Synthesis of dimethyl carbonate from methanol and propylene carbonate over the Ca-Zr catalyst modified by transition metals
-
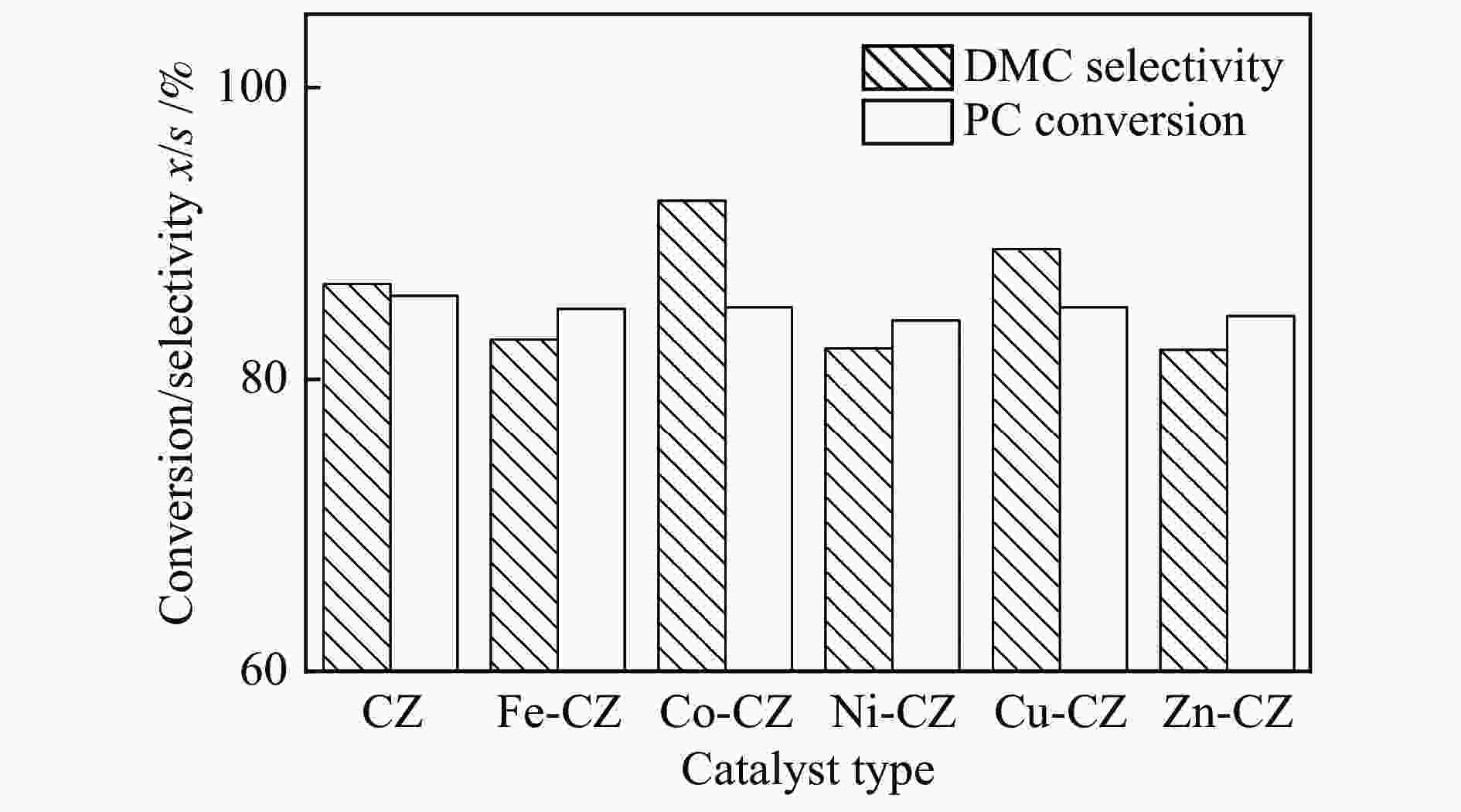
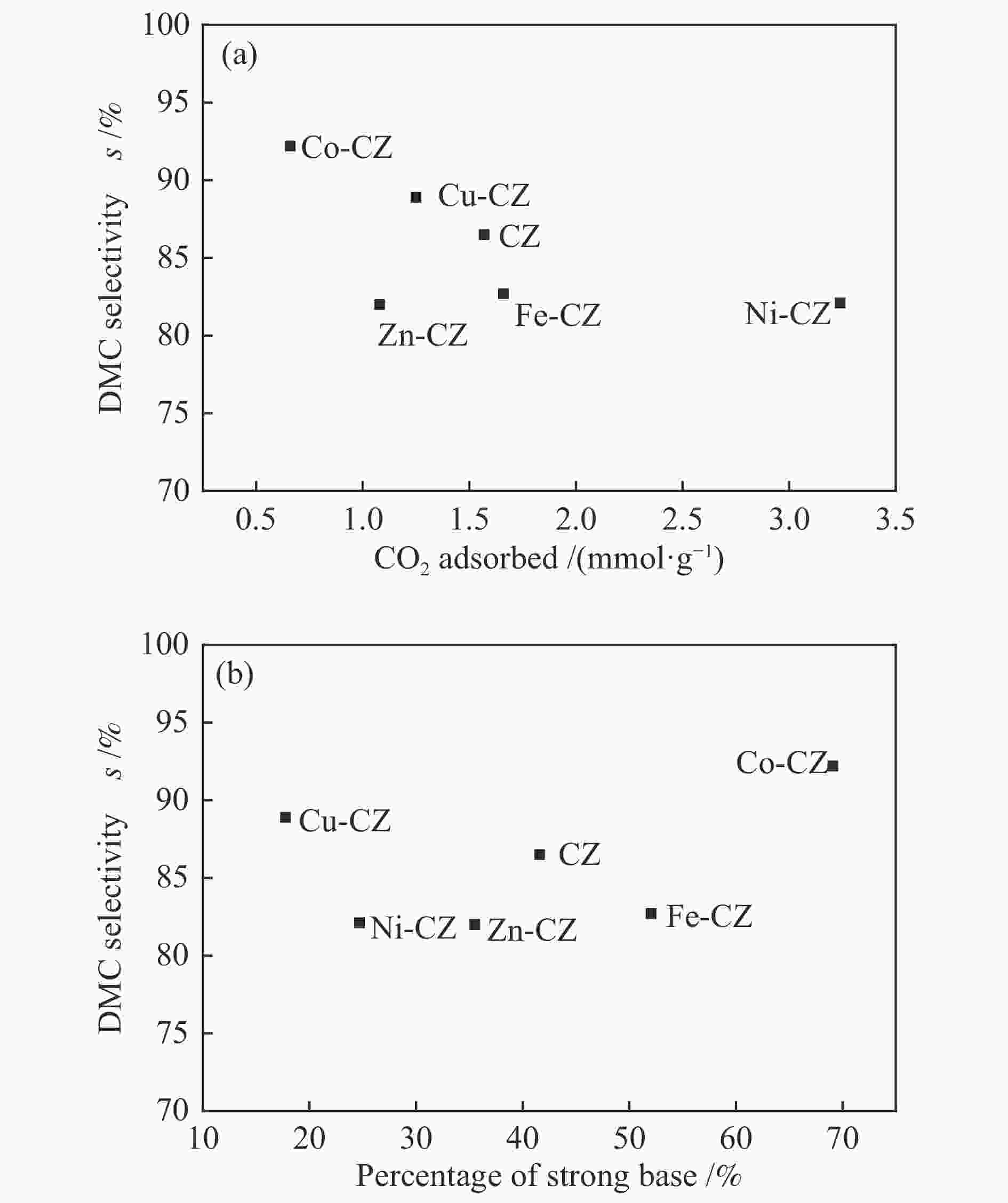
摘要: 采用溶胶凝胶法制备了一系列由过渡金属助剂改性的Ca-Zr催化剂,对其低温下甲醇与碳酸丙烯酯(PC)酯交换反应合成碳酸二甲酯(DMC)的催化性能进行了研究。结果表明,系列过渡金属改性的Ca-Zr催化剂上DMC选择性的顺序依次为Co-Ca-Zr > Cu-Ca-Zr > Ca-Zr > Fe-Ca-Zr > Ni-Ca-Zr > Zn-Ca-Zr。其中,Co改性的Co-Ca-Zr催化剂在35 ℃、甲醇/PC物质的量比为15及催化剂用量为4%的条件下反应2 h,PC的转化率可达84.3%,DMC的选择性可达94.5%。结合XRD、FT-IR、XPS和CO2-TPD等表征结果发现,催化剂的碱性位强度增加可以提高PC的转化率,而总碱性位含量提高则降低DMC的选择性。Co改性的Ca-Zr催化剂具有最少的碱性位和最高的强碱性位站比,因而表现出较高的PC转化率和DMC选择性。Abstract: A series of Ca-Zr catalysts modified by different transition metals were prepared by the sol-gel method and their catalytic performance in the synthesis of dimethyl carbonate (DMC) from methanol and propylene carbonate (PC) by transesterification at low temperature was investigated. The results indicate that the selectivity to DMC of various transition metal-modified Ca-Zr catalysts follows the order of Co-Ca-Zr > Cu-Ca-Zr > Ca-Zr > Fe-Ca-Zr > Ni-Ca-Zr > Zn-Ca-Zr. For the transesterification over the Co-Ca-Zr catalyst, in particular, the conversion of PC reaches 84.3% with a selectivity of 94.5% to DMC after reaction for 2 h under 35 ℃, a methanol/PC molar ratio of 15, and catalyst amount of 4%. Combining with the XRD, FT-IR, XPS and CO2-TPD results, it is revealed that increasing the strength of basic sites can raise the conversion of PC, whereas increasing the density of basic sites leads to a decrease of the selectivity to DMC. As a result, the Co-modified Ca-Zr (Co-Ca-Zr) catalyst, with the lowest density of surface basic sites but the highest fraction of strong basic sites, exhibits a high conversion of PC and a high selectivity to DMC for the transesterification of PC with methanol at a low temperature.
-
Key words:
- transition metals modification /
- Ca-Zr /
- transesterification /
- dimethyl carbonate /
- propylene carbonate /
- methanol
-
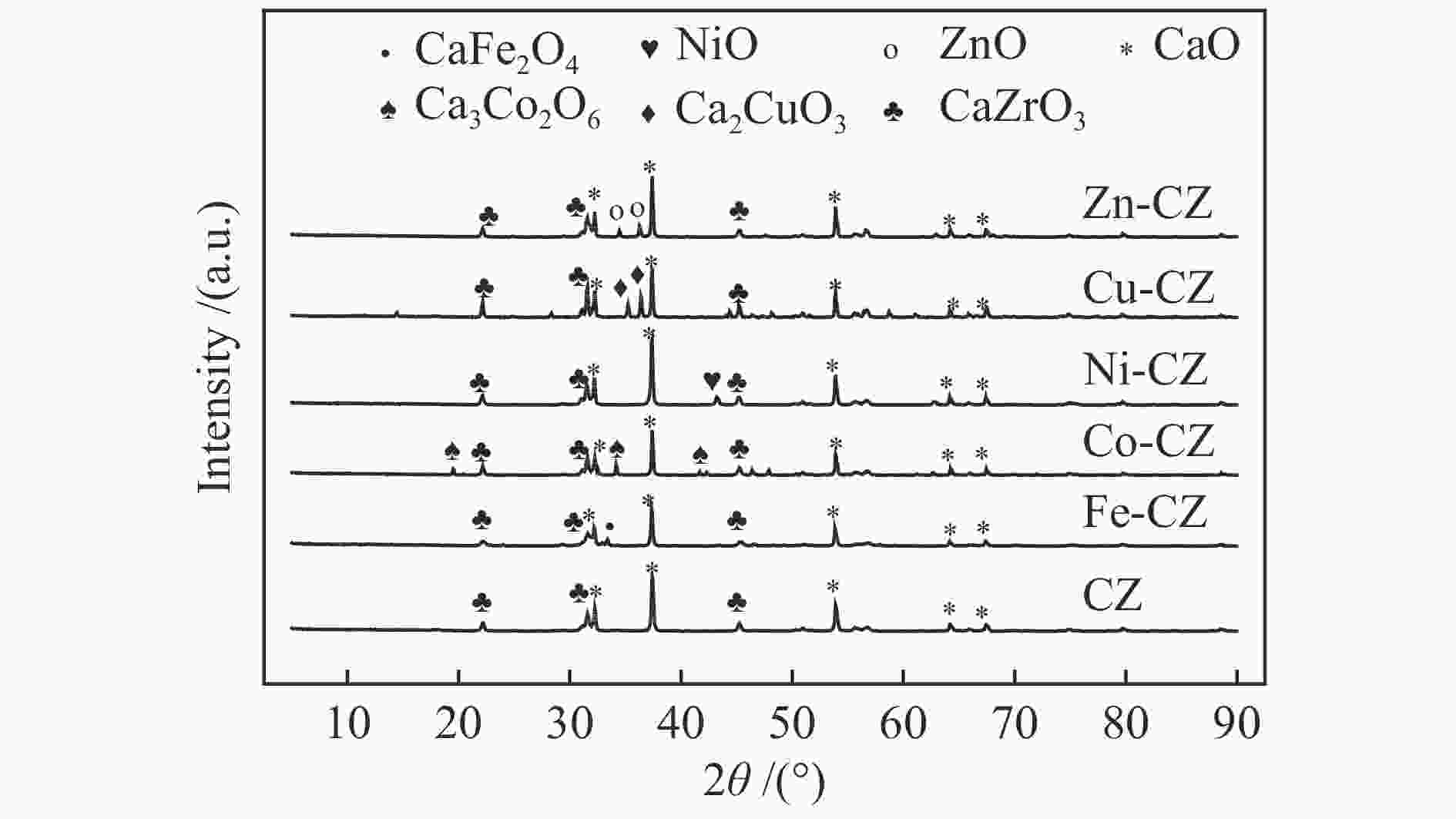
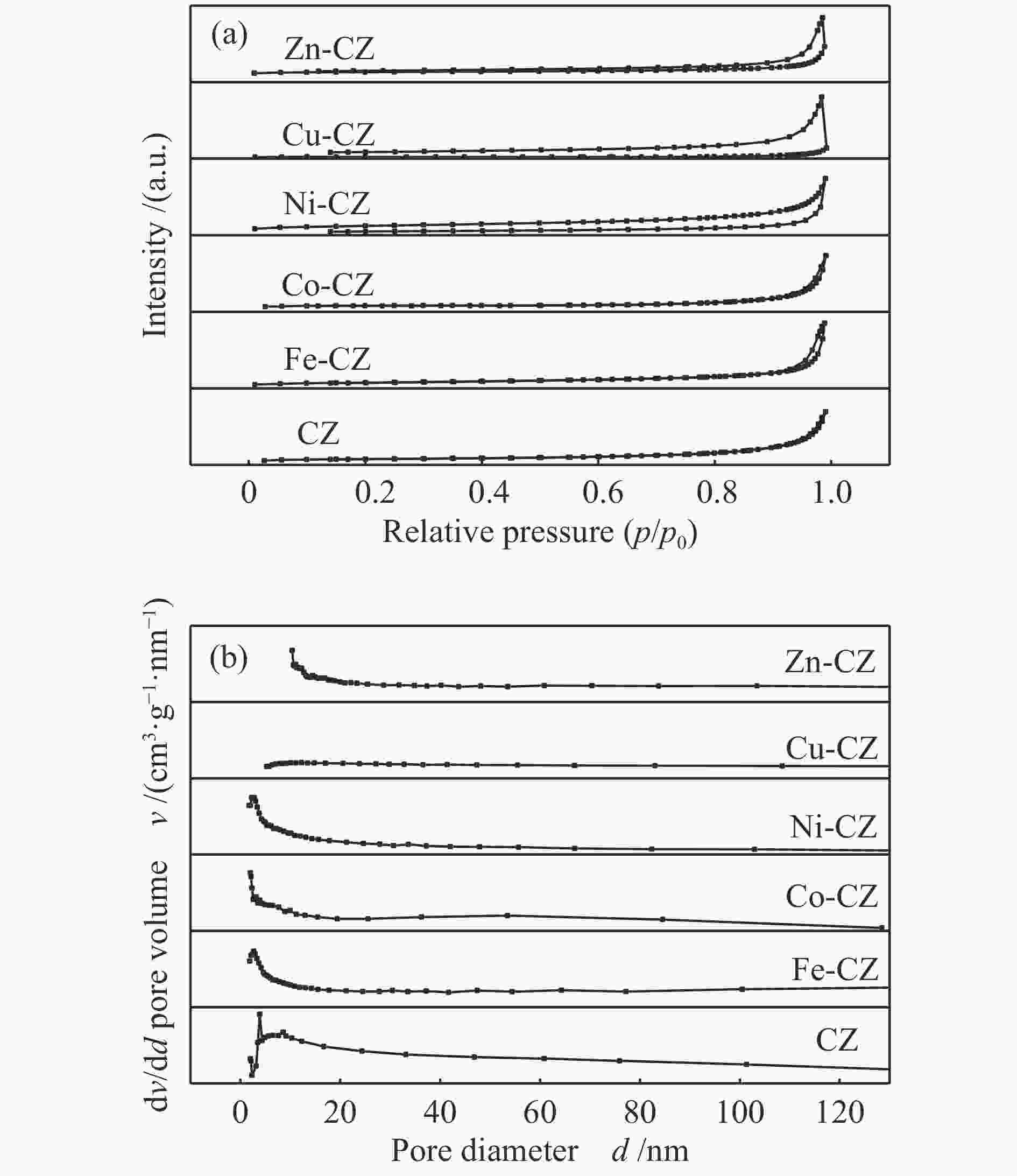
表 1 催化剂的织构参数
Table 1 Texture properties of various catalysts
Catalyst SBET A/(m2·g−1) Pore volume v/(cm3·g−1) Average pore size d/nm CZ 7.5 3.0 × 10−2 16.0 Fe-CZ 10.2 5.0 × 10−2 19.8 Co-CZ 3.1 1.3 × 10−2 16.4 Ni-CZ 7.3 1.9 × 10−2 10.7 Cu-CZ 1.1 0.3 × 10−2 11.2 Zn-CZ 3.5 1.5 × 10−2 17.1 note: the total pore volume was determined at p/p0 = 0.99 and the pore diameter distribution was derived from the desorption branch of the isotherm using the BJH method. 表 2 催化剂的结合能
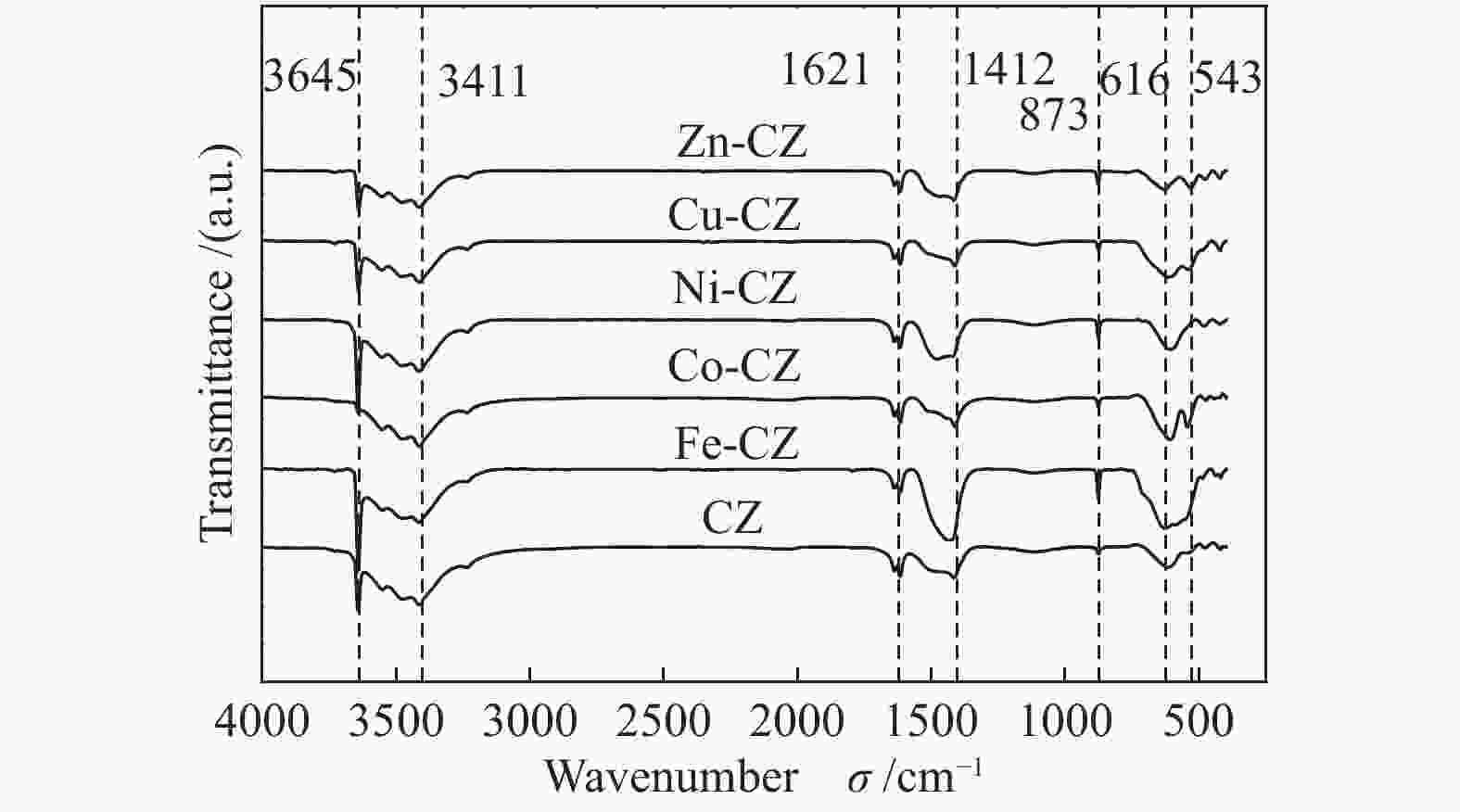
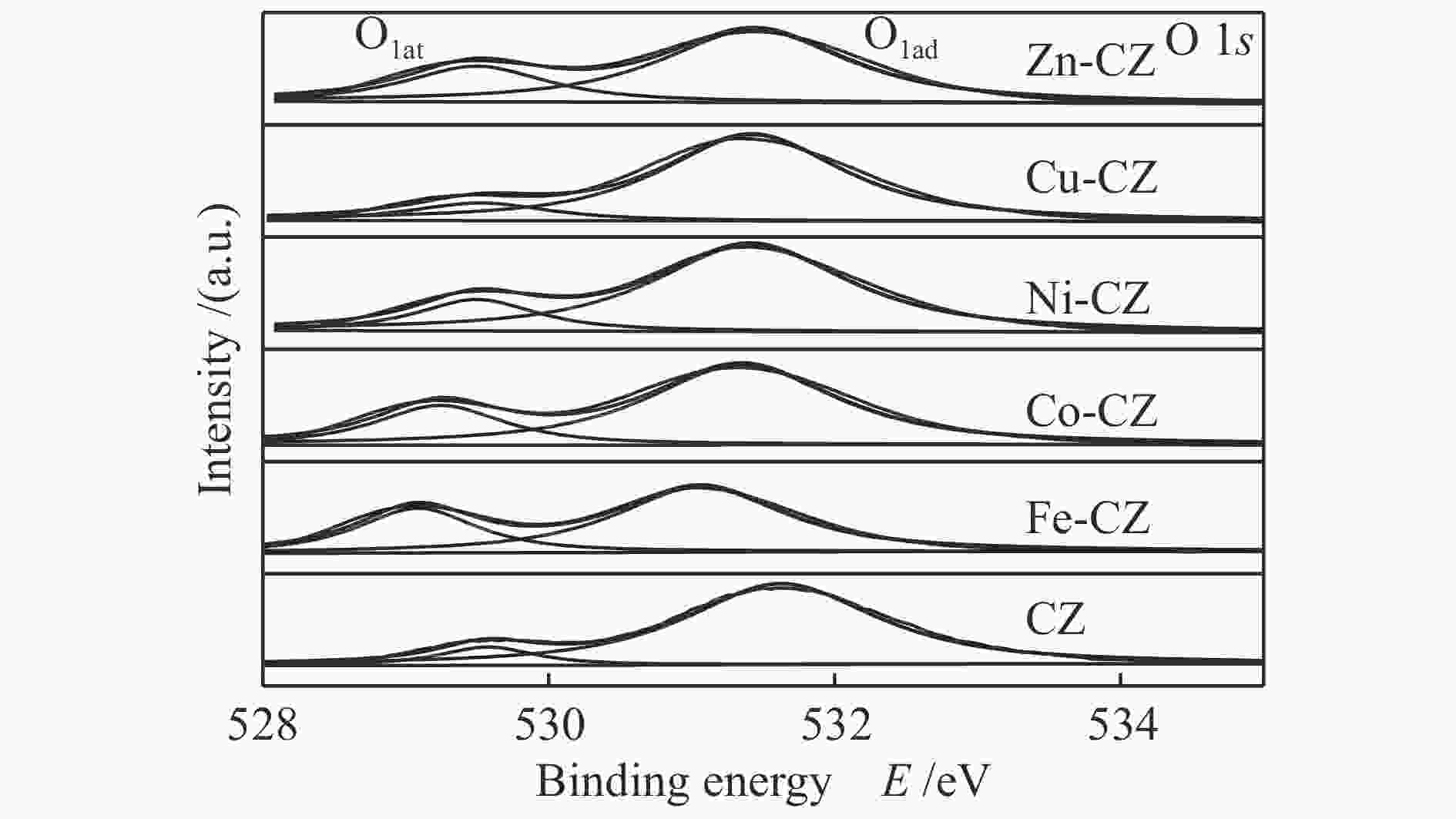
Table 2 Binding energy values of various catalysts
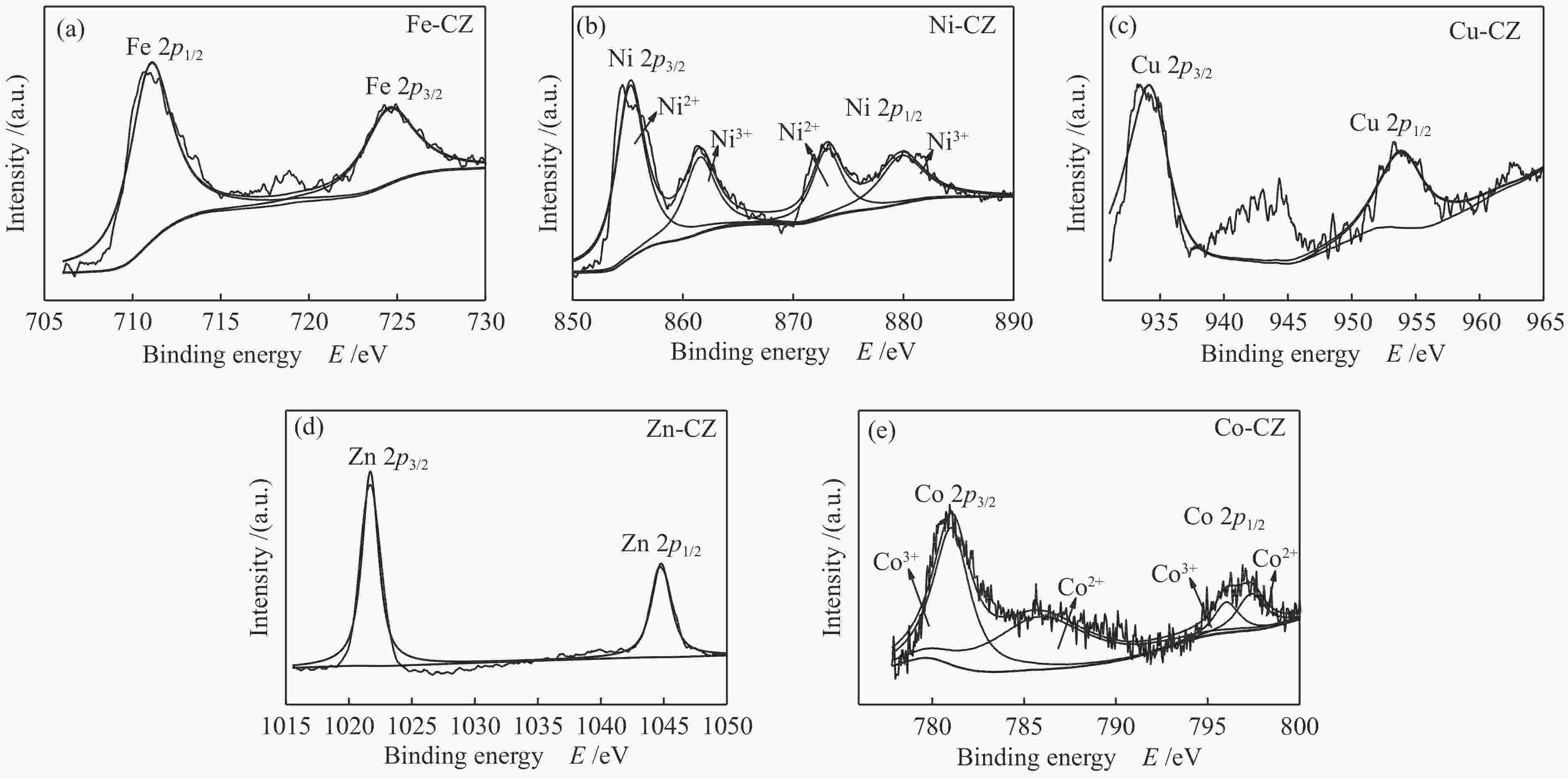
Catalyst Binding energy/eV Ca 2p Zr 3d O 1s CZ 350.7, 347.0 181.7, 184.0 529.5, 531.6 Fe-CZ 350.2, 346.4 181.0, 183.3 529.2, 531.5 Co-CZ 350.2, 346.5 181.3, 183.6 529.2, 531.3 Ni-CZ 350.9, 346.7 181.3, 183.7 529.5, 531.4 Cu-CZ 350.4, 346.7 181.4, 183.7 529.5, 531.4 Zn-CZ 350.3, 346.6 181.4, 183.7 529.5, 531.4 表 3 催化剂的总碱位数量和各碱性位点所占比例
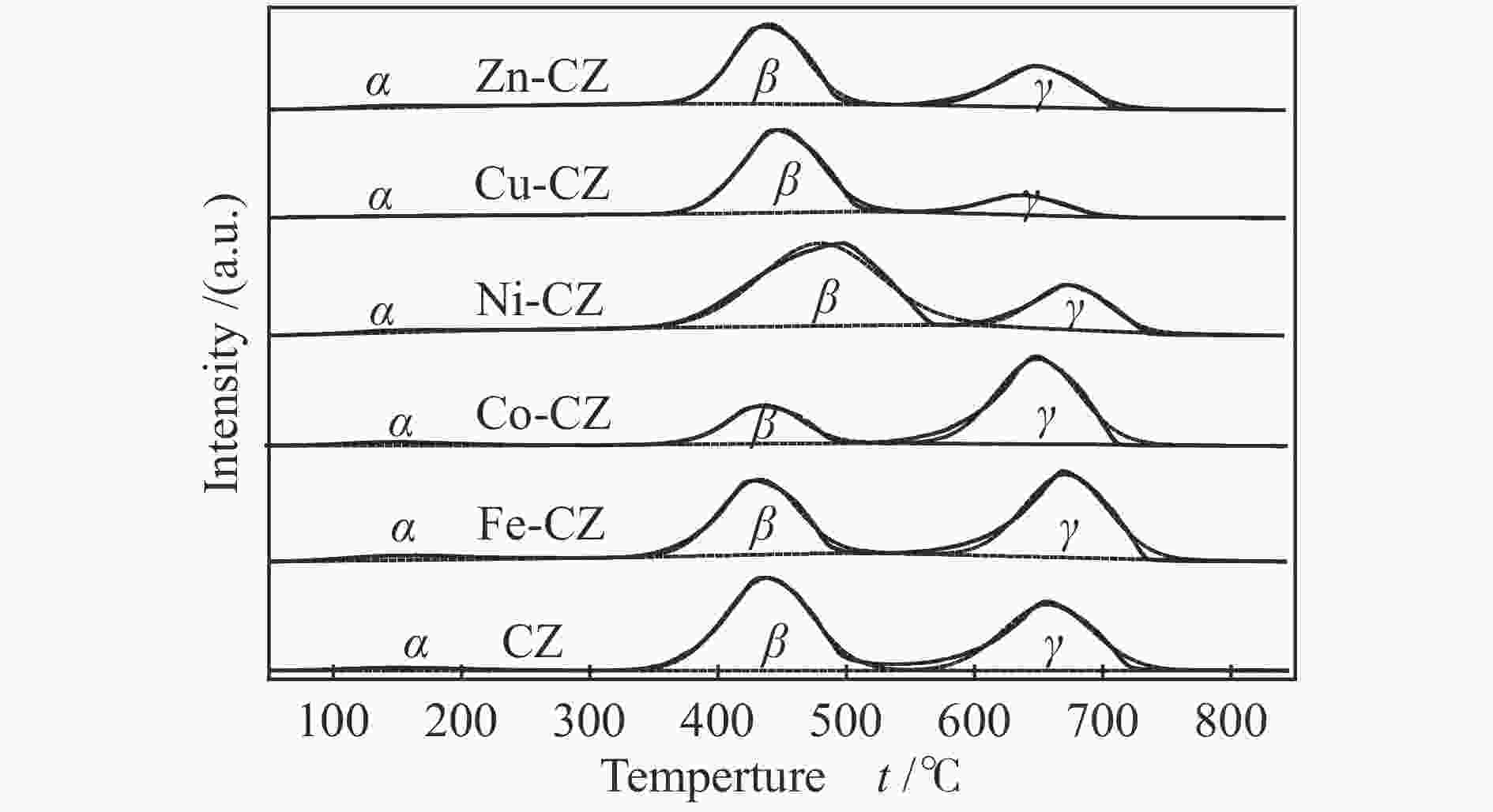
Table 3 Amount of basic sites of different strengths of various catalysts
Catalyst Weak
(α) /%Moderate
(β) /%Strong
(γ) /%Total basicity /
(mmol·g−1)CZ 2.4 56.0 41.6 1.57 Fe-CZ 4.1 43.9 52.0 1.66 Co-CZ 3.5 27.4 69.1 0.66 Ni-CZ 2.3 73.0 24.7 3.24 Cu-CZ 2.9 79.3 17.8 1.25 Zn-CZ 2.9 61.6 35.5 1.08 表 4 合成DMC的催化剂性能
Table 4 Catalyst performance for the synthesis of DMC
Catalyst Reaction
time /hTemp. /
℃PC conv.
x /%DMC sel.
s /%Ref. CaZr 5 60 85.7 86.5 this work CaFeZr 5 60 84.8 82.7 this work CaCoZr 5 60 84.9 92.2 this work CaNiZr 5 60 84.0 82.1 this work CaCuZr 5 60 84.9 88.9 this work CaZnZr 5 60 84.3 82.0 this work CaO 2 60 ~55.0 − [4] ZrO2 6 140 14.0 50.0 [30] CaCo 2 60 71.6 72.9 [11] CaAl 2 60 53.7 92.8 [13] CaMgAl 2 60 55.3 96.3 [13] MgAl 4 65 10.7 20.9 [31] FeMgAl 4 65 66.2 82.6 [31] CuMgAl 4 65 63.8 81.5 [31] -
[1] TUNDO P, MUSOLINO M, ARICO F. The reactions of dimethyl carbonate and its derivatives[J]. Green Chem,2018,20(1):28−85. doi: 10.1039/C7GC01764B [2] KOHLI K, SHARMA B K, PANCHAL C B. Dimethyl carbonate: Review of synthesis routes and catalysts Used[J]. Energies,2022,15(14):5133−5263. doi: 10.3390/en15145133 [3] AN H, ZHANG G, ZHAO X, WANG Y. Preparation of highly stable Ca-Zn-Al oxide catalyst and its catalytic performance for one-pot synthesis of dimethyl carbonate[J]. Catal Today,2018,316:185−192. doi: 10.1016/j.cattod.2018.03.006 [4] WEI T, WANG M, WEI W, SUN Y, ZHONG B. Synthesis of dimethyl carbonate by transesterification over CaO/carbon composites[J]. Green Chem,2003,5(3):343−346. doi: 10.1039/b210716n [5] TIAN X, ZENG Y, XIAO T, YANG C, WANG Y, ZHANG S. Fabrication and stabilization of nanocrystalline ordered mesoporous MgO-ZrO2 solid solution[J]. Microporous Mesoporous Mater,2011,143(2/3):357−361. doi: 10.1016/j.micromeso.2011.03.015 [6] XU J, CHEN Y, MA D, SHANG J K, LI Y X. Simple preparation of MgO/g-C3N4 catalyst and its application for catalytic synthesis of dimethyl carbonate via transesterification[J]. Catal Commun,2017,95:72−76. doi: 10.1016/j.catcom.2017.03.009 [7] KUMAR N, SRIVASTAVA V C. Dimethyl carbonate synthesis via transesterification of propylene carbonate using an efficient reduced graphene oxide-supported ZnO nanocatalyst[J]. Energy Fuels,2020,34(6):7455−7464. doi: 10.1021/acs.energyfuels.0c01091 [8] AHIRE J, BHANAGE B M. Solar light assisted synthesis of CeO2 nanoparticles for transesterification of ethylene carbonate with methanol to dimethyl carbonate[J]. Catal Lett,2022,152(11):3284−3293. doi: 10.1007/s10562-022-03927-2 [9] XUE Y, YU Z, ZHAI S, LI C, WEI X, XU J, WANG F, XUE B. The role of Ce doping on the activity of La2O2CO3 nanosheets catalysts in synthesis of dimethyl carbonate from propylene carbonate and methanol[J]. Catal Commun,2022,171:106526−106530. doi: 10.1016/j.catcom.2022.106526 [10] ZHAO H, SONG H, WANG F, MIAO Z, CHOU L. Low-temperature fabrication of K2O supported mesoporous tetragonal ZrO2 solid base for synthesis of dimethyl carbonate[J]. Mol Catal,2020,495:111141−111150. doi: 10.1016/j.mcat.2020.111141 [11] HUO L, WANG T, PU Y, LI C, LI L, ZHAI M, QIAO C, BAI Y. Effect of cobalt doping on the stability of CaO-based catalysts for dimethyl carbonate synthesis via the transesterification of propylene carbonate with methanol[J]. ChemistrySelect,2021,6(38):10226−10237. doi: 10.1002/slct.202102987 [12] WANG H, WANG M, ZHANG W, ZHAO N, WEI W, SUN Y. Synthesis of dimethyl carbonate from propylene carbonate and methanol using CaO-ZrO2 solid solutions as highly stable catalysts[J]. Catal Today,2006,115(1-4):107−110. doi: 10.1016/j.cattod.2006.02.031 [13] LIAO Y, LI F, DAI X, ZHAO N, SIAO F. Solid base catalysts derived from Ca-M-Al (M = Mg, La, Ce, Y) layered double hydroxides for dimethyl carbonate synthesis by transesterification of methanol with propylene carbonate[J]. Chin J Catal,2017,38(11):1860−1869. doi: 10.1016/S1872-2067(17)62898-5 [14] WEI T, WANG M, WEI W, SUN Y, ZHONG B. Effect of base strength and basicity on catalytic behavior of solid bases for synthesis of dimethyl carbonate from propylene carbonate and methanol[J]. Fuel Process Technol,2003,83(1-3):175−182. doi: 10.1016/S0378-3820(03)00065-1 [15] LUO J, WANG Y, WANG F, LI F, LI L, ZHAO N, XIAO F. Aerobic oxidation of fluorene to fluorenone over copper-doped Co3O4 with a high specific surface area[J]. ACS Sustainable Chem Eng,2020,8(6):2568−2576. doi: 10.1021/acssuschemeng.9b07480 [16] ZHANG Y F, ZHANG J X, LU Q M, ZHANG Q Y. Synthesis and characterization of Ca3Co4O9 nanoparticles by citrate sol-gel method[J]. Mater Lett,2006,60(20):2443−2446. doi: 10.1016/j.matlet.2006.01.013 [17] JI X, YANG J, ZHAO N, WANG F, XIAO F. Synthesis of ethylene carbonate by alcoholysis of urea over Zn-Zr mixed oxides[J]. Inorg Chem Commun,2021,134:109061−109067. doi: 10.1016/j.inoche.2021.109061 [18] THOMMES M, KANEKO K, NEIMARK A V, OLIVIER J P, RODRIGUEZ-REINOSO F, ROUQUEROL J, SING K S W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report)[J]. Pure Appl Chem,2015,87(9/10):1051−1069. doi: 10.1515/pac-2014-1117 [19] DUPIN J C, GONBEAU D, VINATIER P, LEVASSEUR A. Systematic XPS studies of metal oxides, hydroxides and peroxides[J]. Phys Chem Chem Phys,2000,2(6):1319−1324. doi: 10.1039/a908800h [20] SOTO HIDALGO K T, ORITIZ-QUILES E O, BETANCOURT L E, LARIOS E, JOSE YACAMAN M, CABRERA C R. Photoelectrochemical solar cells prepared from nanoscale zerovalent iron used for aqueous Cd2 + removal[J]. ACS Sustainable Chem Eng,2016,4(3):738−745. doi: 10.1021/acssuschemeng.5b00601 [21] LUO J, XUAN K, WANG Y, LI F, WANG F, PU Y, LI L, ZHAO N, XIAO F. Aerobic oxidation of fluorene to fluorenone over Co-Cu bimetal oxides[J]. New J Chem,2019,43(22):8428−8438. doi: 10.1039/C9NJ00499H [22] WEERAKKODY C, BISWAS S, SONG W, HE J, WASALATHANTHRI N, DISSANAYAKE S, KRIZ D, DUTTA B, SUIB S L. Controllable synthesis of mesoporous cobalt oxide for peroxide free catalytic epoxidation of alkenes under aerobic conditions[J]. Appl Catal B: Environ,2018,221:681−690. doi: 10.1016/j.apcatb.2017.09.053 [23] XIE R, FAN G, YANG L, LI F. Hierarchical flower-like Co-Cu mixed metal oxide microspheres as highly efficient catalysts for selective oxidation of ethylbenzene[J]. Chem Eng J,2016,288:169−178. doi: 10.1016/j.cej.2015.12.004 [24] ZHOU M, XIONG W, LI H, ZHANG D, LV Y. Emulsion-template synthesis of mesoporous nickel oxide nanoflowers composed of crossed nanosheets for effective nitrogen reduction[J]. Dalton Trans,2021,50(17):5835−5844. doi: 10.1039/D1DT00213A [25] PAL N, IM S, CHO E B, KIN H, PARK J. Superparamagnetic NiO-doped mesoporous silica flower-like microspheres with high nickel content[J]. J Ind Eng Chem,2020,81:99−107. doi: 10.1016/j.jiec.2019.08.058 [26] DO NASCIMENTO J R, DOLIVEIRA M R, VEIGA A G, XHAGAS C A, SCHMAL M. Synthesis of reduced graphene oxide as a support for nano copper and palladium/copper catalysts for selective NO reduction by CO[J]. ACS Omega,2020,5(40):25568−25581. doi: 10.1021/acsomega.0c02417 [27] VINAY S P, CHANDRASEKHAR N. Structural and biological investigation of green synthesized silver and zinc oxide nanoparticles[J]. J Inorg Organomet Polym Mater,2020,31(2):552−558. [28] WANG Q, ZHANG Y, ZHENG J, WANG Y, HU T, MENG C. Metal oxide decorated layered silicate magadiite for enhanced properties: Insight from ZnO and CuO decoration[J]. Dalton Trans,2017,46(13):4303−4316. doi: 10.1039/C7DT00228A [29] LI H, XIN C, JIAO X, ZHAO N, XIAO F, LI L, WEI W, WUN Y. Direct carbonylation of glycerol with CO2 to glycerol carbonate over Zn/Al/La/X (X=F, Cl, Br) catalysts: The influence of the interlayer anion[J]. J Mol Catal A: Chem,2015,402:71−78. doi: 10.1016/j.molcata.2015.03.012 [30] JUAREZ R, CORMA A, GARCIA H. Gold nanoparticles promote the catalytic activity of ceria for the transalkylation of propylene carbonate to dimethyl carbonate[J]. Green Chem,2009,11(7):949−952. doi: 10.1039/b902850a [31] 王琴, 赵海宏, 匡志奇. 过渡金属改性Mg-Al 基固体碱催化剂上碳酸丙烯酯与甲醇合成碳酸二甲酯的研究[J]. 燃料化学学报,2020,48(4):448−455. doi: 10.3969/j.issn.0253-2409.2020.04.008WANG Qing, ZHAO Hai-hong, KUANG Zi-qi. Preparation of Mg-Al based solid base for the transesterification of propylene carbonate and methanol[J]. J Fuel Chem Technol,2020,48(4):448−455. doi: 10.3969/j.issn.0253-2409.2020.04.008 [32] BRISTOW P A, TILLETT J G. Transesterification of cyclic esters[J]. Tetrahedron Lett,1967,8(10):901−903. doi: 10.1016/S0040-4039(00)90602-6 [33] 魏彤, 王谋华, 魏伟. 氧化钙室温催化碳酸丙烯酯和甲醇的酯交换合成碳酸二甲酯[J]. 催化学报,2003,24(1):52−56. doi: 10.3321/j.issn:0253-9837.2003.01.013WEI Tong, WANG Mou-hua, WEI Wei. Room-temperature catalytic synthesis of dimethyl carbonate from calcium oxide by transesterification of propylene carbonate and methanol[J]. J Catal,2003,24(1):52−56. doi: 10.3321/j.issn:0253-9837.2003.01.013 [34] 冯雪. 碳酸乙烯酯酯交换合成碳酸二甲酯的反应体系研究[D]. 天津: 天津大学, 2016.FENG Xue. Study on the reaction system for the synthesis of dimethyl carbonate by ethylene carbonate ester exchange[D]. Tianjin: Tianjin University, 2016. -





 下载:
下载: