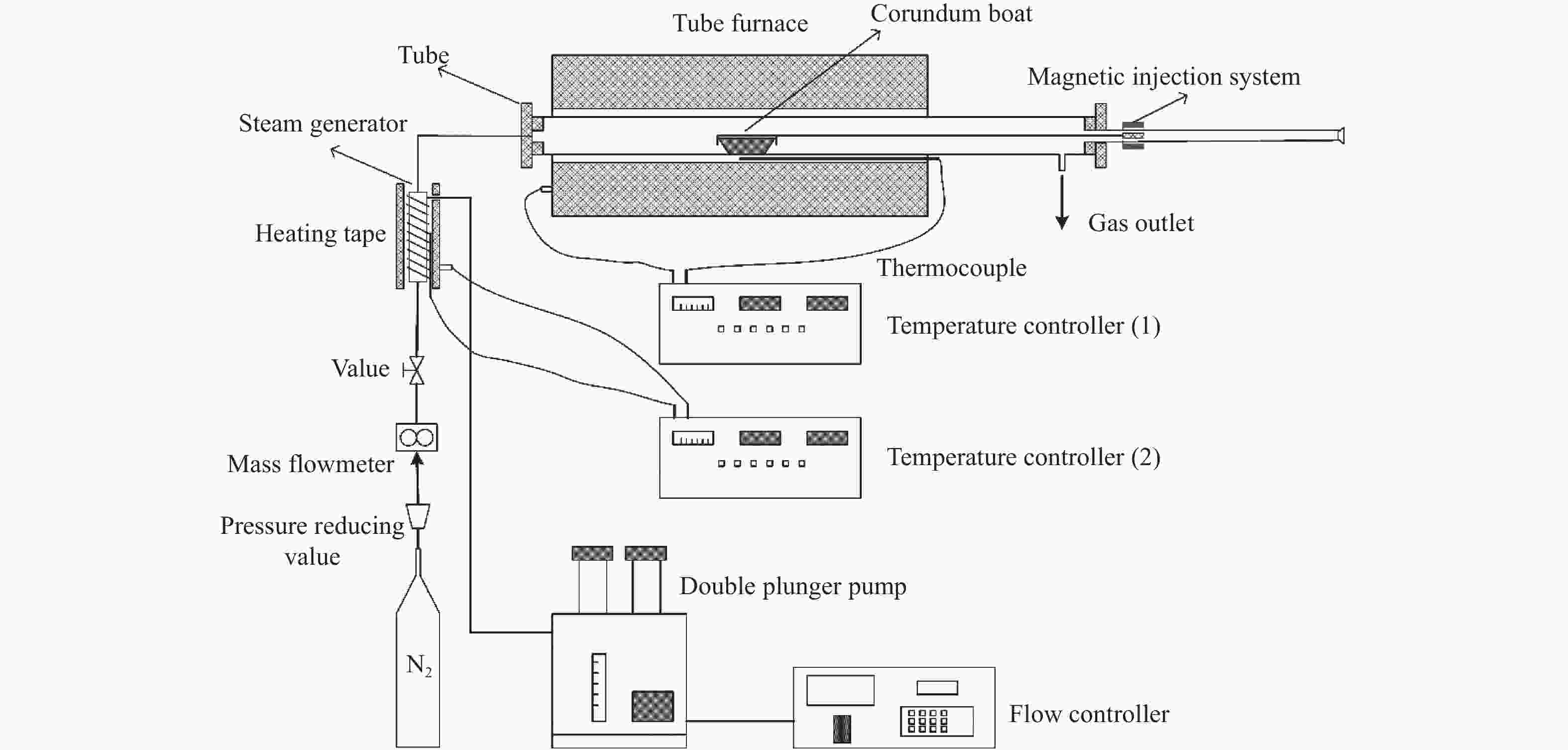
Effect of operating conditions on release and transformation of sodium during CFB gasification of Zhundong coal
-
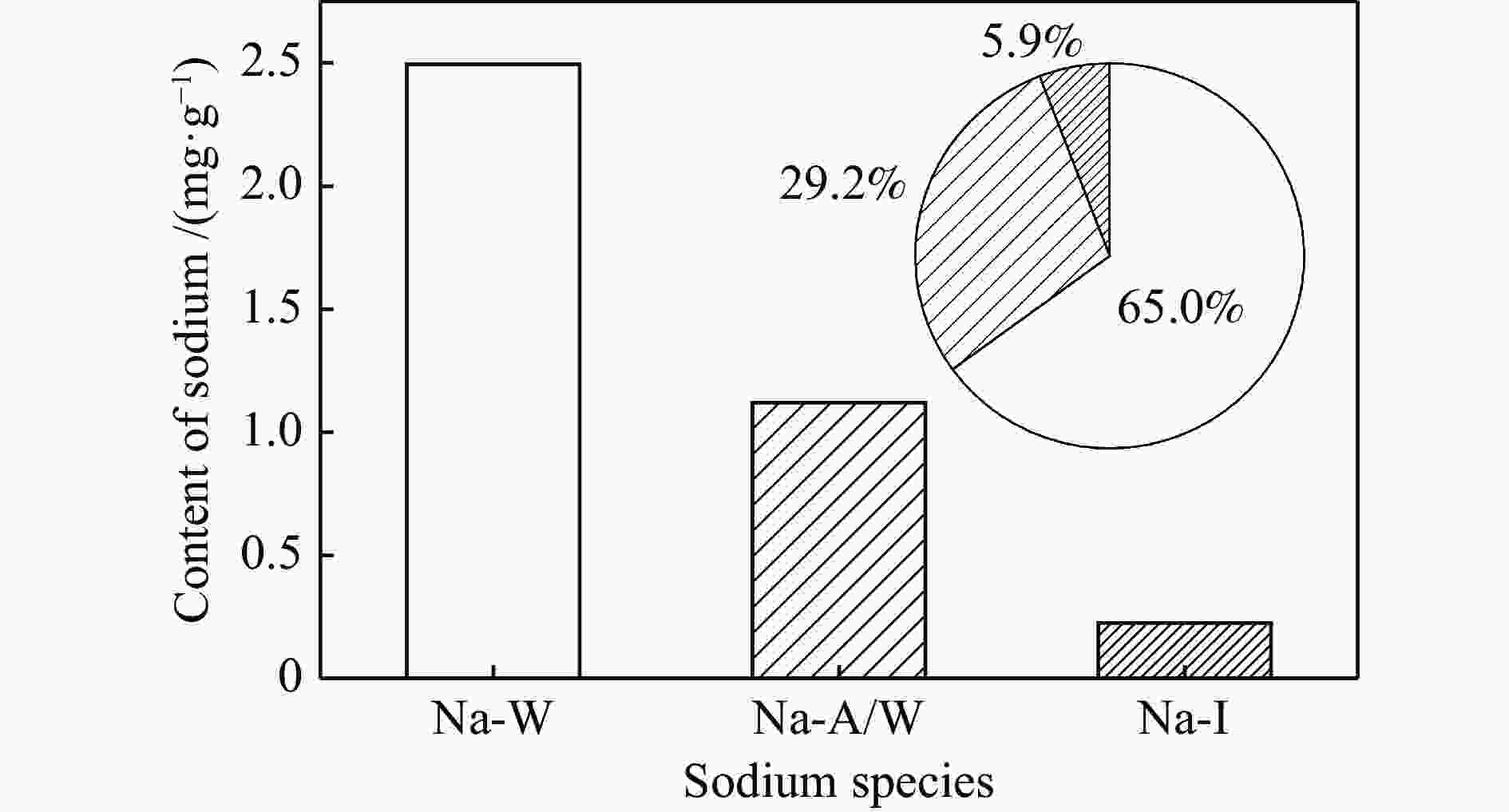
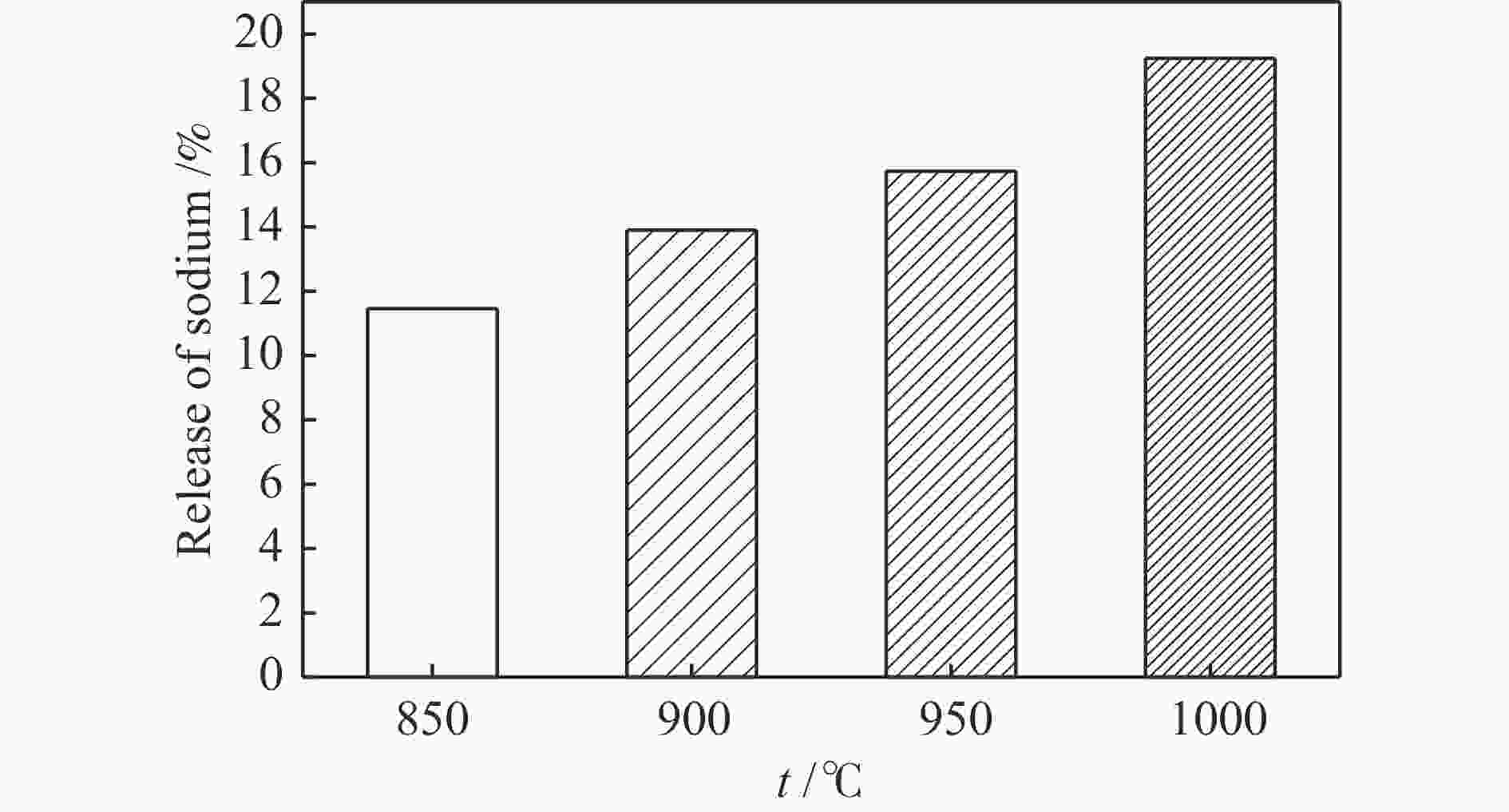
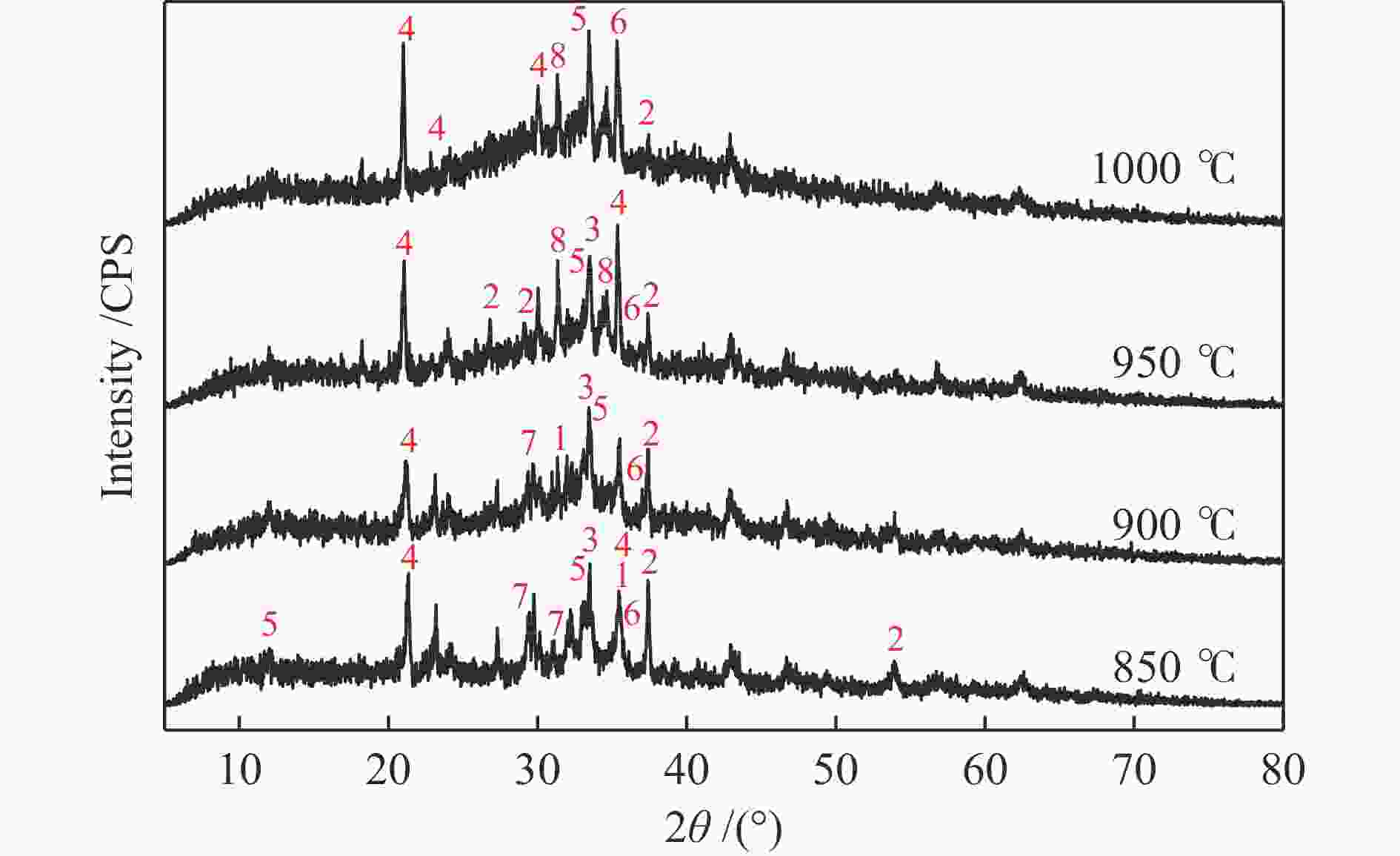
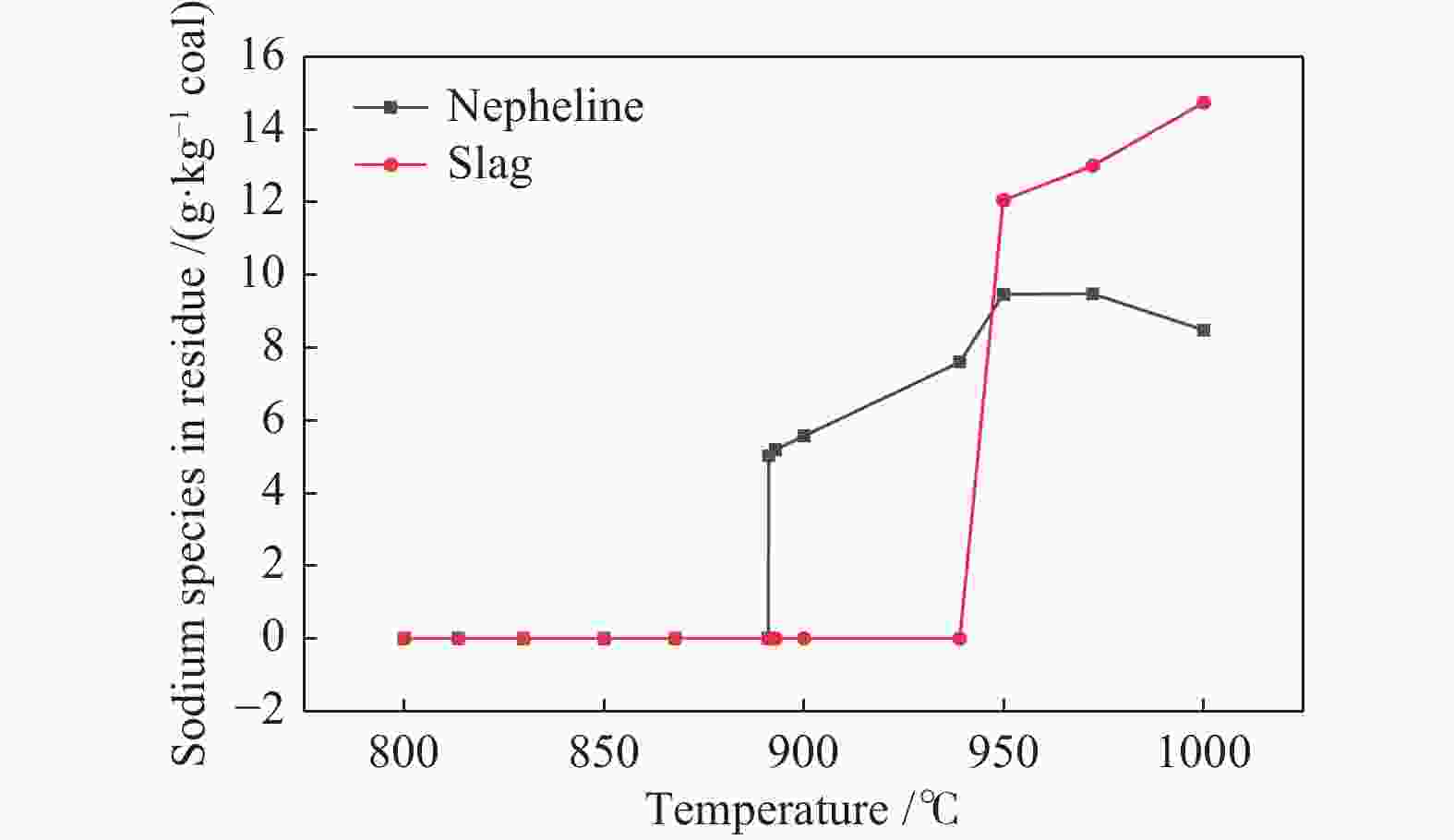
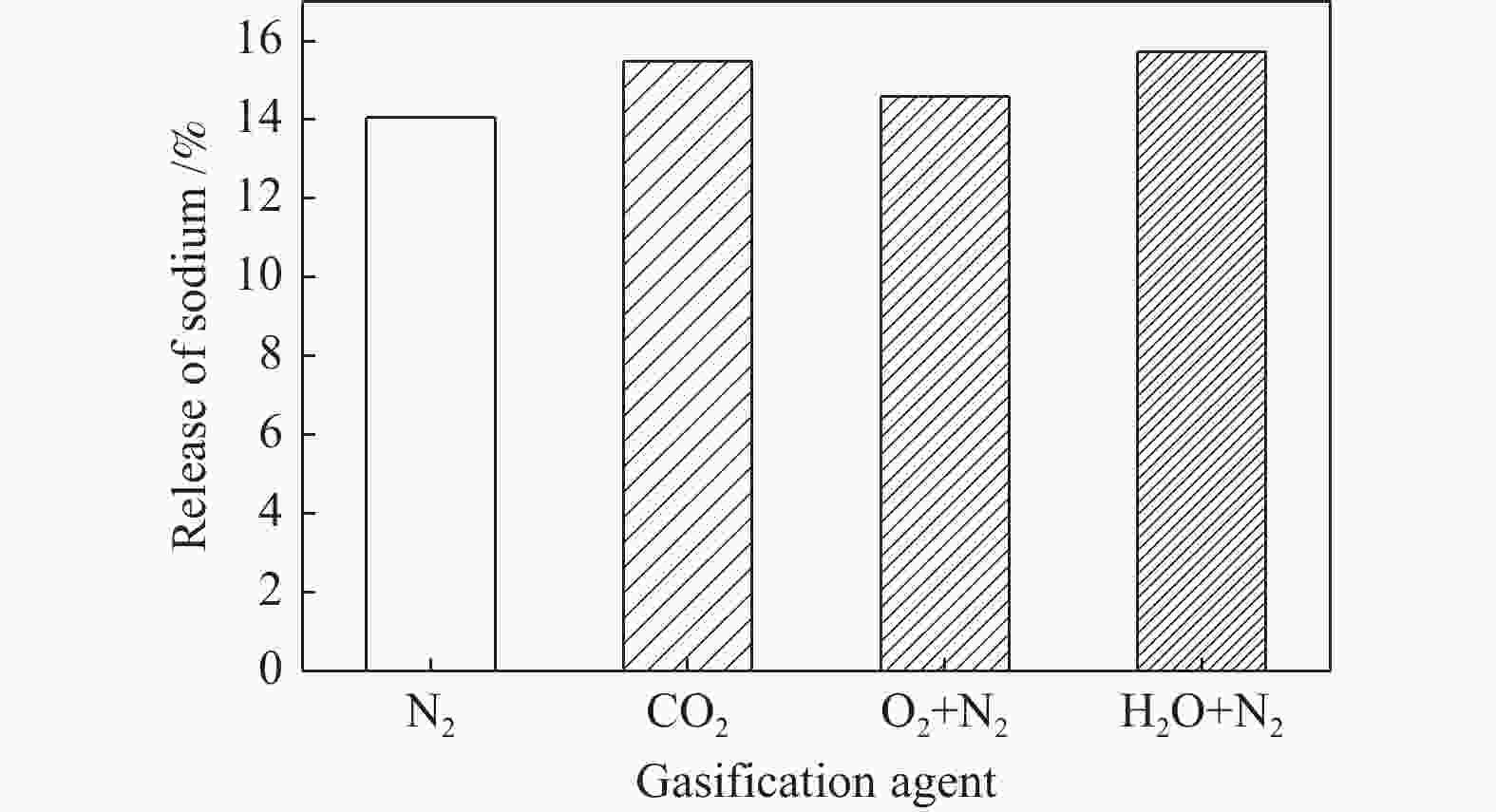
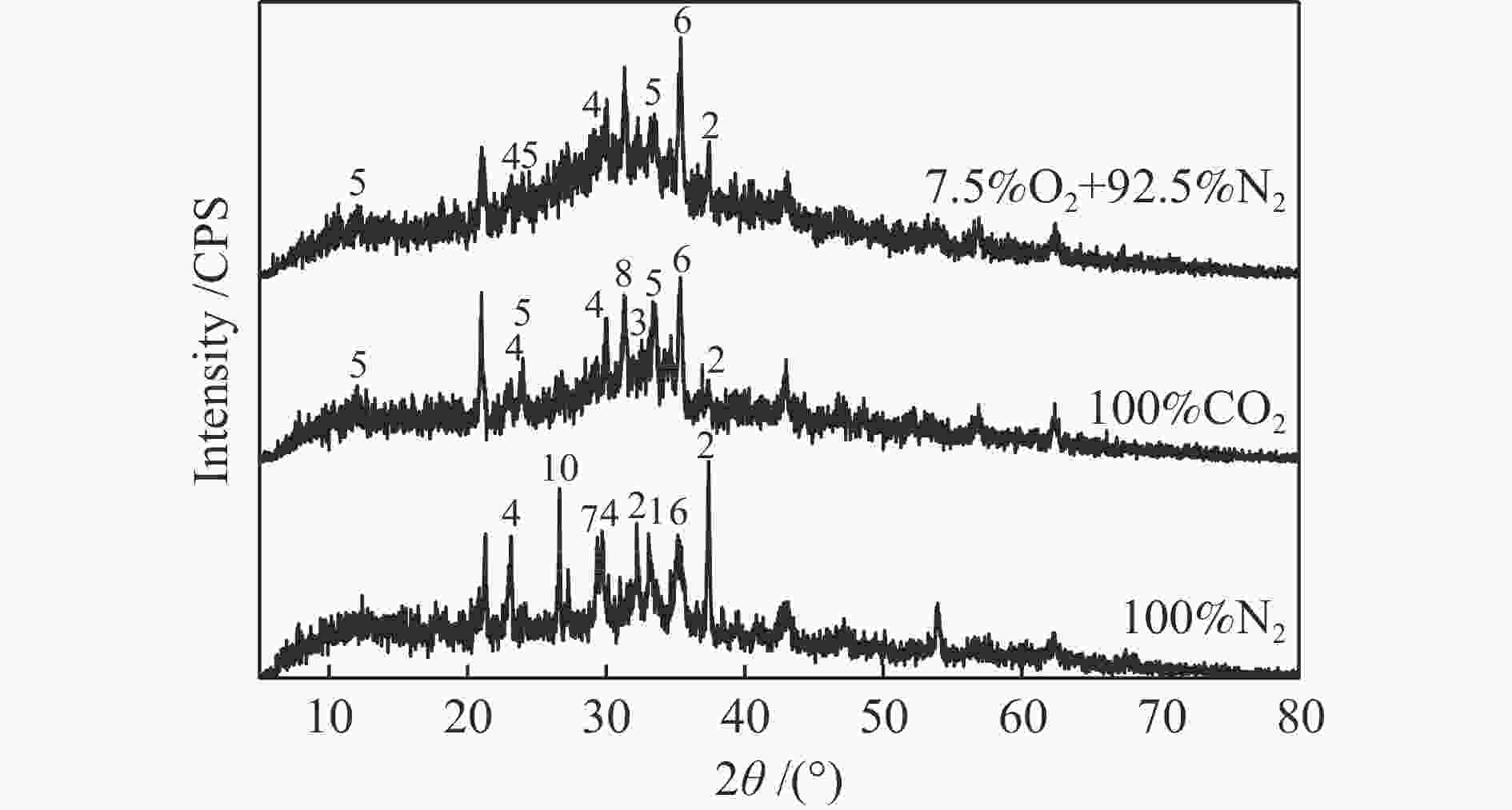
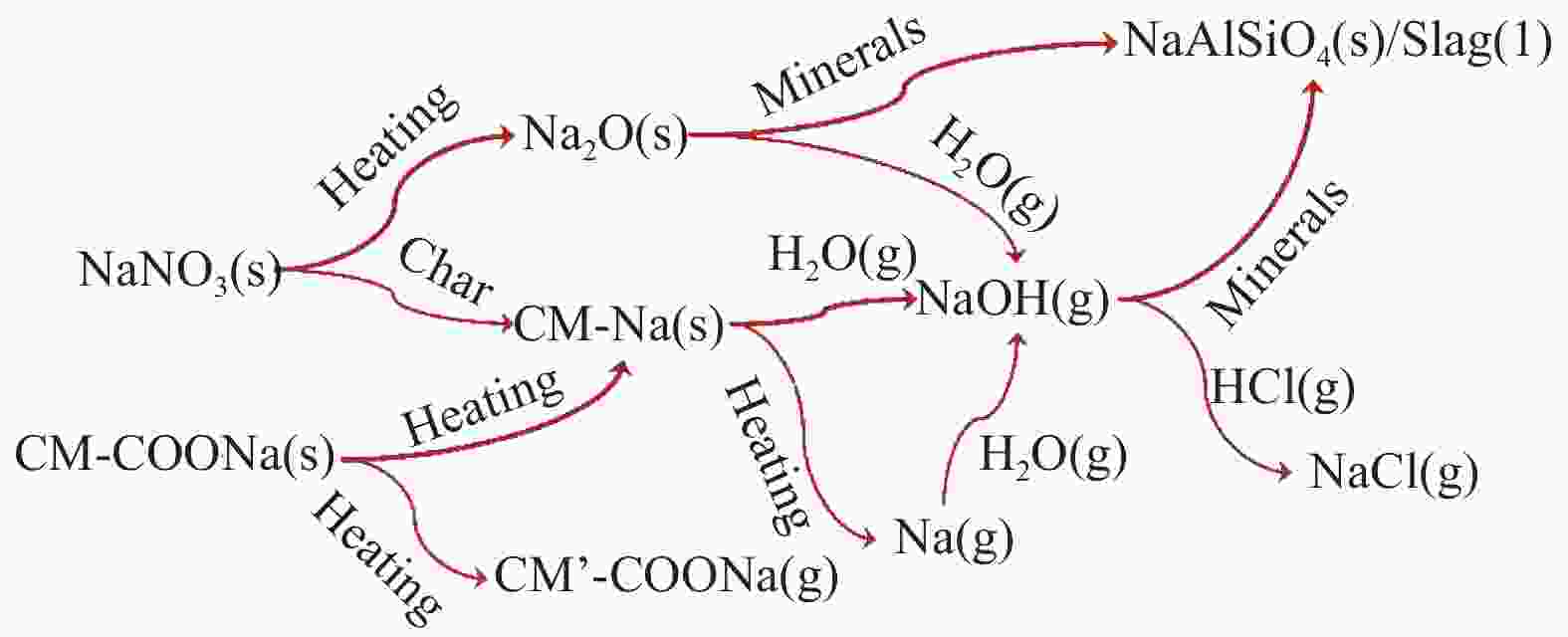
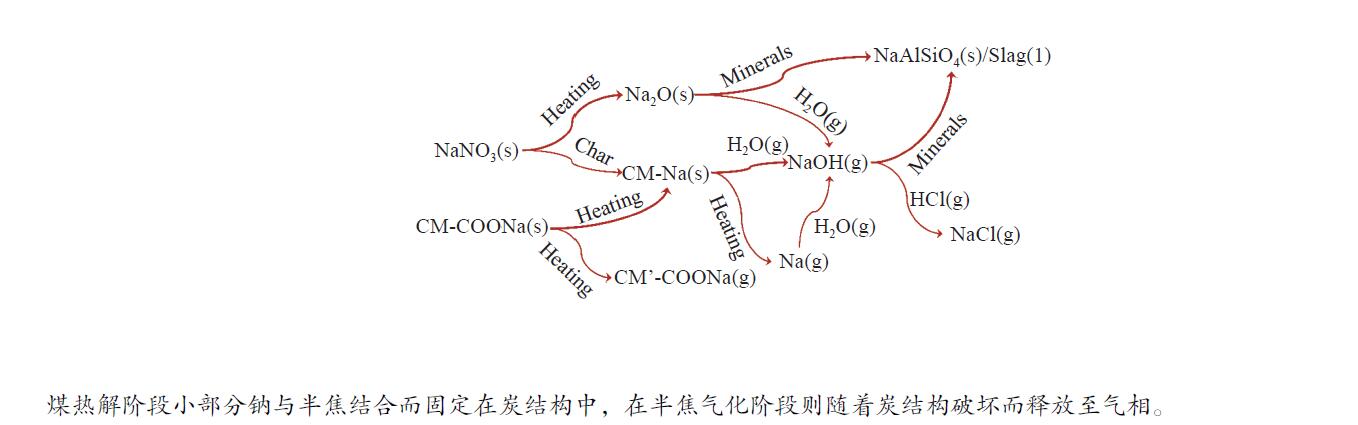
摘要: 为了指导CFB气化炉运行,研究了气化温度、停留时间及气化剂类型对准东煤中钠的挥发和转化的影响,相关研究依托固定床反应器开展,并结合Factsage软件模拟计算。实验结果表明,气化温度是钠挥发与转化的显著性影响因素。温度对钠挥发的促进作用归因于挥发的强化及石灰/偏高岭土的竞争反应。同时,高温可以促进钠霞石和熔渣的形成。熔渣形成的阈值大约950 ℃。气化过程中钠的挥发可以归结为两个阶段:煤热解及半焦气化。在煤热解阶段,部分有机态和水溶性钠被挥发,剩余的钠与煤焦或矿物质结合;在半焦气化阶段,与半焦结合态钠(CM-Na)随着煤焦气化而挥发。由于NaOH形成及熔融温度降低,水蒸气对钠的挥发呈现促进作用;相反,氧气和氮气则表现出抑制作用。前者归因于难挥发Na2SO4形成,而后者是因为煤焦的化学成键和物理束缚。Abstract: To provide some useful suggestions to the operation of circulating fluidized bed (CFB) gasifier, the effect of gasification temperature, residence time and agent on the release and transformation of sodium was studied by using a fixed bed reactor combined with Factsage software. The results indicated that gasification temperature was the significant factor to the release and transformation of sodium. For the promoting effect of sodium release, it was ascribed to the intense of sodium volatilization and competitive reaction between lime and meta-kaolin. Meanwhile, the high temperature promoted the formation of nepheline and slag. The threshold temperature of latter was near 950 °C. It was interesting to find that the release of sodium could be divided into two stages: coal pyrolysis and char gasification. In coal pyrolysis, part of organic and water-soluble sodium was released. The remainder either combined with char structure, or reacted with minerals. In char gasification, Sodium, combined with char structure, was released along with char gasification. Due to the decrease of melting temperature and the formation of NaOH, steam showed a promoting effect on the sodium release. Oppositely, oxygen and nitrogen presented an inhibiting effect. The former was ascribed to the formation of Na2SO4, while the latter was caused by the chemical binding and physical wrapping effect of char.
-
Key words:
- Zhundong coal /
- gasification /
- sodium /
- release and transformation
-
Table 1 Proximate and ultimate analysis
Proximate analysis w/% Ultimate analysis wdaf/% Mad Ad Vdaf C H Oa N St 10.65 3.82 28.93 82.06 3.47 13.25 0.87 0.35 ad: air-dried base; d: dry base; daf: dry and ash free base; a: by difference Table 2 Ash chemical compositions
Content w/% SiO2 Al2O3 Fe2O3 CaO MgO TiO2 SO3 K2O Na2O P2O5 27.02 13.97 13.10 20.28 4.61 0.63 7.94 0.20 12.21 0.05 -
[1] QI X B, SONG G L, SONG W J, LV Q G. Experimental study on alkali metal migration and slagging characteristics during ZD overalkali coal gasification[J]. J Fuel Chem Technol,2015,43(8):906−913. [2] LIU D H, ZHANG S Y, TU S K, JIN T, SHI D Y, PEI Y F. Morphological changes of sodium in Wucaiwan coal during pyrolysis[J]. J Fuel Chem Technol,2014,42(10):1190−1196. [3] HAN K X, HUANG Z Y, WANG Z H, ZHOU J H. Study on the mechanism of Na-based compounds in ZD coal ash sintering and melting process[J]. J Fuel Chem Technol,2015,43(1):22−26. [4] LI L M. Design and application of 350MW supercritical boiler burning Xinjiang ZD coal[J]. Power Stat Syst Eng,2014,30(2):39−41. [5] YANG Z C, LIU J L, HE H G. Study on the characteristics of coal in ZD, Xinjiang and its boiler selection[J]. Therm Power Gener,2010,39(8):38−40. [6] WANG C A, JIN X, WANG Y, YAN Y, CUI J, LIU Y, CHE D. [J]. Release and transformation of sodium during pyrolysis of ZD coals[J]. Energy Fuels,2014,29:78−85. [7] SONG W J, SONG G L, ZHANG H X, FAN J L, LV Q G. Experimental study on the migration characteristics of sodium during pyrolysis of ZD high-sodium coal[J]. J Fuel Chem Technol,2015,43(1):16−21. [8] YANG S B, SONG G L, SONG W J, QI X B. Sodium migration, transformation and fouling characteristics in ZD high-sodium coal under different reaction atmospheres[J]. J Fuel Chem Technol,2016,44(9):1051−1058. [9] LI W, WANG L, QIAO Y, LIN J Y, WANG M, CHANG L. Effect of atmosphere on the release behavior of alkali and alkaline earth metals during coal oxy-fuel combustion[J]. Fuel,2015,139:164−170. doi: 10.1016/j.fuel.2014.08.056 [10] LI C Z, SATHE C, KERSHAW J R, PANG Y. Fates and roles of alkali and alkaline earth metals during the pyrolysis of a Victorian brown coal[J]. Fuel,2000,79(3):427−438. [11] ZHANG H, GUO X, ZHU Z. Effect of temperature on gasification performance and sodium transformation of ZD coal[J]. Fuel,2017,189:301−311. doi: 10.1016/j.fuel.2016.10.097 [12] SONG G, SONG W, QI X, LU Q. Transformation characteristics of sodium of ZD coal combustion/gasification in circulating fluidized bed[J]. Energy Fuels,2016,30(4):3473−3478. [13] WEI Y, LI H, HONMA K, TANOSAKI T, NINOMIYA Y, KAWAGUCHI M, TATARAZAKO N. Effect of additives on slag properties in an entrained bed gasifier[C]//World of Coal Ash Conference. 2011: 9−12. [14] BLÄSING M, MÜLLER M. Mass spectrometric investigations on the release of inorganic species during gasification and combustion of Rhenish lignite[J]. Fuel,2010,89(9):2417−2424. doi: 10.1016/j.fuel.2009.11.042 [15] BLÄSING M, MELCHIOR T, MÜLLER M. Influence of temperature on the release of inorganic species during high temperature gasification of Rhenish lignite[J]. Fuel Process Technol,2011,92(3):511−516. doi: 10.1016/j.fuproc.2010.11.005 [16] VAN E P J, ASHMAN P J, ALWAHABI Z T, NATHAN G J. The release of water-bound and organic sodium from Loy Yang coal during the combustion of single particles in a flat flame[J]. Combust Flame,2011,158(6):1181−1192. doi: 10.1016/j.combustflame.2010.10.024 [17] LI R, CHEN Q, ZHANG H. Detailed investigation on sodium (Na) species release and transformation mechanism during pyrolysis and char gasification of high-Na ZD coal[J]. Energy Fuels,2017,31(6):5902−12. [18] ZHANG C X, SU S, CHEN Y F, LIU T, YIN Z J, WANG Z H, WANG Y, HU S, ZHAO Z G, XIANG J. Study on the effects of steam on the precipitation characteristics of sodium during coal thermal conversion[J]. J Fuel Chem Technol,2020,48(7):769−775. doi: 10.1016/S1872-5813(20)30055-4 [19] WANG L, MAO H, WANG Z, LIN J Y, WANG M, CHANG L. Transformation of alkali and alkaline-earth metals during coal oxy-fuel combustion in the presence of SO2 and H2O[J]. J Energy Chem,2015,24(4):381−387. doi: 10.1016/j.jechem.2015.07.006 [20] KOSMINSKI A, ROSS D P, AGNEW J B. Influence of gas environment on reactions between sodium and silicon minerals during gasification of low-rank coal[J]. Fuel Process Technol,2006,87(11):953−962. doi: 10.1016/j.fuproc.2005.06.005 [21] BLÄSING M, MÜLLER M. Mass spectrometric investigations on the release of inorganic species during gasification and combustion of German hard coals[J]. Combust Flame,2010,157(7):1374−1381. doi: 10.1016/j.combustflame.2010.01.003 [22] GUO S, JIANG Y, LI J, YU Z, ZHAO J, FANG Y. Correlations between coal compositions and sodium release during steam gasification of sodium-rich coals[J]. Energy Fuels,2017,31(6):6025−6033. [23] LI P, ZHU C C, HAN L, LI X, FENG X B, YAO Q, YU S, MENG X L, WANG P, WEI S. Char structure evolution and behaviors of sodium species during catalytic gasification of sodium-rich direct coal liquefaction residue under CO2 atmosphere[J]. J Fuel Chem Technol, 2023, 51(5): 598−607. [24] MEI Y G, WANG Z Q, ZHANG H, ZHANG S J, FANG Y T. In-situ study of effect of migrating alkali metals on gasification reactivity of coal char[J]. J Fuel Chem Technol,2021,49(6):735−741. doi: 10.1016/S1872-5813(21)60031-2 -





 下载:
下载: