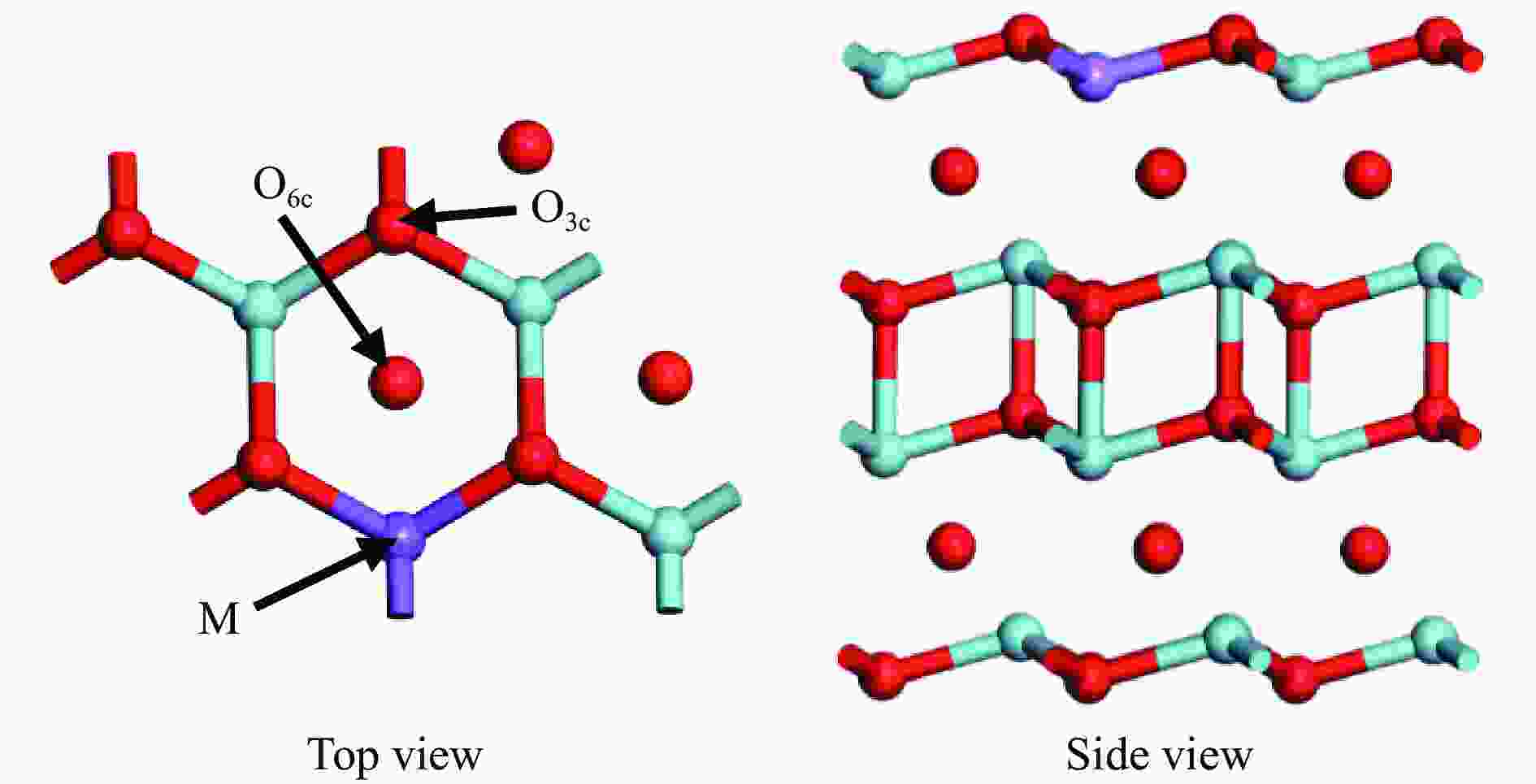
Effect of different valence metals doping on methane activation over La2O3(001) surface
-
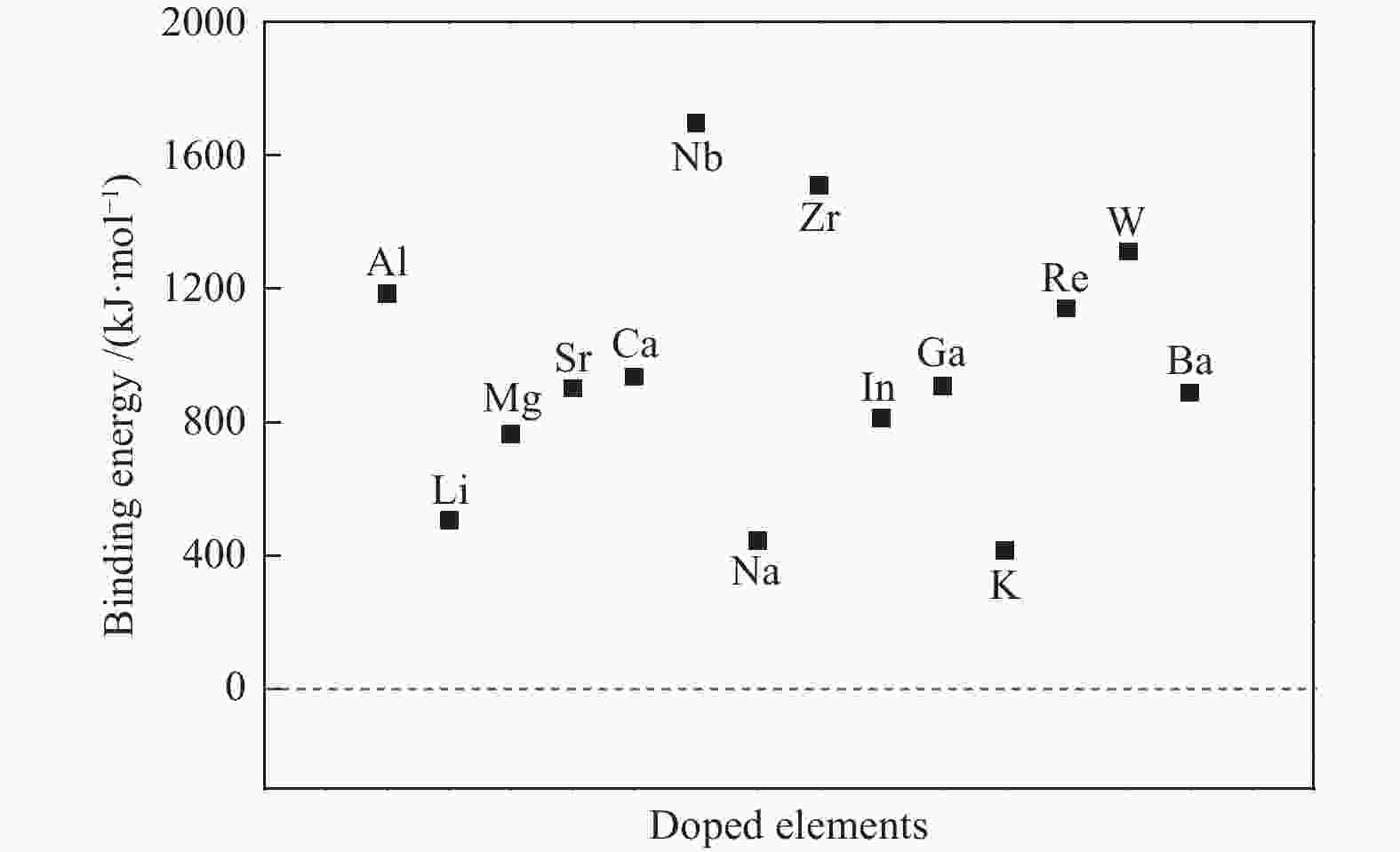
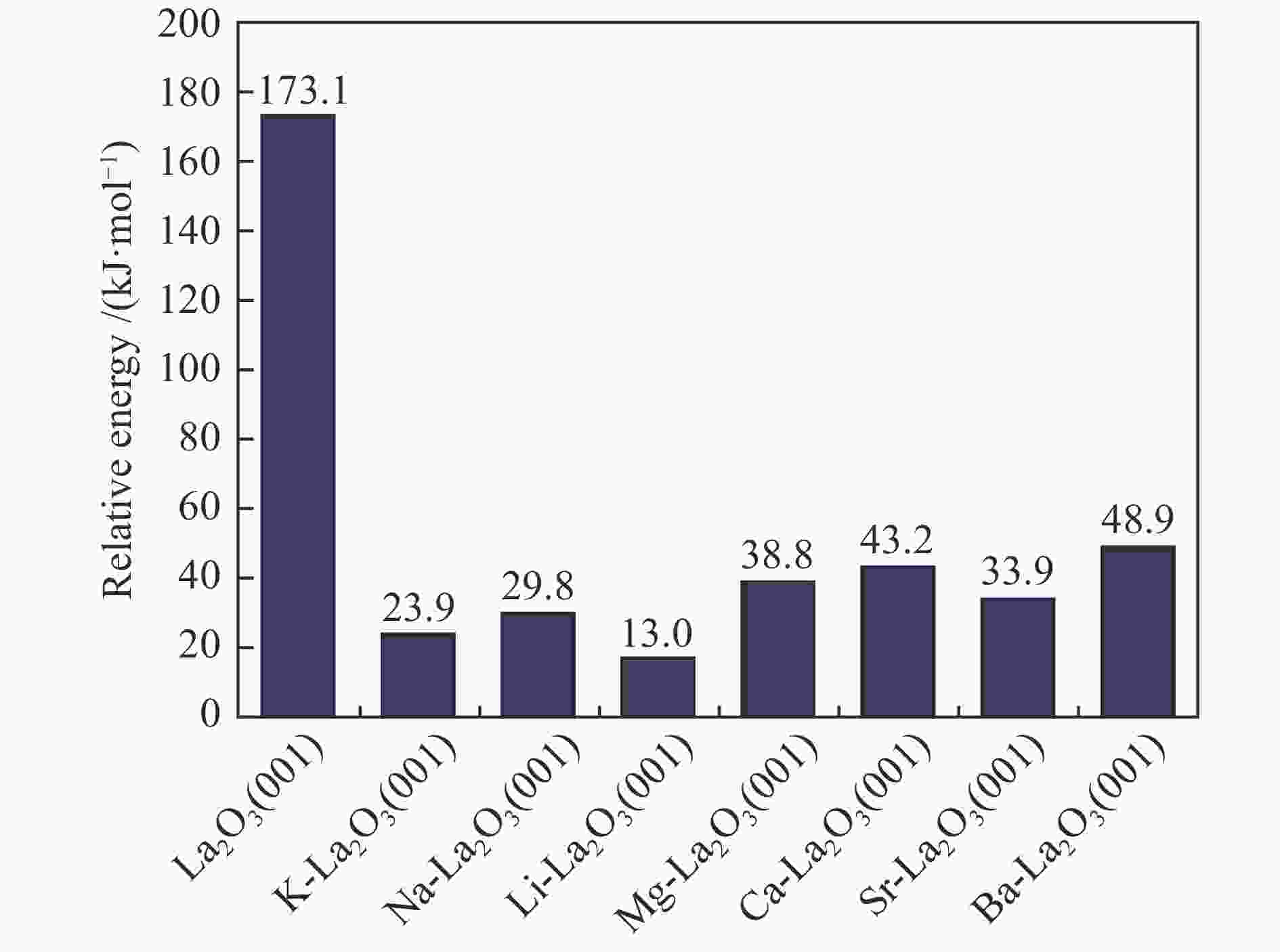
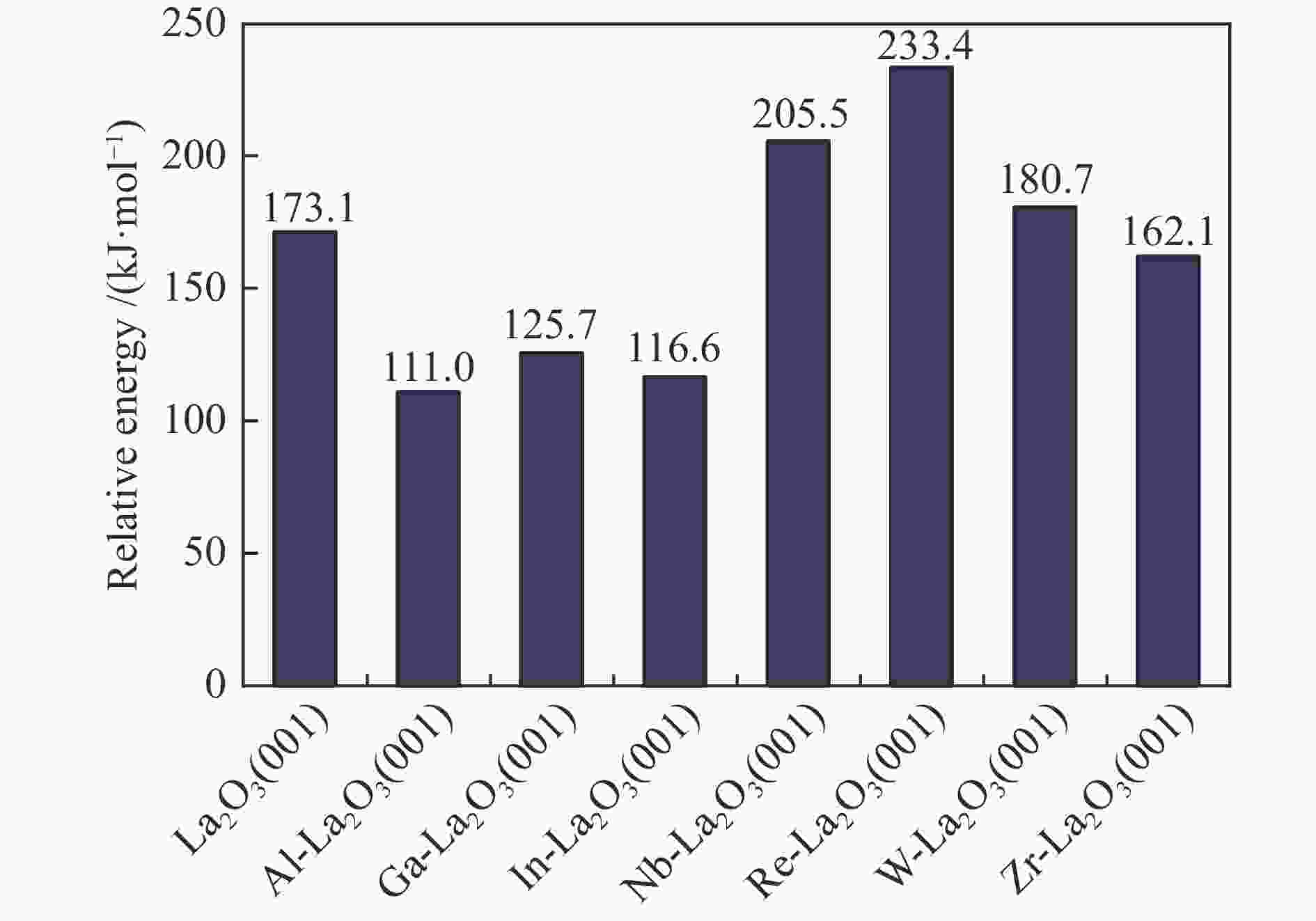
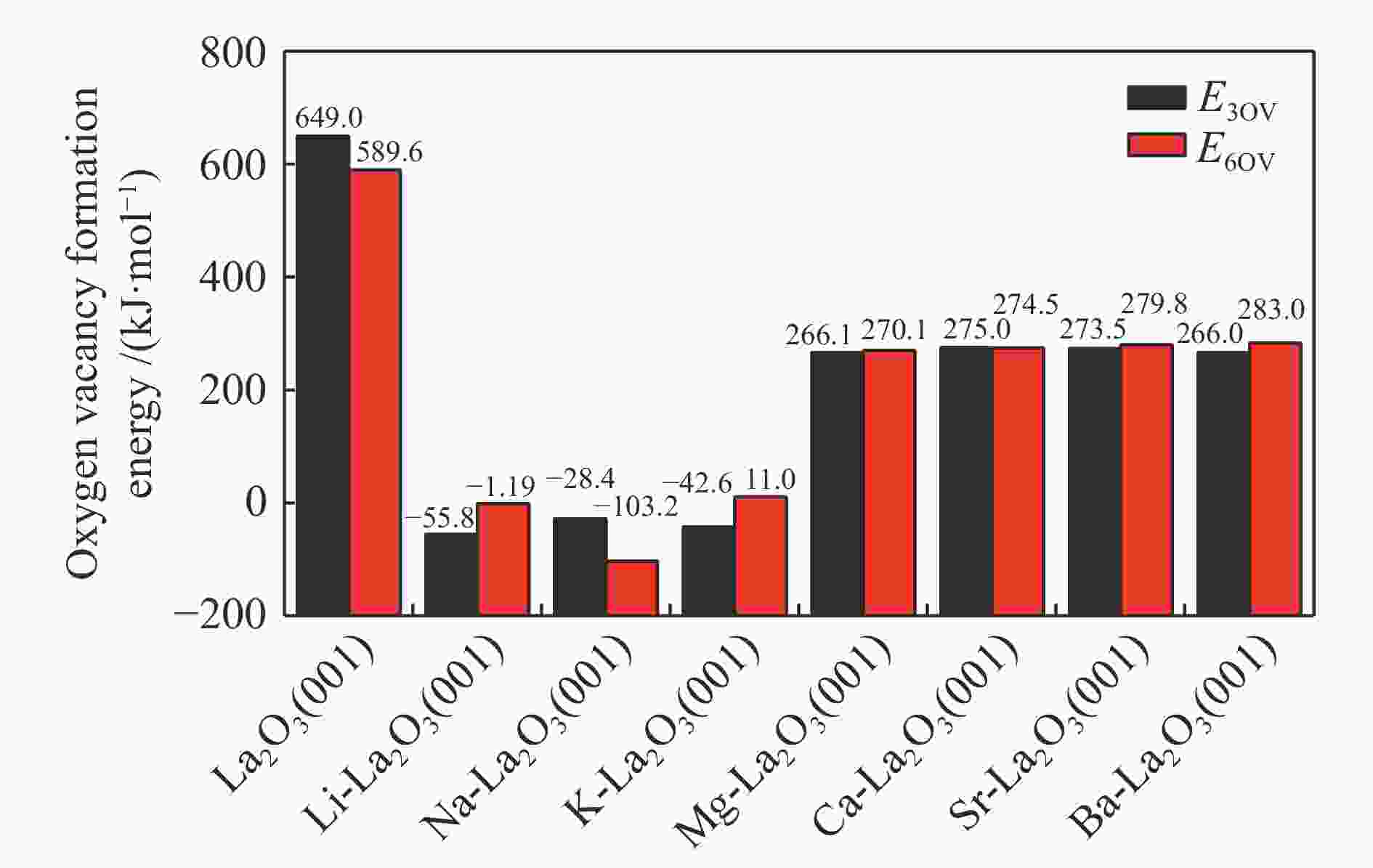
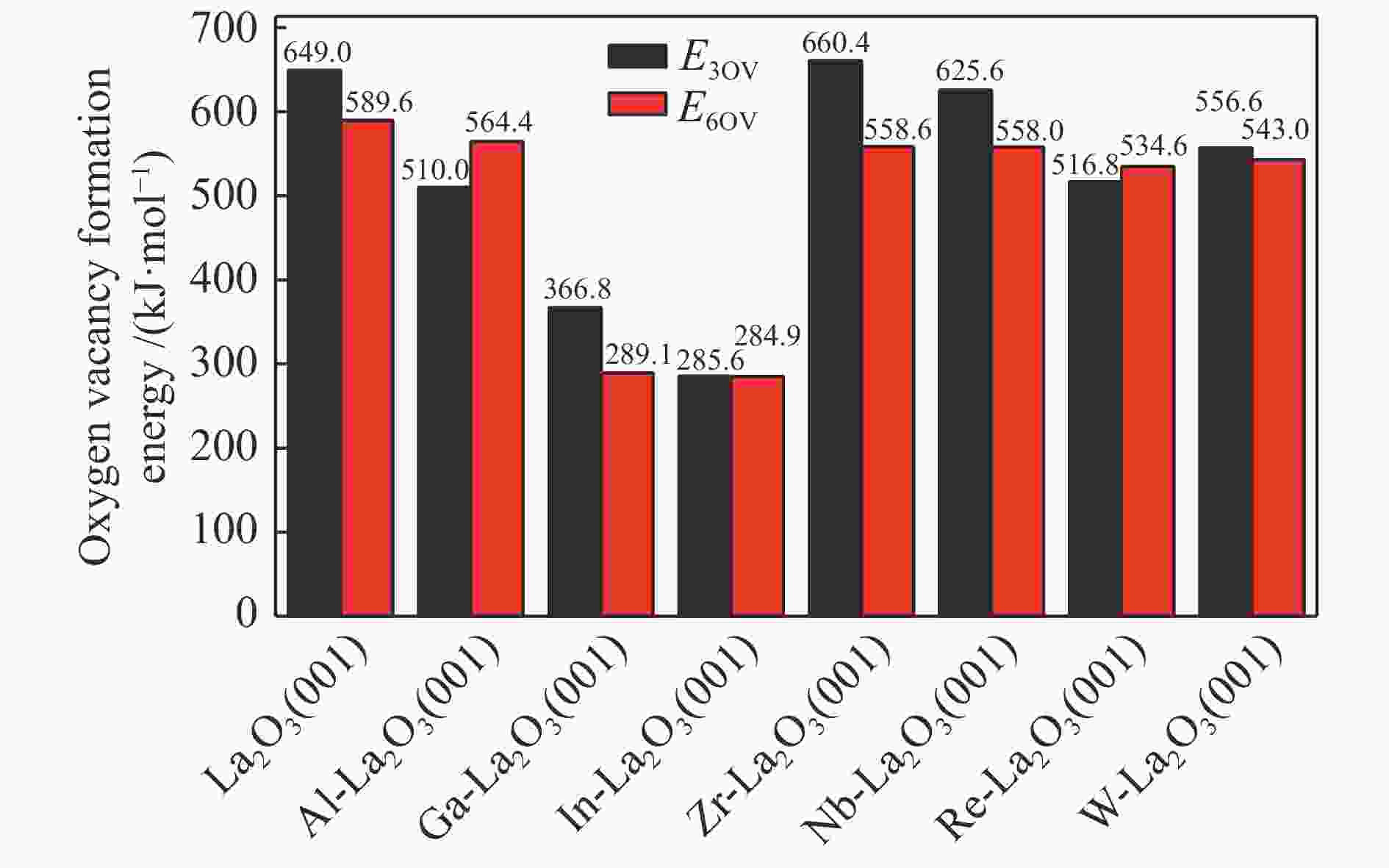
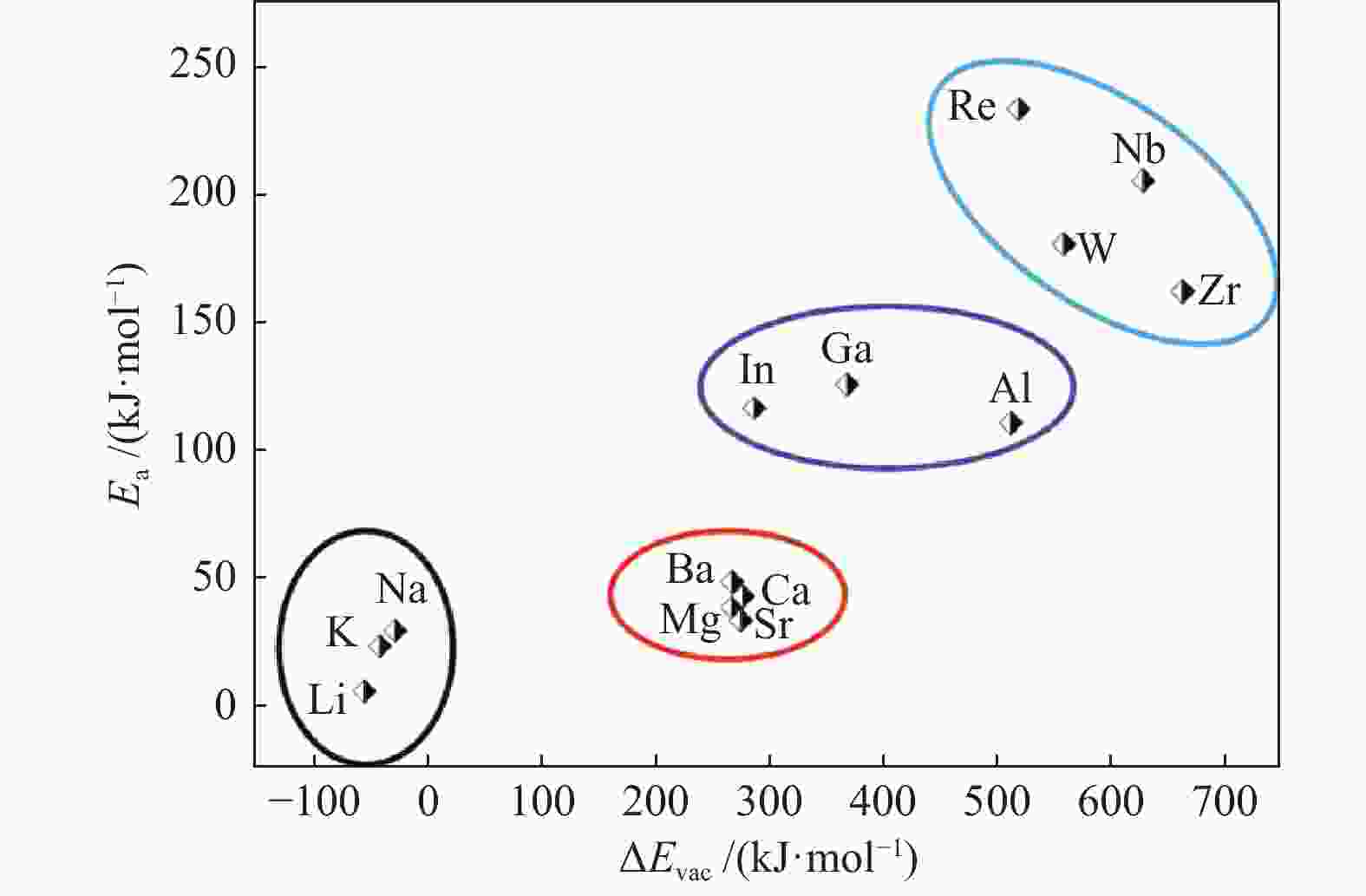
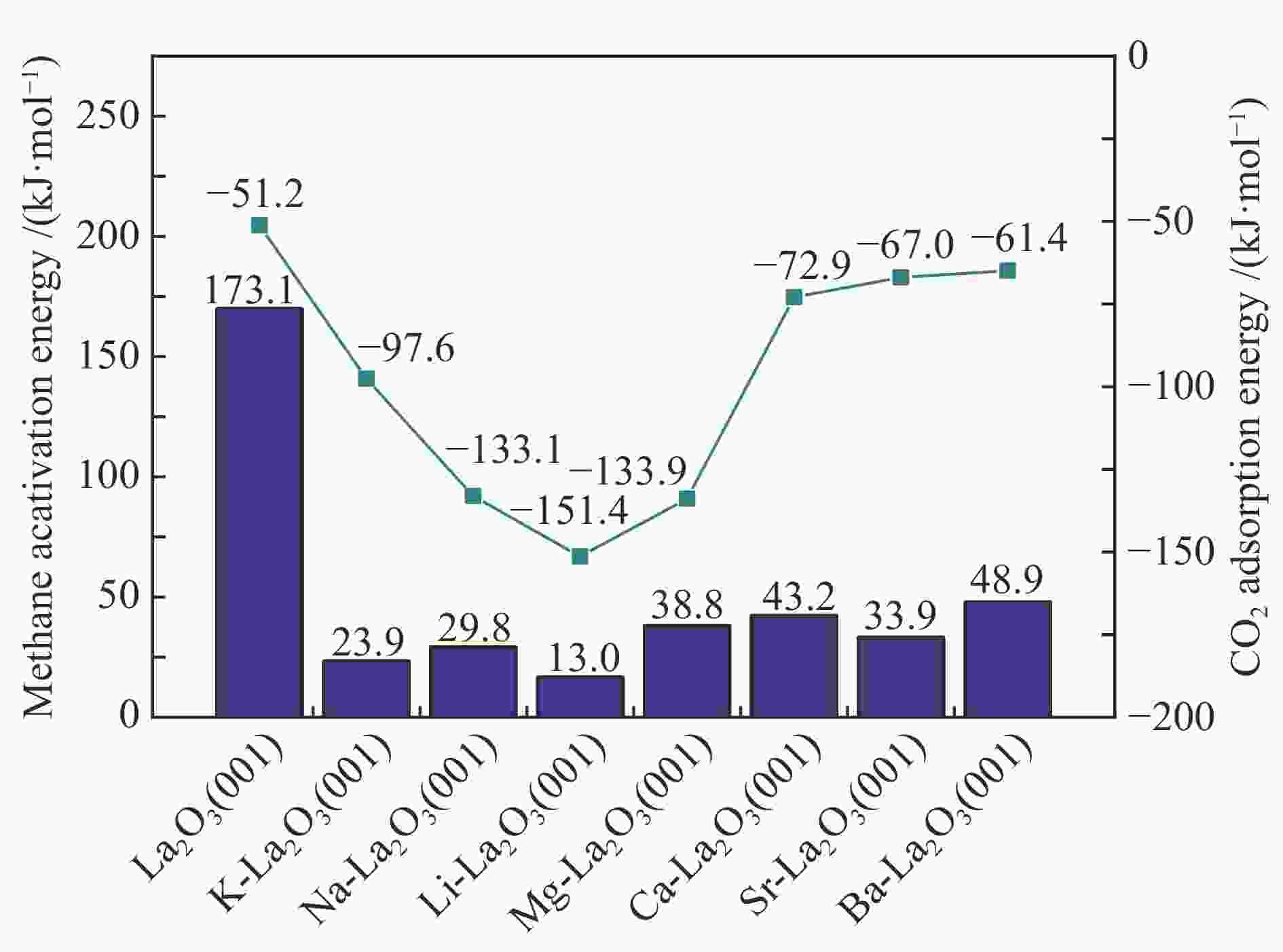
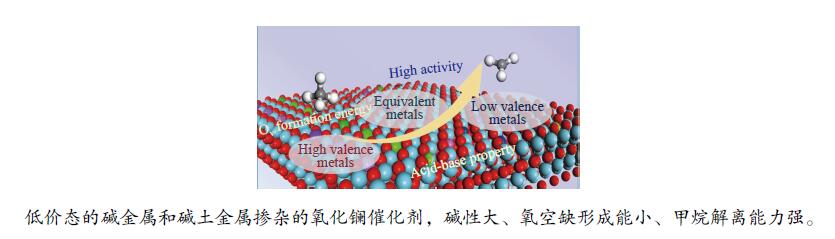
摘要: La2O3因具有优异的稳定性和较高的C2烃选择性,因此,常被用于催化甲烷氧化偶联反应,而较差的甲烷解离活性却限制了其广泛应用。为了提高镧基催化剂活化甲烷的性能,将不同价态的金属掺杂在La2O3(001)表面,并采用密度泛函理论方法对CH4在催化剂表面的活化行为进行了研究。结果表明,低价态金属(Li、Na、K、Mg、Ca、Sr和Ba)和等价态金属(Al、Ga、In)的掺杂可以显著提高La2O3(001)表面的CH4解离活性。其中,CH4在Li-La2O3(001)表面解离的活化能最低,仅为13.0 kJ/mol。而高价态金属(Zr、Nb、Re和W)掺杂不能提高La2O3(001)表面的CH4解离活性。此外,通过研究催化剂表面氧空位形成能、酸碱性与CH4解离活化能之间的关系发现,随着掺杂金属价态的增加,氧空位形成能也逐渐增大,而CH4的解离活性呈现出降低趋势;碱金属和碱土金属的掺杂增大了催化剂表面的碱性,且碱金属掺杂的碱性强于碱土金属,同时,较强的碱性也表现出较高的CH4转化活性。本研究对提高La2O3催化剂的甲烷转化活性具有指导意义。Abstract: La2O3 as a catalyst is used for oxidative coupling of methane (OCM) reactions due to its excellent stability and high C2 selectivity, but poor activity on methane dissociation limits its wide application. Different valence metals are doped on the La2O3(001) surface to improve the methane conversion activity, and the activation of methane on metal-doped La2O3(001) surfaces has been investigated via the density functional theory (DFT) calculations. The relationship between the valence states of doped metals and the methane conversion activities shows that doping low valence metals (Li, Na, K, Mg, Ca, Sr and Ba) and equivalent metals (Al, Ga, In) can significantly improve the conversion activity of methane. Among them, the activation energy of methane on the Li-La2O3(001) surface is the lowest, which is only 13.0 kJ/mol. However, doping of high valence metals (Zr, Nb, Re and W) cannot improve the CH4 dissociation activity. Furthermore, the relationships between surface oxygen vacancy formation energies, acid-base properties and the activation energies of CH4 have also been investigated. The results show that with the increase of metal valence state, the oxygen vacancy formation energy increases, while the dissociation activity of CH4 decreases. The introduction of alkali and alkaline earth metals increases the alkalinity of La2O3(001) surface, and the alkalinity of La2O3(001) doped with the alkali metal is stronger than that with the alkaline earth metal, exhibiting higher dissociation activity of CH4. Our research may provide a guide for improving methane conversion activity on La2O3 catalysts.
-
Key words:
- methane /
- La2O3 catalyst /
- metal doping /
- activity /
- density functional theory
-
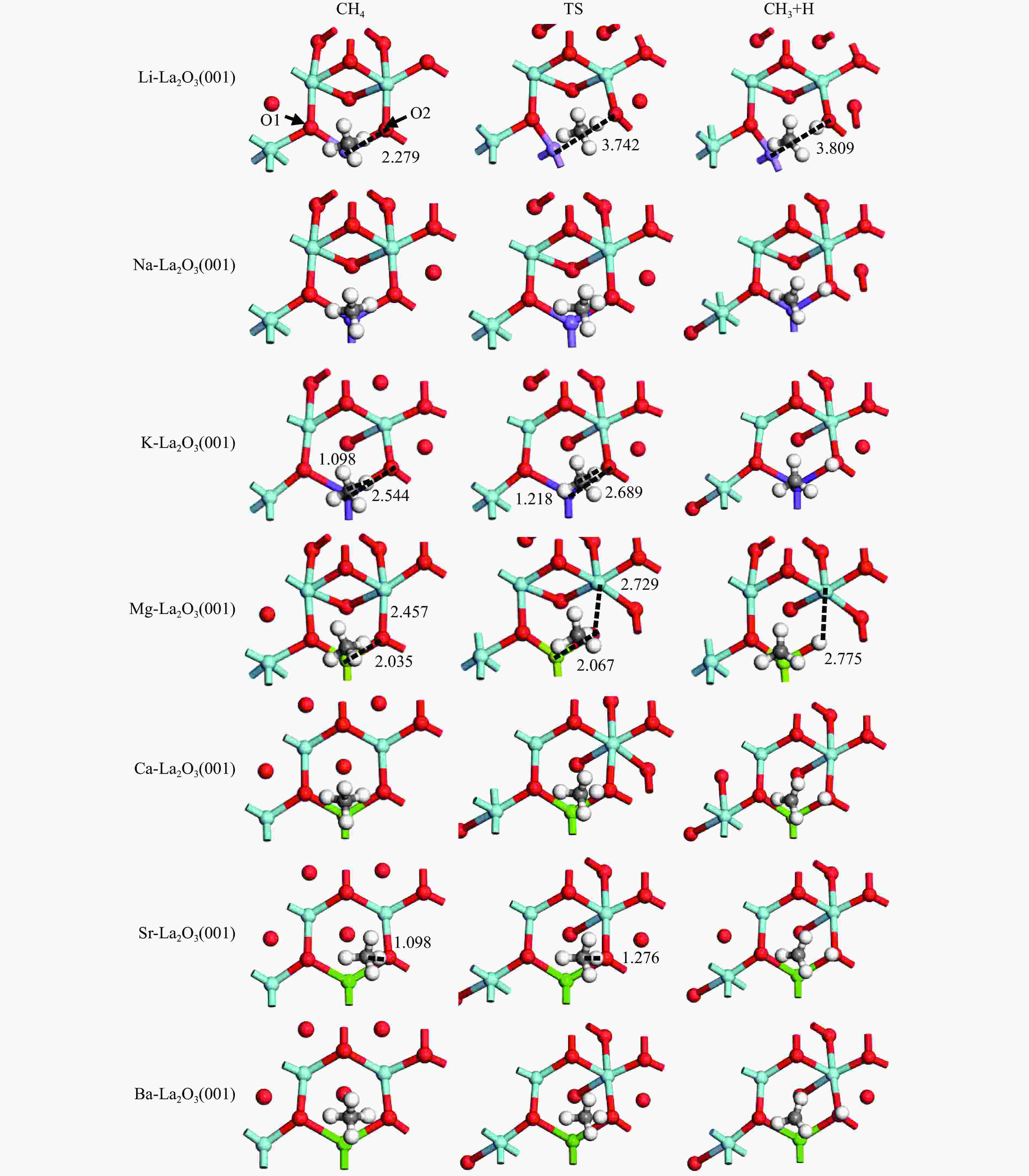
Table 1 Calculated energetics of CH4 activation on the M-La2O3(001) (M = Li, Na, K, Mg, Ca, Sr and Ba) surfaces
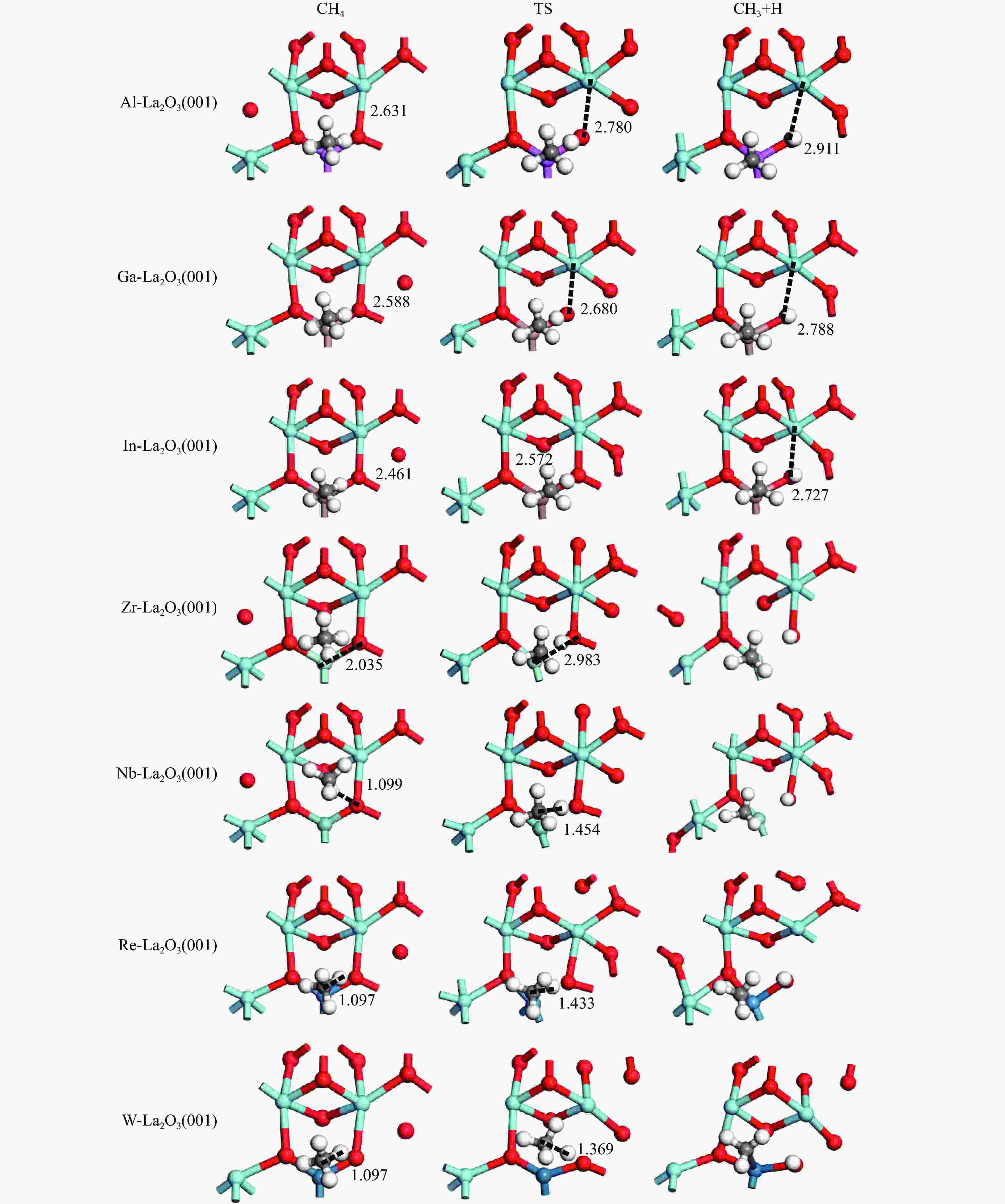
Catalyst E/(kJ·mol−1) Eads(CH4) Ea ΔEr Li-La2O3(001) −4.4 13.0 −28.9 Na-La2O3(001) −4.7 29.8 −21.2 K-La2O3(001) −3.6 23.9 −27.8 Mg-La2O3(001) −3.8 38.8 −3.3 Ca-La2O3(001) −3.9 43.2 −15.5 Sr-La2O3(001) −3.7 33.9 −16.4 Ba-La2O3(001) −3.3 48.9 −11.5 Table 2 Calculated energetics of methane activation on the La2O3(001) surfaces doped with equivalent and high valence metals, including CH4 adsorption energy (Eads), activation energy (Ea), and reaction heat (ΔEr)
Catalyst E/(kJ·mol−1) Eads(CH4) Ea ΔEr Al-La2O3(001) −3.3 111.0 39.5 Ga-La2O3(001) −3.3 125.7 20.4 In-La2O3(001) −2.9 116.6 10.1 Zr-La2O3(001) −2.5 162.1 147.2 Nb-La2O3(001) −3.3 205.5 153.9 Re-La2O3(001) −4.3 233.4 151.0 W-La2O3(001) −4.3 180.7 104.9 -
[1] SCHWACH P, PAN X L, BAO X H. Direct conversion of methane to value-added chemicals over heterogeneous catalysts: challenges and prospects[J]. Chem Rev,2017,117(13):8497−8520. doi: 10.1021/acs.chemrev.6b00715 [2] NWAOHA C, WOOD D A. A review of the utilization and monetization of Nigeria's natural gas resources: Current realities[J]. J Nat Gas Sci Eng,2014,18:412−432. doi: 10.1016/j.jngse.2014.03.019 [3] PULIYALIL H, JURKOVIĆ D L, DASIREDDY V D B C, LIKOZAR B. A review of plasma-assisted catalytic conversion of gaseous carbon dioxide and methane into value-added platform chemicals and fuels[J]. RSC Adv,2018,8:27481−27508. doi: 10.1039/C8RA03146K [4] RAVI M, RANOCCHIARI M, BOKHOVEN J A. The direct catalytic oxidation of methane to methanol-A critical assessment[J]. Angew Chem Int Ed,2017,56(52):16464−16483. doi: 10.1002/anie.201702550 [5] WANG B W, ALBARRACÍN-SUAZO S, PAGÁN-TORRES Y, NIKOLLA E. Advances in methane conversion processes[J]. Catal Today,2017,285:147−158. doi: 10.1016/j.cattod.2017.01.023 [6] SIDIK S M, TRIWAHYONO S, JALIL A A, MAJID Z A, SALAMUN N, TALIB N B, ABDULLAH T A T. CO2 reforming of CH4 over Ni-Co/MSN for syngas production: Role of Co as a binder and optimization using RSM[J]. Chem Eng J,2016,295:1−10. doi: 10.1016/j.cej.2016.03.041 [7] WANG S B, CONG L N, ZHAO C C, LI Y T, PANG Y Q, ZHAO Y H, LI S G, SUN Y H. First principles studies of CO2 and O2 chemisorption on La2O3 surfaces[J]. Phys Chem Chem Phys,2017,19:26799−26811. doi: 10.1039/C7CP05471H [8] GAMBO Y, JALIL A A, TRIWAHYONO S, ABDULRASHEED A A. Recent advances and future prospect in catalysts for oxidative coupling of methane to ethylene: A review[J]. J Ind Eng Chem,2018,59:218−229. doi: 10.1016/j.jiec.2017.10.027 [9] OLIVOS-SUAREZ A I, SZECSENYI A, HENSEN E J M, RUIZ-MARTINEZ J. Strategies for the direct catalytic valorization of methane using heterogeneous catalysis: challenges and opportunities[J]. ACS Catal,2016,6(5):2965−2981. doi: 10.1021/acscatal.6b00428 [10] BECK B, FLEISCHER V, ARNDT S, HEVIA M G, URAKAWA A, HUGO P, SCHOMÄCKER R. Oxidative coupling of methane-A complex surface/gas phase mechanism with strong impact on the reaction engineering[J]. Catal Today,2014,228:212−218. doi: 10.1016/j.cattod.2013.11.059 [11] LOMONOSOV V I, SINEV M Y. Oxidative coupling of methane: Mechanism and kinetics[J]. Kinet Catal,2016,57:647−676. doi: 10.1134/S0023158416050128 [12] LUNSFORD J H. The catalytic oxidative coupling of methane[J]. Angew Chem Int Ed,1995,34:970−980. doi: 10.1002/anie.199509701 [13] SUN J J, THYBAUT J W, MARIN G B. Microkinetics of methane oxidative coupling[J]. Catal Today,2008,137(1):90−102. doi: 10.1016/j.cattod.2008.02.026 [14] SUN X Y, LI B, METIU H. Methane dissociation on Li-, Na-, K-, and Cu-doped flat and stepped CaO(001)[J]. J Phys Chem C,2013,117(14):7114−7122. doi: 10.1021/jp4002803 [15] LUO L, JIN Y, PAN H. Distribution and role of Li in Li-doped MgO catalysts for oxidative coupling of methane[J]. J Catal,2017,346:57−61. doi: 10.1016/j.jcat.2016.11.034 [16] MASUNO A, INOUE H. High refractive index of 0.30 La2O3–0.70 Nb2O5 glass prepared by containerless processing[J]. Appl Phys Express,2010,3(10):102601. doi: 10.1143/APEX.3.102601 [17] SUN Y N, SHEN Y, SONG J J, BA R B, HUANG S S, ZHAO Y H, ZHANG J, SUN Y H, ZHU Y. Facet-controlled CeO2 nanocrystals for oxidative coupling of methane[J]. J Nanosci Nanotechnol,2016,16(5):4692−4700. doi: 10.1166/jnn.2016.11623 [18] HUANG P, ZHAO Y H, ZHANG J, ZHU Y, SUN Y H. Exploiting shape effects of La2O3 nanocatalysts for oxidative coupling of methane reaction[J]. Nanoscale,2013,5(22):10844−10848. doi: 10.1039/c3nr03617k [19] FENG R, NIU P Y, HOU B, WANG Q, JIA L T, LIN M G, LI D B. Synthesis and characterization of the flower-like LaxCe1−xO1.5 + δ catalyst for low-temperature oxidative coupling of methane[J]. J Energy Chem,2022,67:342−353. doi: 10.1016/j.jechem.2021.10.018 [20] PALMER M S, NEUROCK M, OLKEN M M. Periodic density functional theory study of methane activation over La2O3: activity of O2−, O−, O22−, oxygen point defect, and Sr2 + -doped surface sites[J]. J Am Chem Soc,2002,124(28):8452−8461. doi: 10.1021/ja0121235 [21] ZAVYALOVA U, HOLENA M, SCHLÖGL R, BAERNS M. Statistical analysis of past catalytic data on oxidative methane coupling for new insights into the composition of high‐performance catalysts[J]. ChemCatChem,2011,3(12):1935−1947. doi: 10.1002/cctc.201100186 [22] WANG S B, LI S G, DIXON D A. Mechanism of selective and complete oxidation in La2O3-catalyzed oxidative coupling of methane[J]. Catal Sci Technol,2020,10:2602−2614. doi: 10.1039/D0CY00141D [23] ALVAREZ-GALVAN M C, MOTA N, OJEDA M, ROJAS S, NAVARRO R M, FIERRO J L G. Direct methane conversion routes to chemicals and fuels[J]. Catal Today,2011,171(1):15−23. doi: 10.1016/j.cattod.2011.02.028 [24] IGENEGBAI V O, MEYER R J, LINIC S. In search of membrane-catalyst materials for oxidative coupling of methane: performance and phase stability studies of gadolinium-doped barium cerate and the impact of Zr doping[J]. Appl Catal B: Environ,2018,230(15):29−35. [25] SOLLIER B. M, BONNE M, KHENOUSSI N, MICHELIN L, MIRÓ E E, GÓMEZ L. E, BOIX A V, LEBEAU B. Synthesis and characterization of electrospun nanofibers of Sr-La-Ce oxides as catalysts for the oxidative coupling of methane[J]. Ind Eng Chem Res,2020,59(25):11419−11430. doi: 10.1021/acs.iecr.0c01154 [26] SONG J J, SUN Y N, BA R B, HUANG S S, ZHAO Y H, ZHANG J, SUN Y H, ZHU Y. Monodisperse Sr-La2O3 hybrid nanofibers for oxidative coupling of methane to synthesize C2 hydrocarbons[J]. Nanoscale,2015,7(6):2260−2264. doi: 10.1039/C4NR06660J [27] DEBOY J M, HICKS R F. Oxidative coupling of methane over alkaline earth promoted La2O3[J]. J Chem Soc, Chem Commun,1988,982−984. [28] LI B, METIU H. DFT studies of oxygen vacancies on undoped and doped La2O3 surfaces[J]. J Phys Chem C,2010,114(28):12234−12244. doi: 10.1021/jp103604b [29] MCFARLAND E W, METIU H. Catalysis by doped oxides[J]. Chem Rev,2013,113(6):4391−4427. doi: 10.1021/cr300418s [30] LICHTENSTEIN A I, KATSNELSON M I. Ab initio calculations of quasiparticle band structure in correlated systems: LDA + + approach[J]. Phys Rev B,1998,57(12):6884. doi: 10.1103/PhysRevB.57.6884 [31] KRESSE G, FURTHMÜLLER J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set[J]. Comp Mater Sci,1996,6(1):15−50. doi: 10.1016/0927-0256(96)00008-0 [32] PERDEW J P, BURKE K, ERNZERHOF M. Generalized gradient approximation made simple[J]. Phys Rev Lett,1996,77(18):3865−3868. doi: 10.1103/PhysRevLett.77.3865 [33] MONKHORST H J, PACK J D. Special points for brillonin-zone integrations[J]. Phys Rev B,1976,13(12):5188−5192. doi: 10.1103/PhysRevB.13.5188 [34] SHEPPARD D, XIAO P H, CHEMELEWSKI W, JOHNSON D D, HENKELMAN G. A generalized solid-state nudged elastic band method[J]. J Chem Phys,2012,136(7):074103. doi: 10.1063/1.3684549 [35] SHEPPARD D, TERRELL R, HENKELMAN G. Optimization methods for finding minimum energy paths[J]. J Chem Phys,2008,128(13):134106. doi: 10.1063/1.2841941 [36] OLSEN R A, KROES G J, HENKELMAN G, ARNALDSSON A, JÓNSSON H. Comparison of methods for finding saddle points without knowledge of the final states[J]. J Chem Phys,2004,121(20):9776−9792. doi: 10.1063/1.1809574 [37] HENKELMAN G, UBERUAGA B P, JÓNSSON H. A climbing image nudged elastic band method for finding saddle points and minimum energy paths[J]. J Chem Phys,2000,113(22):9901−9904. doi: 10.1063/1.1329672 [38] ALAVI A, HU P J, DEUTSCH T, SLIVESTRELLI P L, HUTTER J. CO oxidation on Pt(111): An ab initio density functional theory study[J]. Phys Rev Lett,1998,80(16):3650−3653. doi: 10.1103/PhysRevLett.80.3650 [39] WANG Z Q, WANG D, GONG X Q. Strategies to improve the activity while maintaining the selectivity of oxidative coupling of methane at La2O3: A density functional theory study[J]. ACS Catal,2020,10(1):586−594. doi: 10.1021/acscatal.9b03066 [40] LI B, METIU H. Dissociation of methane on La2O3 surfaces doped with Cu, Mg, or Zn[J]. J Phys Chem C,2011,115(37):18239−18246. doi: 10.1021/jp2049603 -




 下载:
下载: