surf Sci,2021,550:149179. doi: 10.1016/j.apsusc.2021.149179 [11] LIU G, TIAN Y, ZHANG B, WANG L, ZHANG X. Catalytic combustion of VOC on sandwich-structured Pt@ZSM-5 nanosheets prepared by controllable intercalation[J]. J Hazard Mater,2019,367:568−576. doi: 10.1016/j.jhazmat.2019.01.014 [12] AZIZ A, KIM S, KIM K S. Fe/ZSM-5 zeolites for organic-pollutant removal in the gas phase: Effect of the iron source and loading[J]. J Environ Chem Eng,2016,4(3):3033−3040. doi: 10.1016/j.jece.2016.06.021 [13] SUN P, WANG W, DAI X, WENG X, WU Z. Mechanism study on catalytic oxidation of chlorobenzene over MnxCe1-xO2/H-ZSM5 catalysts under dry and humid conditions[J]. Appl Catal B: Environ,2016,198:389−397. doi: 10.1016/j.apcatb.2016.05.076 [14] CHENG J, SONG L, WU R, LI S, SUN Y, ZHU H, QIU W, HE H. Promoting effect of microwave irradiation on CeO2-TiO2 catalyst for selective catalytic reduction of NO by NH3[J]. J Rare Earth,2020,38(1):59−69. doi: 10.1016/j.jre.2019.04.014 [15] LI S M, HAO Q L, ZHAO R Z, LIU D L, DUAN H Z, DOU B J. Highly efficient catalytic removal of ethyl acetate over Ce/Zr promoted copper/ZSM-5 catalysts[J]. Chem Eng J,2016,285:536−543. doi: 10.1016/j.cej.2015.09.097 [16] ZHENG J, CHEN Z, FANG J F, WANG Z, ZUO S F. MCM-41 supported nano-sized CuO-CeO2 for catalytic combustion of chlorobenzene[J]. J Rare Earth,2020,38(9):933−940. doi: 10.1016/j.jre.2019.06.005 [17] YANG K, SUN Q, XUE F, LIN D. Adsorption of volatile organic compounds by metal-organic frameworks MIL-101: Influence of molecular size and shape[J]. J Hazard Mater,2011,195:124−131. doi: 10.1016/j.jhazmat.2011.08.020 [18] WANG J, LI J, JIANG C, ZHOU P, ZHANG P, YU J. The effect of manganese vacancy in birnessite-type MnO2 on room-temperature oxidation of formaldehyde in air[J]. Appl Catal B: Environ,2017,204:147−155. doi: 10.1016/j.apcatb.2016.11.036 [19] LEI J, WANG S, LI J, XU Y, LI S. Different effect of Y (Y = Cu, Mn, Fe, Ni) doping on Co3O4 derived from Co-MOF for toluene catalytic destruction[J]. Chem Eng Sci,2022,251:117436. doi: 10.1016/j.ces.2022.117436 [20] SHI Y, GUO X, SHI Z, ZHOU R. Transition metal doping effect and high catalytic activity of CeO2-TiO2 for chlorinated VOCs degradation[J]. J Rare Earth,2022,40(5):745−752. doi: 10.1016/j.jre.2021.02.005 [21] ZHANG S L, ZHONG Q, ZHAO W, LI Y T. Surface characterization studies on F-doped V2O5/TiO2 catalyst for NO reduction with NH3 at low-temperature[J]. Chem Eng J,2014,253:207−216. doi: 10.1016/j.cej.2014.04.045 [22] KIM J, JANG E, JEONG Y, BAIK H, CHO S J, KANG C Y, KIM C H, CHOI J. A Cu-impregnated ZSM-5 zeolite for active cold start hydrocarbon removal: Cation-type-dependent Cu species and their synergetic HC adsorption/oxidation functions[J]. Chem Eng J,2022,430:132552. doi: 10.1016/j.cej.2021.132552 [23] XUE H, GUO X, MENG T, MAO D, MA Z. Poisoning effect of K with respect to Cu/ZSM-5 used for NO reduction[J]. Colloid Interfac Sci,2021,44:100465. doi: 10.1016/j.colcom.2021.100465 [24] YASHNIK S A, TARAN O P, SUROVTSOVA T A, AYUSHEEV A B, PARMON V N. Cu- and Fe-substituted ZSM-5 zeolite as an effective catalyst for wet peroxide oxidation of Rhodamine 6 G dye[J]. J Environ Chem Eng,2022,10(3):107950. doi: 10.1016/j.jece.2022.107950 [25] ZHA K, FENG C, HAN L, LI H, YAN T, KUBOON S, SHI L, ZHANG D. Promotional effects of Fe on manganese oxide octahedral molecular sieves for alkali-resistant catalytic reduction of NOx: XAFS and in situ DRIFTs study[J]. Chem Eng J,2020,381:122764. doi: 10.1016/j.cej.2019.122764 [26] WU Y S, FENG R, SONG C J, XING S T, GAO Y Z, MA Z C. Effect of reducing agent on the structure and activity of manganese oxide octahedral molecular sieve (OMS-2) in catalytic combustion of o-xylene[J]. Catal Today,2017,281:500−506. doi: 10.1016/j.cattod.2016.05.024 [27] MA Y, LI W, WANG H, CHEN J, WEN J, XU S, TIAN X, GAO L, HOU Z, ZHANG Q, YANG H. Enhanced performance of iron-cerium NO reduction catalysts by sulfuric acid treatment: The synergistic effect of surface acidity and redox capacity[J]. Appl Cacal A: Gen,2021,621:118200. doi: 10.1016/j.apcata.2021.118200 [28] CAO X, LU J, ZHENG X, HE D, ZHU W, ZHAO Y, ZHANG W, TIAN R, LUO Y. Regulation of the reaction pathway to design the high sulfur/coke-tolerant Ce-based catalysts for decomposing sulfur-containing VOCs[J]. Chem Eng J,2022,429:132473. doi: 10.1016/j.cej.2021.132473 [29] WANG J, GUO X, SHI Y, ZHOU R. Synergistic effect of Pt nanoparticles and micro-mesoporous ZSM-5 in VOCs low-temperature removal[J]. J Environ Sci,2021,107:87−97. doi: 10.1016/j.jes.2021.01.033 [30] YAN Y, WANG L, ZHANG H P. Catalytic combustion of volatile organic compounds over Co/ZSM-5 coated on stainless steel fibers[J]. Chem Eng J,2014,255:195−204. doi: 10.1016/j.cej.2014.05.141 [31] ZHANG C, HUANG H, LI G, WANG L, SONG L, LI X. Zeolitic acidity as a promoter for the catalytic oxidation of toluene over MnOx/HZSM-5 catalysts[J]. Catal Today,2019,327:374−381. doi: 10.1016/j.cattod.2018.03.019 [32] ZHANG Z X, GONG Y, XU J W, ZHANG Y, XIAO Q Y, XI R, XU X L, FANG X Z, WANG X. Dissecting La2Ce2O7 catalyst to unravel the origin of the surface active sites devoting to its performance for oxidative coupling of methane (OCM)[J]. Catal Today,2022,400-401:73−81. doi: 10.1016/j.cattod.2021.11.012 [33] WANG C P, WANG Z, MAO S J, CHEN Z R, WANG Y. Coordination environment of active sites and their effect on catalytic performance of heterogeneous catalysts[J]. Chin J Catal,2022,43(4):928−955. doi: 10.1016/S1872-2067(21)63924-4 [34] VELLINGIRI K, KUMAR P, DEEP A, KIM K-H. Metal-organic frameworks for the adsorption of gaseous toluene under ambient temperature and pressure[J]. Chem Eng J,2017,307:1116−1126. doi: 10.1016/j.cej.2016.09.012 [35] PAN H, CHEN Z, MA M, GUO T, LING X, ZHENG Y, HE C, CHEN J. Mutual inhibition mechanism of simultaneous catalytic removal of NO and toluene on Mn-based catalysts[J]. J Colloid Interface Sci,2022,607:1189−1200. doi: 10.1016/j.jcis.2021.09.110 [36] WANG Z, XIE K, ZHENG J, ZUO S. Studies of sulfur poisoning process via ammonium sulfate on MnO2/γ-Al2O3 catalyst for catalytic combustion of toluene[J]. Appl Catal B: Environ,2021,298:120595. doi: 10.1016/j.apcatb.2021.120595 [37] HU P, WENG Q, LI D, LV T, WANG S, ZHUO Y. Effects of O2, SO2, H2O and CO2 on As2O3 adsorption by gamma-Al2O3 based on DFT analysis[J]. J Hazard Mater,2021,403:123866. doi: 10.1016/j.jhazmat.2020.123866 [38] HOU Z, DAI L, LIU Y, DENG J, JING L, PEI W, GAO R, FENG Y, DAI H. Highly efficient and enhanced sulfur resistance supported bimetallic single-atom palladium-cobalt catalysts for benzene oxidation[J]. Appl Catal B: Environ,2021,285:119844. doi: 10.1016/j.apcatb.2020.119844 [39] LIU H L, YE C, XU Y S, WANG Q S. Effect of activation conditions and iron loading content on the catalytic cracking of toluene by biochar[J]. Energy,2022,247:123409. doi: 10.1016/j.energy.2022.123409 [40] AHMADI M, HAGHIGHI M, KAHFOROUSHAN D. Influence of active phase composition (Mn, Ni, MnxNi10−x ) on catalytic properties and performance of clinoptilolite supported nanocatalysts synthesized using ultrasound energy toward abatement of toluene from polluted air[J]. Process Saf Environ Prot,2017,106:294−308. doi: 10.1016/j.psep.2016.06.029 [41] WANG Z, YANG H, LIU R, XIE S, LIU Y, DAI H, HUANG H, DENG J. Probing toluene catalytic removal mechanism over supported Pt nano- and single-atom-catalyst[J]. J Hazard Mater,2020,392:122258. doi: 10.1016/j.jhazmat.2020.122258 [42] XU W C, WANG N, CHEN Y D, CHEN J D, XU X X, YU L, CHEN L M, WU J L, FU M L, ZHU A M, YE D Q. In situ FT-IR study and evaluation of toluene abatement in different plasma catalytic systems over metal oxides loaded gamma-AL(2)O(3)[J]. Catal Commun,2016,84:61−66. doi: 10.1016/j.catcom.2016.06.004 [43] WEI G C, ZHANG Q L, ZHANG D H, WANG J, TANG T, WANG H M, LIU X, SONG Z X, NING P. The influence of annealing temperature on copper-manganese catalyst towards the catalytic combustion of toluene: The mechanism study[J]. Appl Surf Sci,2019,497:143777. doi: 10.1016/j.apsusc.2019.143777 [44] WANG Z W, MA P J, ZHENG K, WANG C, LIU Y X, DAI H X, WANG C C, HSI H C, DENG J G. Size effect, mutual inhibition and oxidation mechanism of the catalytic removal of a toluene and acetone mixture over TiO2 nanosheet-supported Pt nanocatalysts[J]. Appl Catal B: Environ,2020,274:118963. doi: 10.1016/j.apcatb.2020.118963 -





 下载:
下载: