Experimental study on preparation of bio-oil by hydrothermal liquefaction of three kinds of lignin
-
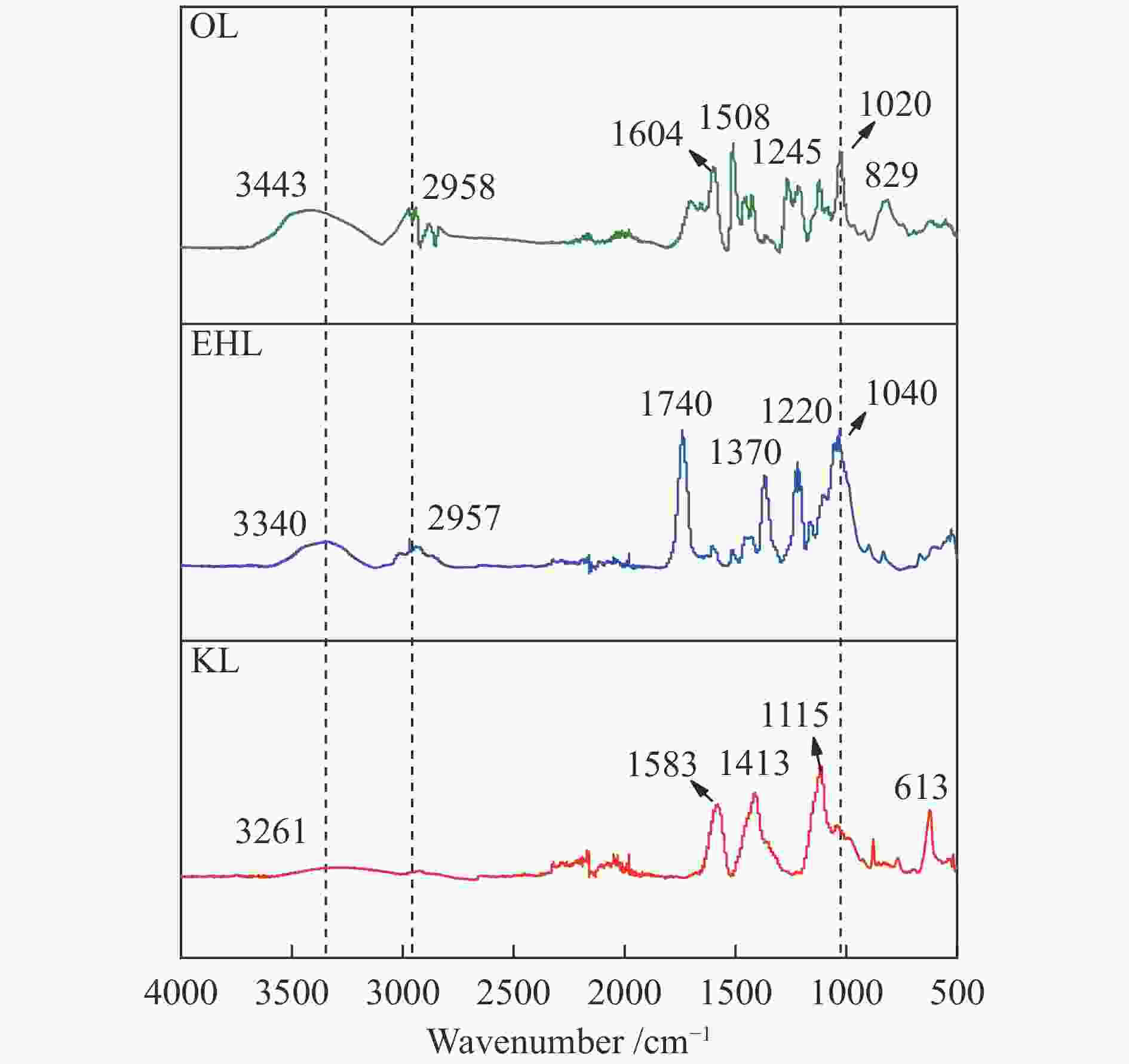
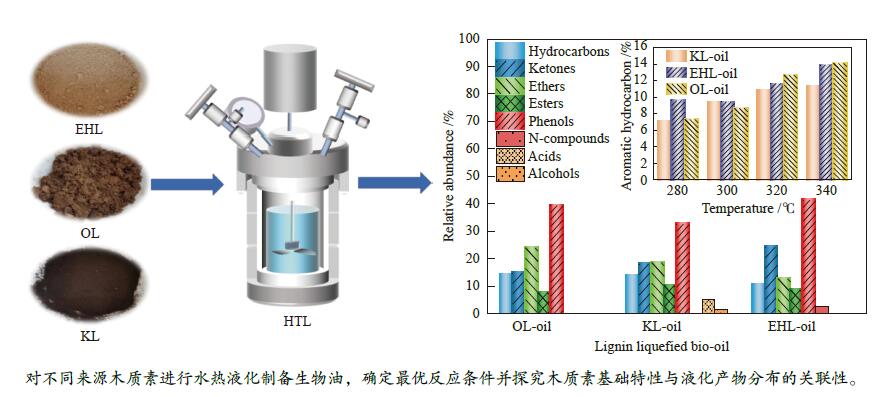
摘要: 木质素是具有芳香族结构的天然可再生资源,可以通过水热液化技术将其转化为生物油。不同种类的木质素结构特点和反应活性存在差异,故本研究选取三种木质素(工业碱木质素(KL)、酶解木质素(EHL)和乙醇木质素(OL))为原料,首先对三种原料理化特性进行分析;其次考察反应条件对三种木质素水热液化生物油特性的影响。在三种木质素中,EHL、OL为愈创木基型结构。OL的C、H元素含量最高,其高位热值为23.54 MJ/kg,芳香特征更加明显,酚羟基含量相对较高。KL为紫丁香基型结构,甲氧基与酚羟基含量较少。液化实验结果显示,反应温度为300 ℃时,木质素生物油产率及能量回收率最高,该温度下产率OL>KL>EHL,生物油的H/C比值为1.0–1.4。三种生物油化学成分不同,OL生物油中含有9.14%的芳香烃,EHL生物油酚类物质含量达到41.34%,KL生物油中酸类含量较高。Abstract: Lignin is a natural and renewable resource with aromatic structure. It can be converted into bio-oil by hydrothermal liquefaction. Due to the complex structure of wood fiber, the structural characteristics and reactivity of different kinds of lignin are different. Therefore, three typical lignin (kraft lignin (KL), enzymatic hydrolysis lignin (EHL) and ethanol lignin (OL)) were selected as raw materials. Firstly, physical and chemical properties of the raw materials were analyzed. Secondly, effects of reaction conditions on characteristics of their hydrothermal liquefaction bio-oil were investigated. Among them, EHL and OL are guaiacyl units. OL has the highest content of carbon and hydrogen elements, and its higher heating value reaches 23.54 MJ/kg. The aromatic characteristics are more obvious, and the phenolic hydroxyl content is relatively high. KL is mainly syringyl unit with less methoxy and phenolic hydroxyl groups. The results of liquefaction experiment show that when the reaction temperature was 300 ℃, yield and energy recovery rate of lignin bio-oil were the highest. The bio-oil yield ranked in the order of OL>KL>EHL. H/C ratio of bio-oil was concentrated within 1.0-1.4. Chemical composition of the three bio-oils was different. OL bio-oil contains 9.14% aromatic hydrocarbons, EHL bio-oil contains 41.34% phenolic species, and KL bio-oil has a higher acid content.
-
Key words:
- lignin /
- hydrothermal liquefaction /
- bio-oil /
- aromatic compound
-
表 1 木质素的元素分析及高位热值
Table 1 Ultimate analysis and higher heating value of lignin
Sample Ultimate analysis w/% QHHV /(MJ·kg−1) C H O* N S EHL 47.26 5.74 44.49 0.53 0 18.87 KL 39.02 4.42 21.75 0.38 2.03 17.08 OL 58.04 5.78 34.48 1.09 0 23.60 *: by difference 表 2 木质素热解特性
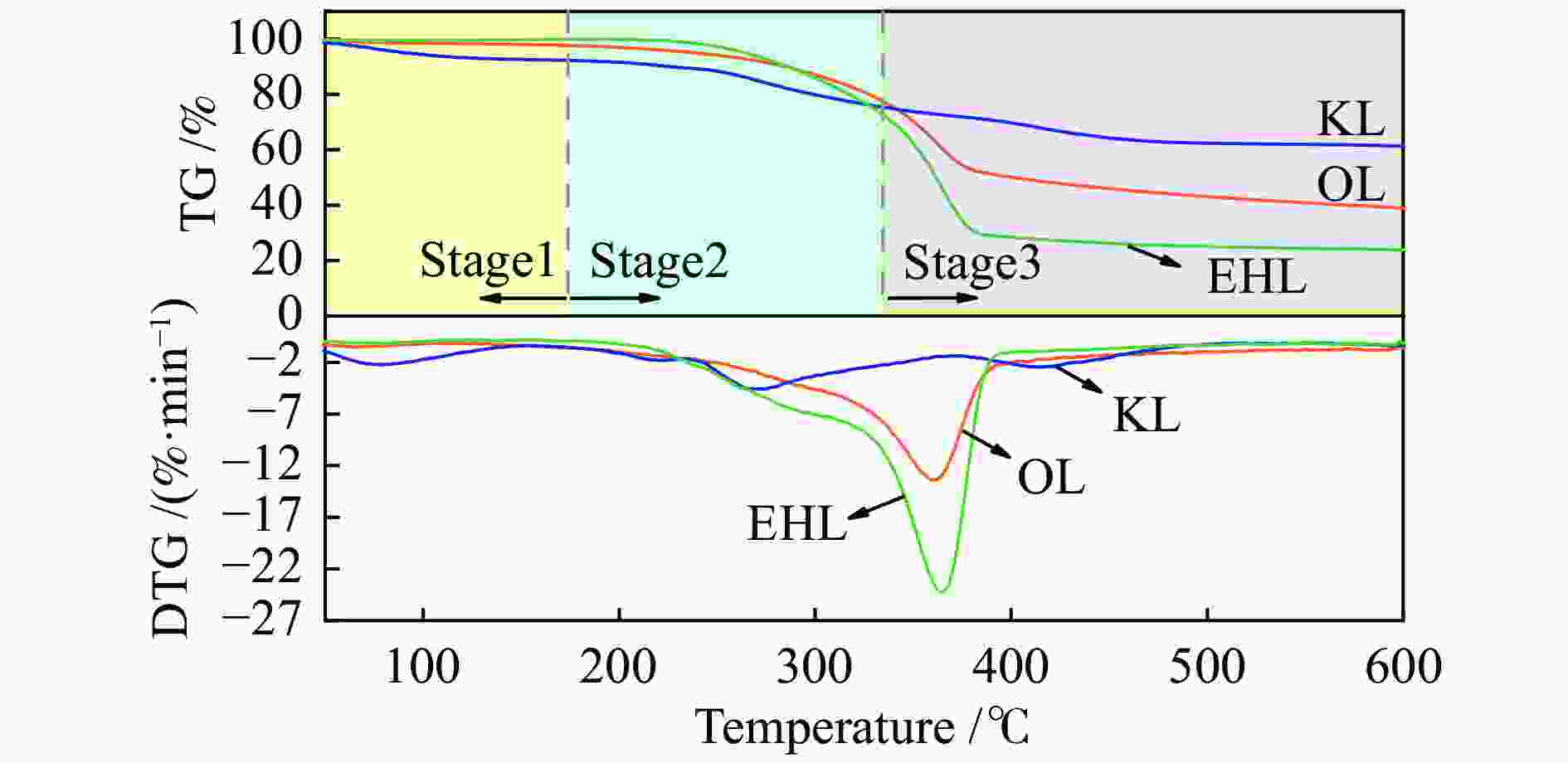
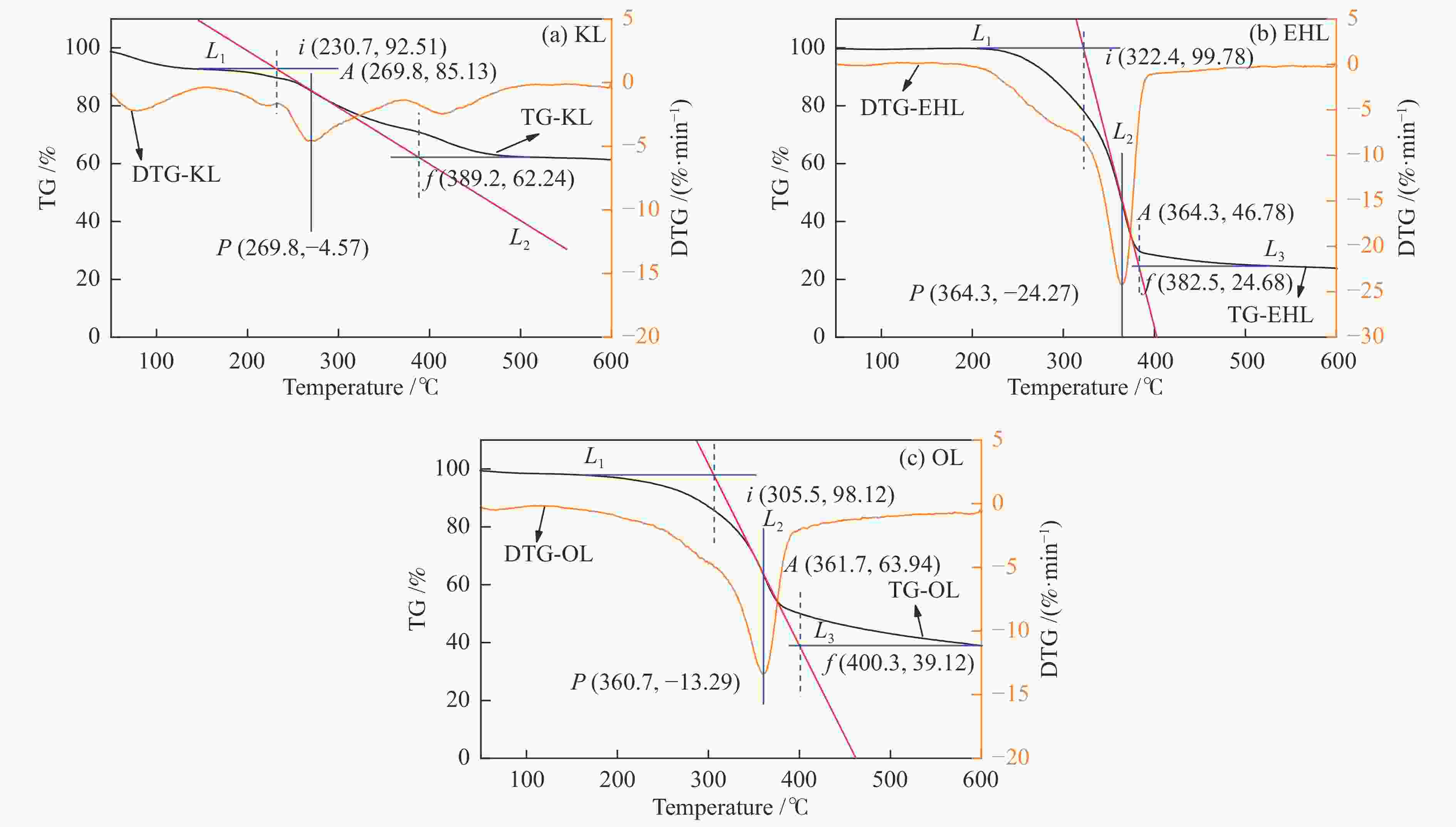
Table 2 Pyrolysis characteristics of lignin
Sample Start temperature
t/℃End temperature
t/℃Maximum pyrolysis rate Vp/(%·min−1) Maximum pyrolysis rate temperature
tp/℃Average pyrolysis rate
Va/(%·min−1)KL 230.7 389.2 4.57 269.8 3.82 EHL 322.4 382.5 24.27 364.3 24.99 OL 305.5 400.3 13.29 360.7 12.45 表 3 液化反应的质量衡算
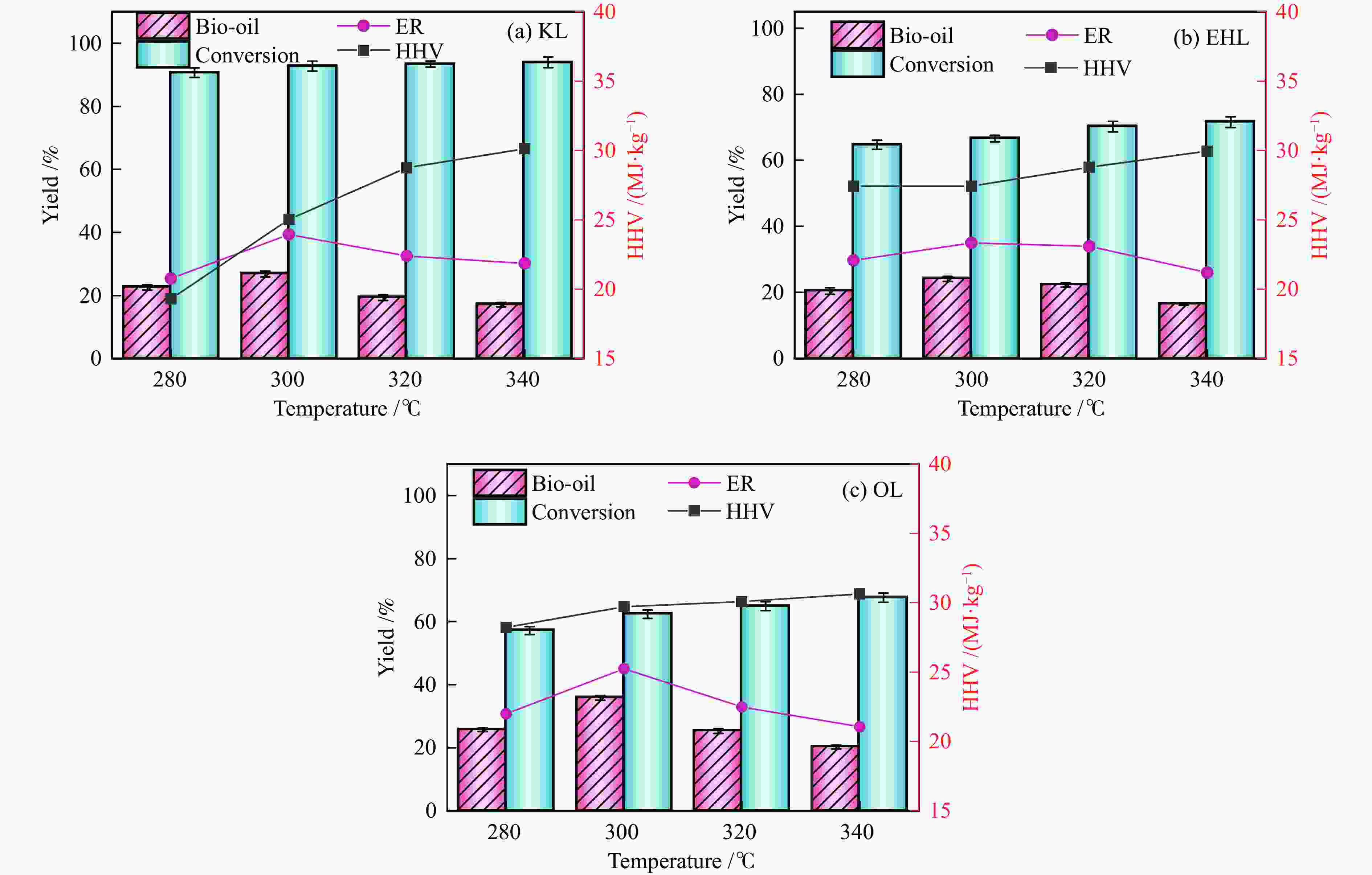
Table 3 Mass balance of liquefaction reaction
Sample Bio-oil
yield /%Solid residue
yield /%Gas + aqueous
yield /%KL (280 ℃) 22.5 9.34 68.16 KL (300 ℃) 26.84 7.28 65.88 KL (320 ℃) 19.32 6.46 74.22 KL (340 ℃) 17.1 6.04 76.86 EHL (280 ℃) 20.44 35.3 44.26 EHL (300 ℃) 24.08 33.4 42.52 EHL (320 ℃) 22.28 29.8 47.92 EHL (340 ℃) 16.4 28.42 55.18 OL (280 ℃) 25.7 42.86 31.44 OL (300 ℃) 35.8 37.64 26.56 OL (320 ℃) 25.28 35.1 39.62 OL (340 ℃) 20.18 32.44 47.38 表 4 不同反应条件下液化生物油的元素分析
Table 4 Elemental analysis of liquefied bio-oil under different reaction conditions
Sample Elemental analysis w/% H/C O/C QHHV /(MJ·kg−1) C H O N S KL (280 ℃) 49.33 5.39 44.18 0.44 0.67 1.31 0.67 19.3 KL (300 ℃) 60.37 6.07 32.42 0.65 0.49 1.21 0.4 25.03 KL (320 ℃) 68.56 6.23 24.3 0.81 0.11 1.09 0.27 28.75 KL (340 ℃) 70.8 6.51 21.68 0.78 0.23 1.1 0.23 30.13 EHL (280 ℃) 66.64 5.95 27.06 0.36 0 1.07 0.3 27.42 EHL (300 ℃) 66.75 5.91 26.95 0.39 0 1.06 0.3 27.42 EHL (320 ℃) 69.27 6.11 24.21 0.42 0 1.06 0.26 28.79 EHL (340 ℃) 71.26 6.32 22.01 0.42 0 1.06 0.23 29.95 OL (280 ℃) 66.37 6.56 26.07 1.01 0 1.19 0.29 28.22 OL (300 ℃) 68.34 7.09 27.15 0.39 0 1.24 0.3 29.7 OL (320 ℃) 70.48 7.04 21.25 1.23 0 1.2 0.23 30.71 OL (340 ℃) 72.05 6.97 20.56 0.43 0 1.16 0.21 31.17 表 5 木质素液化生物油的化学成分分析(反应温度300 ℃)
Table 5 GC-MS analysis of lignin liquefaction bio-oil (reaction temperature 300 ℃)
No. RT Compound Formula KL-oil EHL-oil OL-oil 1 5.87 acetic acid, methyl ester C3H6O2 4.37 2.38 2 6.67 acetic acid C2H4O2 2.29 3 6.74 pentane, 3-methyl- C6H14 2.65 1.93 4 6.79 ethane, 1,1-dimethoxy- C4H10O2 3.22 12.72 5 6.86 2-butanone C4H8O 6.38 1.40 6 7.48 cyclopentane, methyl- C6H12 0.58 7 9.31 propane, 1,1-dimethoxy- C5H12O2 1.27 8 9.38 2-butanone, 3-methyl- C5H10O 1.33 9 9.39 2-pentanone C5H10O 0.48 10 9.54 1,3-dioxolane, 2,2-dimethyl- C5H10O2 1.81 11 10.65 butanoic acid, methyl ester C5H10O2 0.39 2.62 12 12.39 toluene C7H8 1.23 5.59 13 12.85 butanoic acid, 2-methyl-, methyl ester C6H12O2 0.47 14 13.67 furan, 2-methoxy- C5H6O2 24.01 15 14.37 acetic acid, butyl ester C6H12O2 0.57 3.06 16 15.15 cyclohexane, ethyl- C8H16 0.57 1.88 0.52 17 15.28 1,1,4-trimethylcyclohexane C9H18 0.53 18 15.48 2-pentanone, 4-hydroxy-4-methyl- C6H12O2 8.43 5.55 19 16.34 ethylbenzene C8H10 3.57 7.53 20 17.06 acetamide, N-(cyanomethyl)- C4H6N2O 2.64 21 17.07 3-penten-2-one, 4-methyl- C6H10O 0.97 22 17.07 2-hexanone, 6-(acetyloxy)- C8H14O3 2.55 23 17.63 ethylbenzene C8H10 2.96 24 18.30 2-pentanone, 4-methoxy-4-methyl- C7H14O2 3.88 13.89 6.89 25 19.91 alpha-hydroxyisobutyric acid, acetate C6H10O4 2.99 26 20.88 phenol C6H6O 6.74 2.22 27 21.501 benzene, 1,2,4-trimethyl- C9H12 1.83 1.20 28 23.51 dl-erythro-1-phenyl-1,2-propanediol C9H12O2 1.00 29 24.19 p-cresol C7H8O 0.82 30 24.69 benzene, 1-methyl-3-(1-methylethyl)- C10H14 0.57 1.78 31 24.7 o-cymene C10H14 1.26 32 24.85 phenol, 2-methoxy- C7H8O2 7.75 2.55 33 25.75 benzene, 1-ethyl-2,4-dimethyl- C10H14 0.57 34 25.89 benzene, 1,2,4,5-tetramethyl- C10H14 1.64 35 26.54 benzene, 1,2-dimethoxy- C8H10O2 2.98 36 27.93 phenol, 4-methoxy-3-methyl- C8H10O2 1.48 37 27.13 phenol, 4-ethyl- C8H10O 13.36 14.55 38 28.13 2-methoxy-5-methylphenol C8H10O2 5.54 0.98 39 29.37 2,3-dimethoxytoluene C9H12O2 2.49 40 30.70 phenol, 4-ethyl-2-methoxy- C9H12O2 5.83 5.63 4.74 41 30.78 ethanone, 1-(2,4-dihydroxyphenyl)- C8H8O3 1.75 42 31.52 1,2,3-trimethoxybenzene C9H12O3 1.46 43 31.99 1-methylindan-2-one C10H10O 0.83 44 32.68 2,3-dimethoxyphenol C8H10O3 2.61 7.27 45 32.69 phenol, 2,6-dimethoxy-, acetate C10H12O4 12.87 46 33.18 phenol, 2-methoxy-4-propyl- C10H14O2 1.12 47 33.97 benzene, 1,2,3-trimethoxy-5-methyl- C10H14O3 1.28 48 35.21 3,5-dimethoxy-4-hydroxytoluene C9H12O3 2.01 1.54 49 35.48 dimethyl phthalate C10H10O4 3.87 1.49 50 35.94 ethanone, 1-(4-hydroxy-3,5-dimethoxyphenyl)- C10H12O4 0.91 6.54 51 36.37 ethanone, 1-(3-hydroxy-4-methoxyphenyl)- C9H10O3 0.71 52 37.21 2,5-dihydroxy-4-methoxyacetophenone C9H10O4 1.58 53 37.22 4-ethyl-2,6-dimethoxyphenol C10H14O3 2.94 1.62 54 38.78 7-methylnaphthalen-2-ol, me derivative C12H12O 0.49 55 39.25 2,6-dimethoxy-4-propylphenol C11H16O3 2.30 56 42.15 4,5-dimethoxy-2-hydroxyacetophenone C10H12O4 1.65 57 42.57 1-naphthol, 6,7-dimethyl- C12H12O 0.77 58 45.86 hexadecanoic acid, methyl ester C17H34O2 2.93 3.01 59 49.71 methyl stearate C19H38O2 0.76 -
[1] RAGAUSKAS A J, BECKHAM G T, BIDDY M J. Lignin valorization: Improving lignin processing in the biorefinery[J]. Science,2014,344:6185. [2] BISWAS B, KUMAR A A, BISHT Y, SINGH R, KUMAR J, BHASKAR T. Effects of temperature and solvent on hydrothermal liquefaction of Sargassum tenerrimum algae[J]. Bioresour Technol,2017,242:344−350. doi: 10.1016/j.biortech.2017.03.045 [3] XU D, WANG Y, LIN G, GUO S, WANG S, WU Z. Co-hydrothermal liquefaction of microalgae and sewage sludge in subcritical water: Ash effects on bio-oil production[J]. Renewable Energy,2019,138:1143−1151. doi: 10.1016/j.renene.2019.02.020 [4] WEN J L, CHEN T Y, SUN R C. Progress in research methods of biomass lignin separation and structure[J]. J Eng,2017,2(5):76−84. [5] JIN Y, RUAN X, CHENG X, LV Q F. Liquefaction of lignin by polyethyleneglycol and glycerol[J]. Bioresour Technol,2011,102(3):3581−3583. doi: 10.1016/j.biortech.2010.10.050 [6] VANHOLME R, MORREEL K, RALPH J, BOERJAN W. Lignin engineering[J]. Curr Opin Plant Biol,2008,11(3):278−285. doi: 10.1016/j.pbi.2008.03.005 [7] VANHOLME R, DEMEDETS B, MORREEL K, JOHN R, WOUT B. Lignin biosynthesis and structure[J]. Plant Physiol,2010,153(3):895−905. doi: 10.1104/pp.110.155119 [8] RALPH J. Hydroxycinnamates in lignification[J]. Phytochem Rev,2010,9(1):65−83. doi: 10.1007/s11101-009-9141-9 [9] WANG S R, RU B, LIN H Z, SUN W X, LUO Z Y. Pyrolysis behaviors of four lignin polymers isolated from the same pine wood[J]. Bioresour Technol,2015,182:120−127. doi: 10.1016/j.biortech.2015.01.127 [10] GUO Z, JIANG X Y, LIAO Y, ZHANG M, HUANG Y. Fast pyrolysis of Masson Pine lignin and analysis of pyrolysis products[J]. J Cent South Univ Forest Technol,2017,37(6):106−107. [11] 黄明, 朱亮, 马中青, 周秉亮, 刘晓欢, 叶结旺, 赵超. 金属改性分子筛催化热解木质素制取轻质芳烃[J]. 燃料化学学报,2021,49(3):292−302. doi: 10.19906/j.cnki.JFCT.2021021HUANG Ming, ZHU Liang, MA Zhong-qing, ZHOU Bing-liang, LIU Xiao-huan, YE Jie-wang, ZHAO Chao. Production of light aromatics from the fast pyrolysis of lignin catalyzed by metal-modified H-ZSM-5 zeolite[J]. J Fuel Chem Technol,2021,49(3):292−302. doi: 10.19906/j.cnki.JFCT.2021021 [12] CAPRARIIS D, BENEDETTA, FILIPPIS D, FILIPPIS, PAOLO, PETRULLO A, SCARSELLA M. Hydrothermal liquefaction of biomass: Influence of temperature and biomass composition on the bio-oil production[J]. Fuel,2017,208:618−625. doi: 10.1016/j.fuel.2017.07.054 [13] CAO Y, GAO J, ZHANG C, DANIEL C W T, FAN J, JAMES H C, LUO G, ZHU X D, ZHANG S C. Fast and selective production of aromatics via efficient lignin depolymerization: Critical factors and mechanism studies[J]. ACS Sustainable Chem Eng,2022,10(46):15273−15283. doi: 10.1021/acssuschemeng.2c05018 [14] BARBIER J, CHARON N, DUPASSIEUX N. Hydrothermal conversion of lignin compounds: A detailed study of fragmentation and condensation reaction pathways[J]. Biomass Bioenergy,2012,46:479−491. doi: 10.1016/j.biombioe.2012.07.011 [15] SHEEHAN J D, SAVAGE P E. Modeling the effects of microalga biochemical content on the kinetics and biocrude yields from hydrothermal liquefaction[J]. Bioresour Technol,2017,239:144−150. doi: 10.1016/j.biortech.2017.05.013 [16] ZHU Z, ROSENDAHL L, TOOR S S, YU D, CHEN G. Hydrothermal liquefaction of barley straw to bio-crude oil: Effects of reaction temperature and aqueous phase recirculation[J]. Appl Energy,2015,137(jan.1):183−192. [17] DURAK H, AYSU T. Structural analysis of bio-oils from subcritical and supercritical hydrothermal liquefaction of Datura stramonium L[J]. J Supercrit Fluid,2016,108:123−135. doi: 10.1016/j.supflu.2015.10.016 [18] KIM S J, UM B H. Biocude production from Korean native kenaf through suberitical hydrothermal liquefaction under mild alkaline catalytic conditions[J]. Ind Crop Prod,2020,145(1):1−7. [19] PIŃKOWSKA H, WOLAK P, ZŁOCIŃSKA A. Hydrothermal decomposition of alkali lignin in sub-and supercritical water[J]. Chem Eng J,2012,187:410−414. doi: 10.1016/j.cej.2012.01.092 [20] YANG T H, WU K Y, LI B S, DU C Z, WANG J, LI R D. Conversion of lignin into phenolic-rich oil by two-step liquefaction in sub-supercritical ethanol system assisted by carbon dioxide[J]. J Energy Inst,2021,94:329−336. doi: 10.1016/j.joei.2020.10.001 [21] LI B S, YANG T H, LI R D, KAI X P. Co-generation of liquid biofuels from lignocellulose by integrated biochemical and hydrothermal liquefaction process[J]. Energy,2020,200:117524. [22] LIN X H, CHEN L H, LI H Y, LV Y C, LIU Y F. Mild depolymerization of the sinocalamus oldhami alkali lignin to phenolic monomer with base and activated carbon supported nickel-tungsten carbide catalyst composite system[J]. Bioresour Technol,2021,333:125136. doi: 10.1016/j.biortech.2021.125136 [23] CHAUDHARY R, DHEPE P L. Depolymerization of lignin using a solid base catalyst[J]. Energy Fuels,2019,33(MAY):4369−4377. [24] FENG X B, CAO J P, ZHAO X Y, SONG C, LIU T L, WANG J X, FAN X, WEI X Y. Organic oxygen transformation during pyrolysis of Baiyinhua lignite[J]. J Anal Appl Pyrolysis,2016,117:106−115. [25] 徐昊, 王冠宇, 李允超, 陆凯锋, 王树荣. 生物质液相解聚残渣热解行为对比研究[J]. 工程热物理学报,2021,42(12):3045−3053.XU Hao, WANG Guan-yu, LI Yun-chao, LU Kai-feng, WANG Shu-rong. Comparative study on pyrolysis behavior of biomass liquid depolymerization residues[J]. J Eng Thermophys,2021,42(12):3045−3053. [26] 刘素敏, 杨海平, 胡俊豪, 邹俊, 陈汉平, 王晨光. 典型木质素气化动力学及产物析出特性[J]. 燃料化学学报,2022,50(4):428−435.LIU Su-min, YANG Hai-ping, HU Jun-hao, ZOU Jun, CHEN Han-ping, WANG Chen-guang. Study on gasification kinetics and product characteristics of typical lignin[J]. J Fuel Chem Technol,2022,50(4):428−435. [27] 叶俊, 李广学, 周博涵, 曹从伟, 杨和彦, 李家鸣. ZnCl2催化木质素磺酸盐加氢液化的溶剂效应[J]. 燃料化学学报,2012,40(3):321−325. doi: 10.3969/j.issn.0253-2409.2012.03.011YE Jun, LI Guang-xue, ZHOU Bo-han, CAO Cong-wei, YANG He-yan, LI Jia-ming. Solvent effect of lignosulfonate liquefaction by ZnCl2 catalyzed hydrogenation[J]. J Fuel Chem Technol,2012,40(3):321−325. doi: 10.3969/j.issn.0253-2409.2012.03.011 [28] MA Z Q, WANG J H, ZHOU H Z. Relationship of thermal degradation behavior and chemical structure of lignin isolated from palm kernel shell under different process severities[J]. Fuel Process Technol,2018,181:142−156. doi: 10.1016/j.fuproc.2018.09.020 [29] WANG S R, LIN H Z, R B, SUN W X, WANG Y R, LUO Z Y. Comparison of the pyrolysis behavior of pyrolytic lignin and milled wood lignin by using TG-FTIR analysis[J]. J Anal Appl Pyrolysis, 2014, 2014, 108(-): 78–85. [30] ZHANG B, CHEN J X, KANDASAMY S, HE Z X. Hydrothermal liquefaction of fresh lemon-peel and Spirulina platensis blending-operation parameter and biocrude chemistry investigation[J]. Energy,2020,193:116645. doi: 10.1016/j.energy.2019.116645 [31] 郭大亮, 王林芳, 郭惠萍, 陈梦薇, 薛国新, 武书彬. 结合态与无机态钠对木质素半焦气化特性的影响[J]. 农业机械学报,2017,48(3):332−337. doi: 10.6041/j.issn.1000-1298.2017.03.042GUO Da-liang, WANG Lin-fang, GUO Hui-ping, CHEN Meng-wei, XUE Guo-xin, WU Shu-bin. Influence of inorganic and organic bound Na on char gasification characteristics of lignin[J]. Trans Chin Soc Agric Mach,2017,48(3):332−337. doi: 10.6041/j.issn.1000-1298.2017.03.042 [32] LIU Z C, WANG Z W, GAO S, TONG Y X, LE X, HU N W, YAN Q S, ZHOU X G, HE Y R, WANG L. Isolation and Fractionation of the Tobacco Stalk Lignin for Customized Value-Added Utilization[J]. Front Bioeng Biotechnol,2021,9:811287. [33] FARAVELLI T, FRASSOLDATI A, MIGLIAVACCA G, RANZI E. Detailed kinetic modeling of the thermal degradation of lignins[J]. Biomass Bioenergy,2010,34(3):290−301. doi: 10.1016/j.biombioe.2009.10.018 [34] KRUSE A, DINJUS E. Hot compressed water as reaction medium and reactant 2. Degradation reactions[J]. J Supercrit Fluid,2007,41(3):361−379. doi: 10.1016/j.supflu.2006.12.006 [35] YANG J, HE Q, CORSCADDEN K. Advanced models for the prediction of product yield in hydrothermal liquefaction via a mixture design of biomass model components coupled with process variables[J]. Appl Energy, 2019, 233–234: 906–915. [36] YANG T H, WANG J, LI B S, KAI X P, XING W L. Behaviors of rice straw two-step liquefaction with sub/supercritical ethanol in carbon dioxide atmosphere[J]. Bioresour Technol,2018,258:287−294. doi: 10.1016/j.biortech.2018.02.099 [37] LI B S, LIU Y X, LI R D, YANG T H, KAI X P. Aluminum-water reactions assisted in situ hydrodeoxygenation of enzymolysis lignin from bioconversion of rice straw over NiMo catalyst[J]. Ind Crop Prod,2020,154:112727. doi: 10.1016/j.indcrop.2020.112727 [38] CHEN P, ZHANG Q, SHU R, XU Y, MA L L. Catalytic depolymerization of the hydrolyzed lignin over mesoporous catalysts[J]. Bioresour Technol,2017,226(1):125−131. [39] ABOULKAS A, HAMMANI H, ACHABY M EL, BILAL E, BARAKAT A, HARFI K EL. Valorization of algal waste via pyrolysis in a fixed-bed reactor: production and characterization of bio-oil and bio-char[J]. Bioresour Technol,2017,243:400−408. doi: 10.1016/j.biortech.2017.06.098 [40] KUMAR B, BHARDWAJ N, AGRAWAL K, CHATURVEDI V, VERMA P. Current perspective on pretreatment technologies using lignocellulosic biomass: An emerging biorefinery concept[J]. Fuel Process Technol,2020,199:106244. doi: 10.1016/j.fuproc.2019.106244 -





 下载:
下载: