Influence of dopants on the structure and catalytic features of the Cu/ZnO catalyst for dimethyl oxalate hydrogenation to ethylene glycol
-
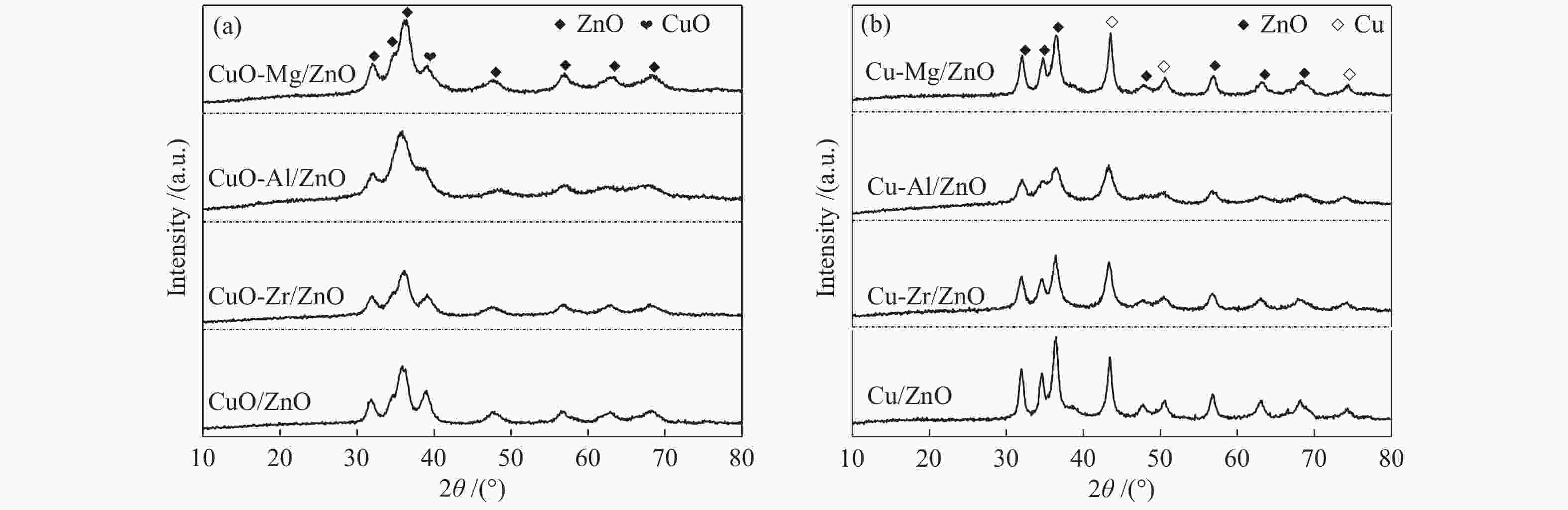
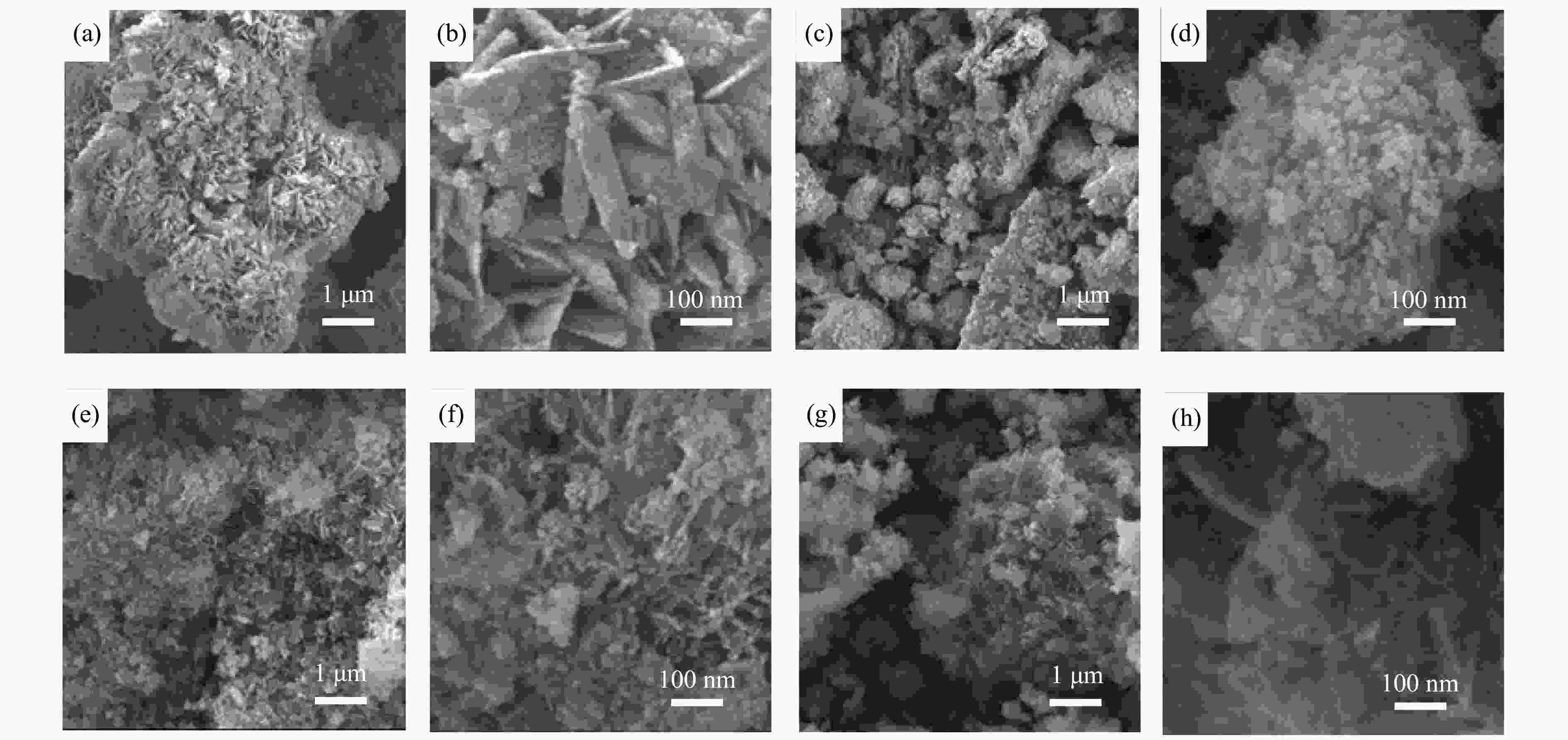
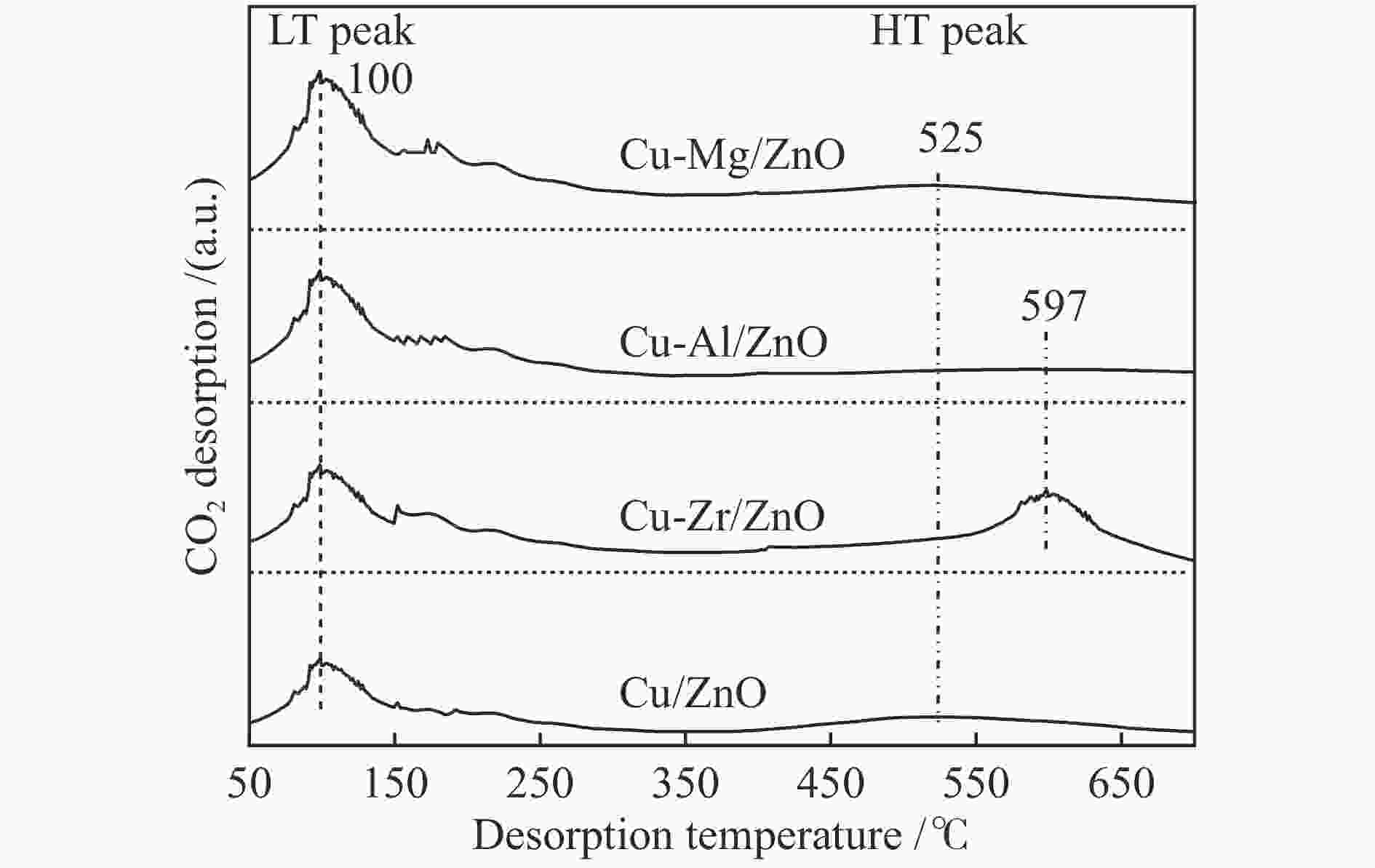
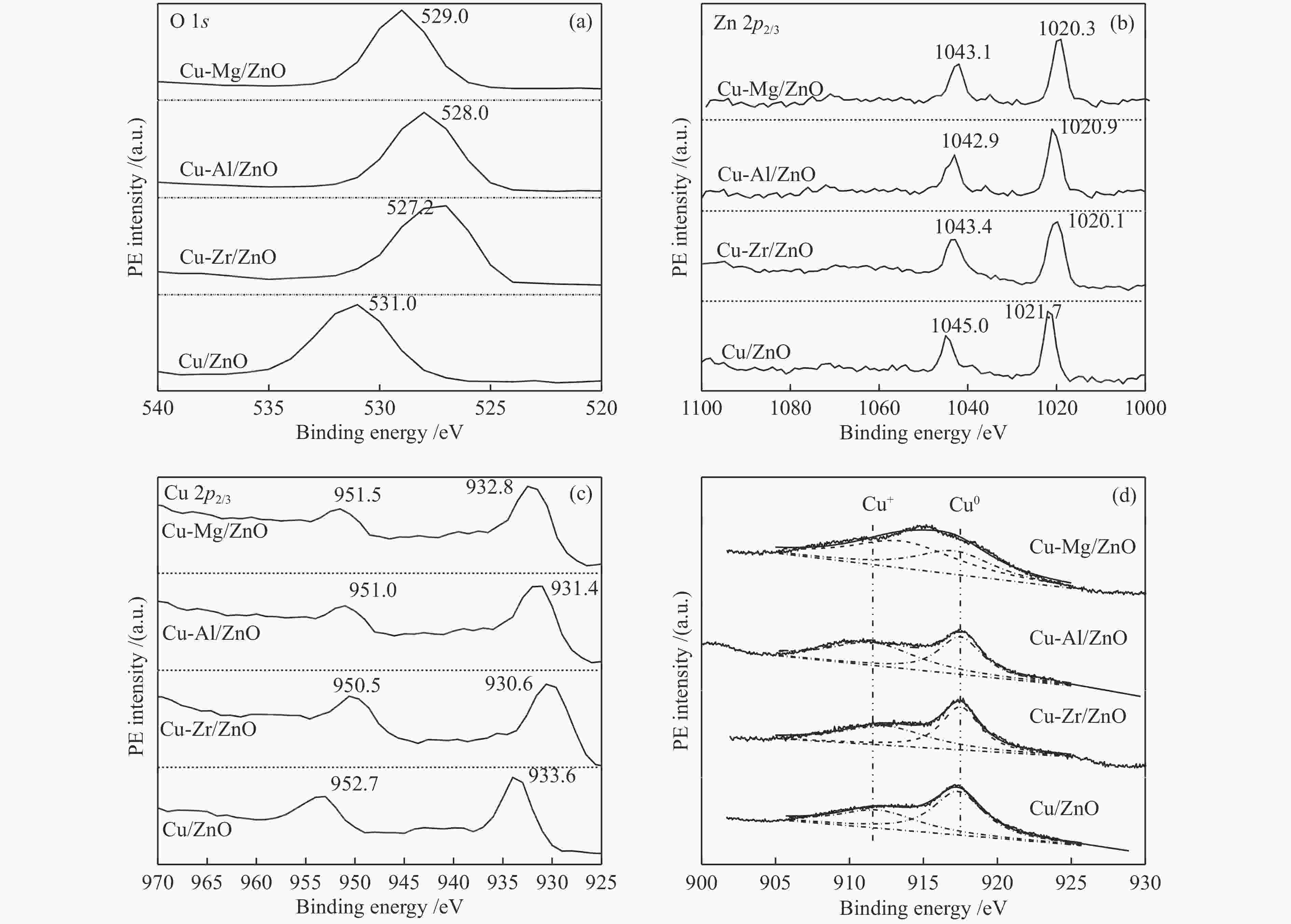
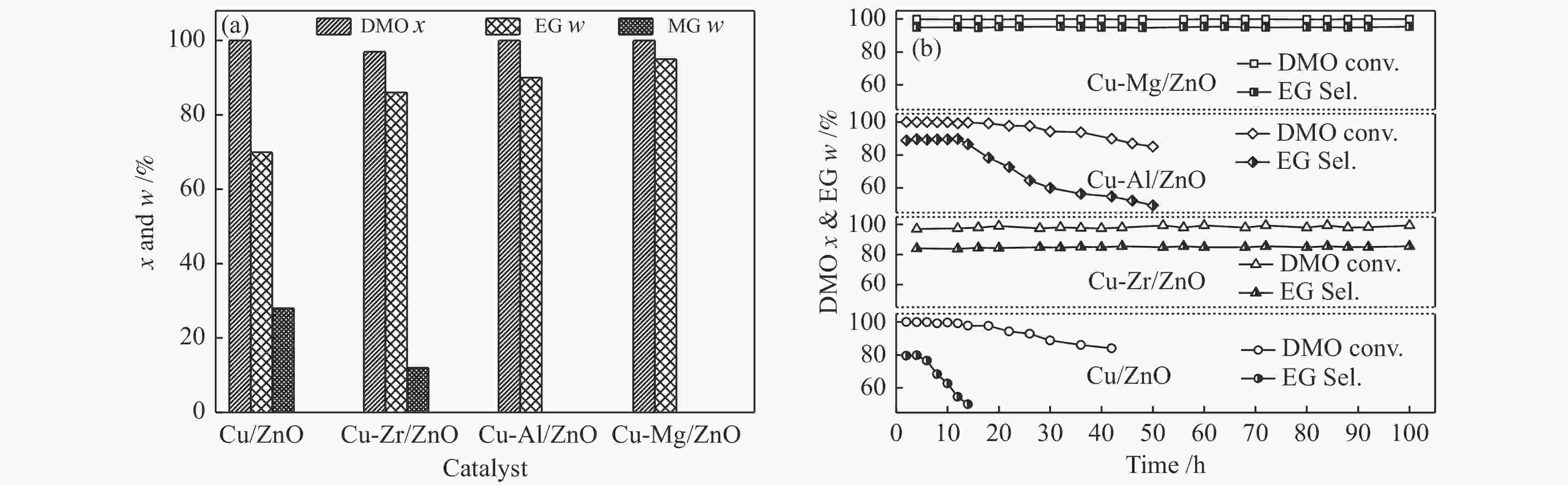
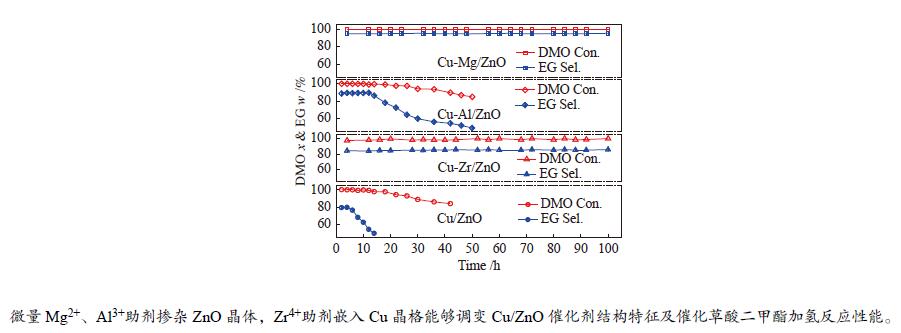
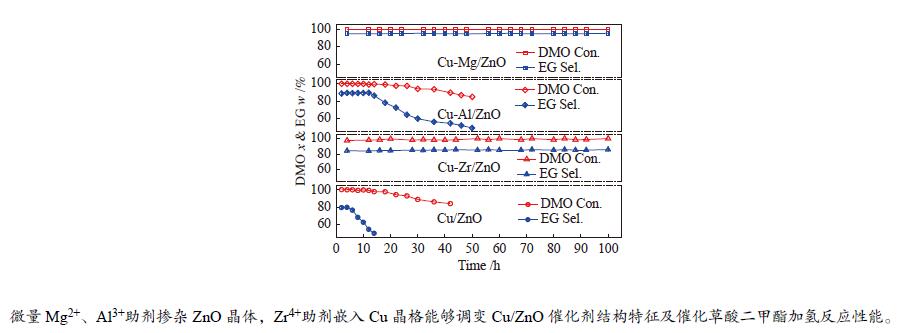
摘要: 采用共沉淀法合成了掺杂不同助剂的Cu-M/ZnO (Cu: ZnO物质的量比=5∶4,M = Zr4 + 、Al3 + 、Mg2 + ,助剂含量为4.0%)用于催化草酸二甲酯(Dimethyl oxalate , DMO)选择加氢反应催化剂。结果表明,微量掺杂Al3 + 、Mg2 + 助剂嵌入于ZnO晶相,Zr4 + 助剂嵌入Cu晶相均能显著促进Cu/ZnO催化剂中铜分散;其中,Mg2 + 助剂能够有效增强Cu、ZnO物相间相互作用,Zr4 + 助剂能够有效增强Cu、ZrO2物相间相互作用。催化DMO加氢选择加氢反应,Cu/ZnO催化剂乙二醇(Ethylene glycol,EG)收率仅为75.0%,Cu-Al/ZnO、Cu-Zr/ZnO和Cu-Mg/ZnO催化剂的EG收率分别为90.0%、85.0%、95.0%。相比Cu/ZnO和Cu-Al/ZnO催化剂催化DMO选择加氢反应易于失活,Cu-Zr/ZnO和Cu-Mg/ZnO催化剂显现出优异稳定性,稳定反应时长超过100 h。催化剂构-效关系表明,Cu/ZnO和Cu-Mg/ZnO催化剂表面较高Cu + 活性位以及充足Cu0活性位协同效应是其显现优异催化活性的主要因素。此外,Cu-Zr/ZnO和Cu-Mg/ZnO中较强的金属/氧化物相互作用能够有效抑制催化剂中铜纳米粒子于强放热反应中发生迁移、烧结,赋予催化剂优异的稳定性。Abstract: The Cu-M/ZnO catalysts (M = Zr4+, Al3+ and Mg2+) for dimethyl oxalate (DMO) selective hydrogenation to ethylene glycol (EG) were synthesized by the co-precipitation method. The properties of the as-synthesized catalysts were characterized by N2-physisorption, N2O-titration, XRD, H2-TPR, CO2-TPD, SEM, FT-IR and XPS. It was found that the Cu dispersion could be effectively promoted by the dopants incorporated in the Cu/ZnO catalyst. Particularly, a trace amount of Mg2+ and Al3+ dopants could significantly reinforce the chemical interaction between the Cu and ZnO phases by embedding into the ZnO lattice, while the Cu/ZrO2 interaction could be improved with the introduction of Zr4+. For DMO gas-phase hydrogenation, the EG yield of the Cu/ZnO catalyst increased from 75.0% to 85.0% and 90.0% in the presence of Zr4+ and Al3+ dopants, respectively. Particularly, the EG selectivity of Cu-Mg/ZnO catalyst reached up to 95.0% with DMO completely converted for more than 100 h. The correlation between the catalytic behavior and physicochemical features of the Cu/ZnO based catalysts suggested that the surface Cu+ sites was vital for the catalytic behavior with adequate Cu0 sites. Additionally, the strengthened Cu/oxide interaction favored the outstanding stability of the Cu-Zr/ZnO and Cu-Mg/ZnO catalyst for DMO hydrogenation.
-
Key words:
- dopant /
- Cu/ZnO catalyst /
- dimethyl oxalate /
- hydrogenation /
- ethylene glycol
-
表 1 Cu/ZnO和添加不同助剂Cu-M/ZnO催化剂的织构参数
Table 1 Textures and copper dispersion of Cu-M/ZnO catalysts with different dopants
Catalyst SBET /(m2·g−1) a vp /(cm3·g−1)a Dp /nma Cu crystallite size /nm b Cu dispersion /% c SCu0/(m2·g−1)c Cu/ZnO 50.3 0.21 16.4 11.3 13.0 2.40 Cu-Zr/ZnO 72.2 0.27 15.1 8.6 60.1 7.00 Cu-Al/ZnO 76.0 0.44 23.1 7.4 42.3 3.69 Cu-Mg/ZnO 93.1 0.27 11.4 11.1 13.4 2.45 a: SBET specific surface area; vp: pore volume; Dp: average pore determined by N2 physical adsorption; b: Cu crystallite size determined by Scherrer formula; c: Cu dispersion and SCu0(Cu surface area) determined by the N2O titration 表 2 还原活化催化剂还原后的XPS测试及拟合参数
Table 2 Surface Cu component of the reduced samples based on Cu LMM deconvolution
Catalyst EB /eV EB of Cu
2p3/2 /eVCu + /% a SCu+/(m2·g−1) b Cu + Cu0 Cu/ZnO 916.2 918.6 933.0 38.6 0.9 Cu-Zr/ZnO 915.7 918.1 935.6 45.5 3.1 Cu-Al/ZnO 914.5 917.0 932.0 49.6 1.9 Cu-Mg/ZnO 913.7 916.1 934.0 55.2 2.7 a: Cu+ /(Cu+ + Cu0) intensity ratio obtained by deconvolution of Cu LMM XAES spectra, b: Calculated based on xCu+ and $S_{{\rm{Cu}}}^{0}$ assuming that the Cu+ ion occupies the same area and has the same atomic sensitivity factor as those of the Cu0 atom -
[1] ZHU J, ZHAO G F, MENG C, CHEN P J, SHI X R, LU Y. Superb Ni-foam-structured nano-intermetallic InNi3C0.5 catalyst for hydrogenation of dimethyl oxalate to ethylene glycol[J]. Chem Eng J,2021,426:130857. doi: 10.1016/j.cej.2021.130857 [2] CUI G Q, ZHANG X, WANG H, LI Z Y, WANG W L, YU Q, ZHENG L R, WANG Y D, ZHU J H, WEI Min. ZrO2−x modified Cu nanocatalysts with synergistic catalysis towards carbonoxygen bond hydrogenation[J]. Appl Catal B: Environ,2021,280:119406. doi: 10.1016/j.apcatb.2020.119406 [3] CHEN C C, LIN L, YE R P, HUANG L, ZHU L B, HUANG Y Y, QIN Y Y, YAO Y G. Construction of Cu-Ce composite oxides by simultaneous ammonia evaporation method to enhance catalytic performance of Ce-Cu/SiO2 catalysts for dimethyl oxalate hydrogenation[J]. Fuel,2021,290:120083. doi: 10.1016/j.fuel.2020.120083 [4] JIN E, ZHANG Y L, HE L L, HARRIS H G, TENG B T, FAN M H. Indirect coal to liquid technologies[J]. Appl Catal A: Gen,2014,476:158−174. doi: 10.1016/j.apcata.2014.02.035 [5] ZHAO Y J, ZHANG H H, XU Y X, WANG S N, Yan Xu, WANG S P, MA X B. Interface tuning of Cu + /Cu0 by zirconia for dimethyl oxalate hydrogenation to ethylene glycol over Cu/SiO2 catalyst[J]. J Energy Chem,2020,49:248−256. doi: 10.1016/j.jechem.2020.02.038 [6] WEN C, LI F Q, CUI Y Y, DAI W L, FAN K N. Investigation of the structural evolution and catalytic performance of the CuZnAl catalysts in the hydrogenation of dimethyl oxalate to ethylene glycol[J]. Catal Today,2014,233:117−126. doi: 10.1016/j.cattod.2013.10.075 [7] WANG X P, CHEN M, CHEN X K, LIN R H, ZHU H J, HUANG C Q, YANG W S, TAN Y, WANG S S, DU Z N, DING Y. Constructing copper-zinc interface for selective hydrogenation of dimethyl oxalate[J]. J Catal,2020,383:254−263. [8] BEHRENS M, LOLLII G, MURATOVA N, KASATKIN I, HAVECJER M, ALNONCOURT R N, STORCHEVA O, KOHLER K, MUHLERD M, SCHLOGLA R. The effect of Al-doping on ZnO nanoparticles applied as catalyst support[J]. Phys Chem Chem Phys,2013,15:1374−1381. doi: 10.1039/C2CP41680H [9] KONG X P, CHEN Z, WU Y H, WANG R H, CHEN J G, DING L F. Synthesis of Cu-Mg/ZnO catalysts and catalysis in dimethyl oxalate hydrogenation to ethylene glycol: Enhanced catalytic behavior in the presence of a Mg2 + dopant[J]. RSC Adv,2017,7:49548−49561. doi: 10.1039/C7RA09435C [10] PENG S Y, Xu Z N, CHEN Q S, WANG Z Q, LV D M, SUN J, CHEN Y, GUO G C. Enhanced stability of Pd/ZnO catalyst for CO oxidative coupling to dimethyl oxalate: Effect of Mg2 + doping[J]. ACS Catal,2015,5(7):4410−4417. doi: 10.1021/acscatal.5b00365 [11] JULIA S, MAIK E, THOMAS L, THOMAS N, GALVAN M C Á, SCHOGL R, BEHRENS M. Promoting strong metal support interaction: Doping ZnO for enhanced activity of Cu/ZnO: M (M = Al, Ga, Mg) catalysts[J]. ACS Catal,2015,5:3260−3270. [12] LI K Z, CHEN J G. CO2 hydrogenation to methanol over ZrO2-containing catalysts: Insights into ZrO2 induced synergy[J]. ACS Catal,2019,9(9):7840−7861. [13] ZHANG S Y, LIU Q Y, FAN G L, LI F. Highly-dispersed copper-based catalysts from Cu-Zn-Al layered double hydroxide precursor for Gas-phase hydrogenation of dimethyl oxalate to ethylene glycol[J]. Catal Lett,2012,142:1121−1127. doi: 10.1007/s10562-012-0871-8 [14] ZHANG S Y, HU Q, FAN G L, LI F. The relationship between the structure and catalytic performance Cu/ZnO/ZrO2 catalysts for hydrogenation of dimethyl 1, 4-cyclohexane dicarboxylate[J]. Catal Commun,2013,39:96−101. doi: 10.1016/j.catcom.2013.05.011 [15] YUAN Z L, WANG L N, WANG J H, XIA S X, CHEN P, HOU Z, ZHENG X M. Hydrogenolysis of glycerol over homogenously dispersed copper on solid base catalysts[J]. Appl Catal B: Environ,2011,101:431−440. doi: 10.1016/j.apcatb.2010.10.013 [16] QIAN J F, LIU Z T, LIN S, LI X L, ALI M. Study on microstructure characteristics of material evidence in coal dust explosion and its significance in accident investigation[J]. Fuel,2020,265:116992. doi: 10.1016/j.fuel.2019.116992 [17] KONG X P, WU Y H, DING L F, WANG R H, CHEN J G. Effect of Cu loading on the structural evolution and catalytic activity of Cu-Mg/ZnO catalysts for dimethyl oxalate hydrogenation[J]. New J Chem,2020,44:4486−4493. doi: 10.1039/C9NJ06085E [18] CHAWLA S, JAYANTHI K, CHANDER H, HARANATH D, HALDER S K Halder, MAR M. Synthesis and optical properties of ZnO/MgO nanocomposite[J]. J Alloy Compd,2008,459:457−460. doi: 10.1016/j.jallcom.2007.04.303 [19] KONG X P, MA C L, ZHANG J, SUN J Q, CHEN J G, LIU K F. Effect of leaching temperature on structure and performance of Raney Cu catalysts for hydrogenation of dimethyl oxalate[J]. Appl Catal A: Gen,2016,509:153−160. doi: 10.1016/j.apcata.2015.10.029 [20] AGRELL J, BIRGERSSON H, BOUTONNET M, MELIAN-CABRERA I, NAVARRO R M, FIERRO J L G. Production of hydrogen from methanol over Cu/ZnO catalysts promoted by ZrO2 and Al2O3[J]. J Catal,2003,219:389−403. [21] TU Y J, CHEN Y W. Effects of alkaline-earth oxide additives on silica-supported copper catalysts in ethanol dehydrogenation[J]. Ind Eng Chem Res,1998,37:2618−2622. doi: 10.1021/ie9708135 [22] FRUSTER F, CORDARO M, CANNILLA C, BONURA G. Multifunctionality of Cu-ZnO-ZrO2/H-ZSM5 catalysts for the one-step CO2-to-DME hydrogenation reaction[J]. Appl Catal B: Environ,2015,162:57−65. doi: 10.1016/j.apcatb.2014.06.035 [23] WANG H F, RHYS D, MARTIN S, HANS-JOACHIM F. Surface science approach to catalyst preparation-Pd deposition onto thin Fe3O4(111) films from PdCl2 precursor[J]. J Catal,2012,286:1−5. doi: 10.1016/j.jcat.2011.09.026 [24] CHAMINAND J, DJAKOVITCH L, GALLEZOT P, MARION P, PINEL C, ROSIER C. Glycerol hydrogenolysis on heterogeneous catalysts[J]. Green Chem,2004,6:359−361. doi: 10.1039/b407378a [25] SUI X M, LIU Y C, SHAO C L, LIU Y X, XU C S. Structural and photoluminescent properties of ZnO hexagonal nanoprisms synthesized by microemulsion with polyvinyl pyrrolidone served as surfactant and passivant[J]. Chem Phys Lett,2006,424:340−344. doi: 10.1016/j.cplett.2006.04.053 [26] SARAVANAN R, KARTHIKRYAN S, GUPTA V K, SEKARAN G, NARAYANAN V, STEPHEN A. Enhanced photocatalytic activity of ZnO/CuO nanocomposite for the degradation of textile dye on visible light illumination[J]. Mater Sci Eng A,2013,33:91−98. doi: 10.1016/j.msec.2012.08.011 [27] GAO X Y, WANG S Y, LI J, ZHENG Y X, ZHANG R J, ZHOU P, YANG Y M, CHEN L Y. Study of structure and optical properties of silver oxide films by ellipsometry, XRD and XPS methods[J]. Thin Solid Films,2004,455−456:438−442. [28] SHIM J B, CHANG Hyukg, KIM S O. Rapid hydrothermal synthesis of zinc oxide nanowires by annealing methods on seed layers[J]. J Nanomater,2011,582764. [29] QIU X Q, LI L P, ZHENG J, LIU J J, SUN X F, LI G S. Origin of the enhanced photocatalytic activities of semiconductors: A case study of ZnO doped with Mg2 + [J]. J Phys Chem C,2008,112(32):12242−12248. doi: 10.1021/jp803129e [30] ZHAO Y J, ZHANG Y Q, WANG Y, ZHANG J, XU Y, WANG S P, MA X B. Structure evolution of mesoporous silica supported copper catalyst for dimethyl oxalate hydrogenation[J]. Appl Catal A: Gen,2017,539:59−69. doi: 10.1016/j.apcata.2017.04.001 [31] LU Z P, YIN H B, WANG A L, HU J, XUE W P, YIN H X, LIU S X. Hydrogenation of ethyl acetate to ethanol over Cu/ZnO/MOx (MOx = SiO2, Al2O3, and ZrO2) catalysts[J]. J Ind Eng Chem,2016,37:208−215. doi: 10.1016/j.jiec.2016.03.028 [32] ZHENG J, HAO Z P, YU J J, HOU H X, HU C, SU J X. Catalytic combustion of methane on novel catalysts derived from Cu-Mg/Al-hydrotalcites[J]. Catal Lett,2005,99:157−163. doi: 10.1007/s10562-005-2108-6 [33] YAO Y Q, WU X Q, GUTIERREZ O Y, JI J, JIN P, WANG S N, XU Y, ZHAO Y J, WANG S P, MA X B, LERCHERBE J A. Roles of Cu+ and Cu0 sites in liquid-phase hydrogenation of esters on core-shell CuZnx@C catalysts[J]. Appl Catal B: Environ,2020,267:118698. doi: 10.1016/j.apcatb.2020.118698 -




 下载:
下载: