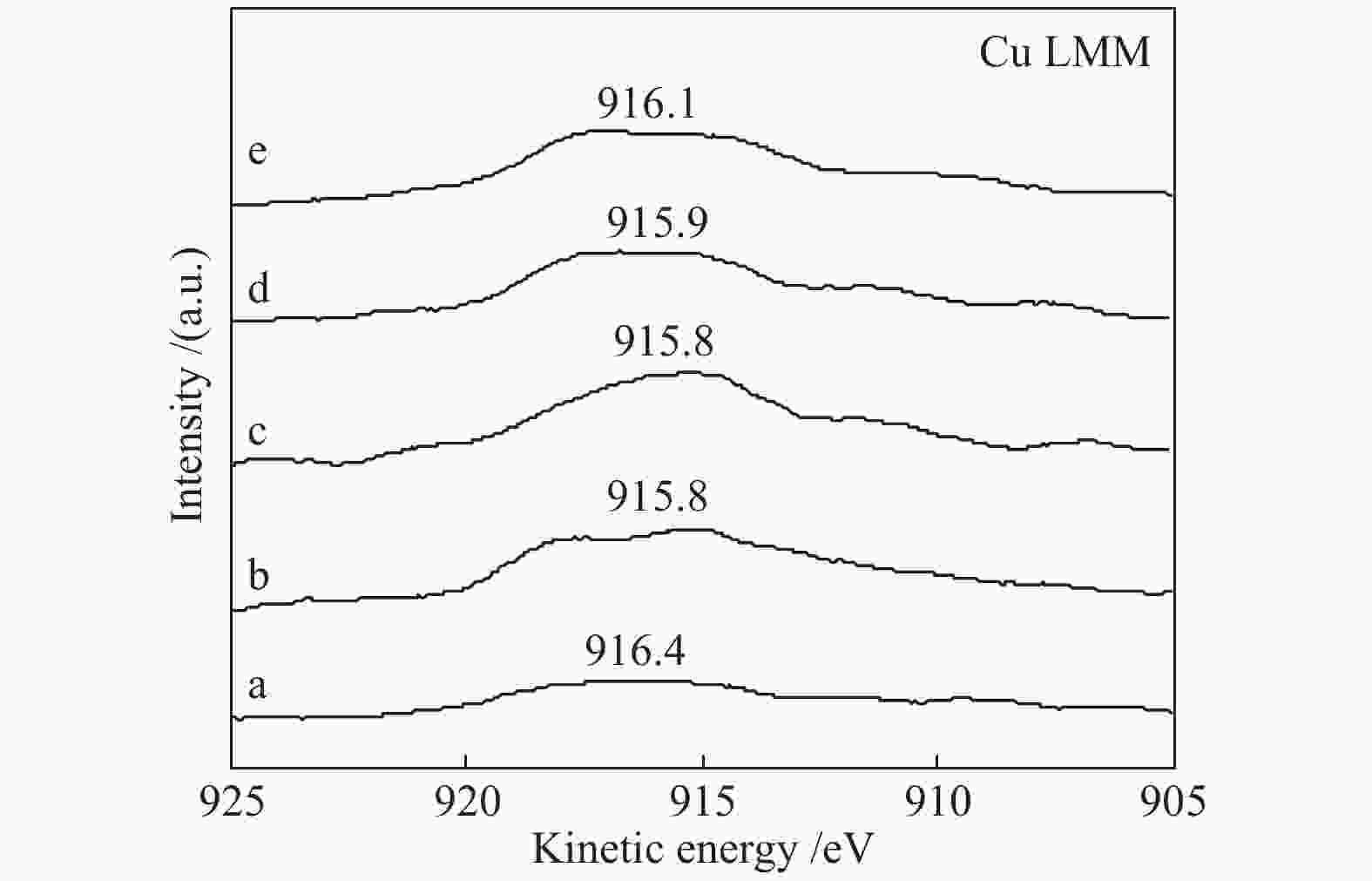
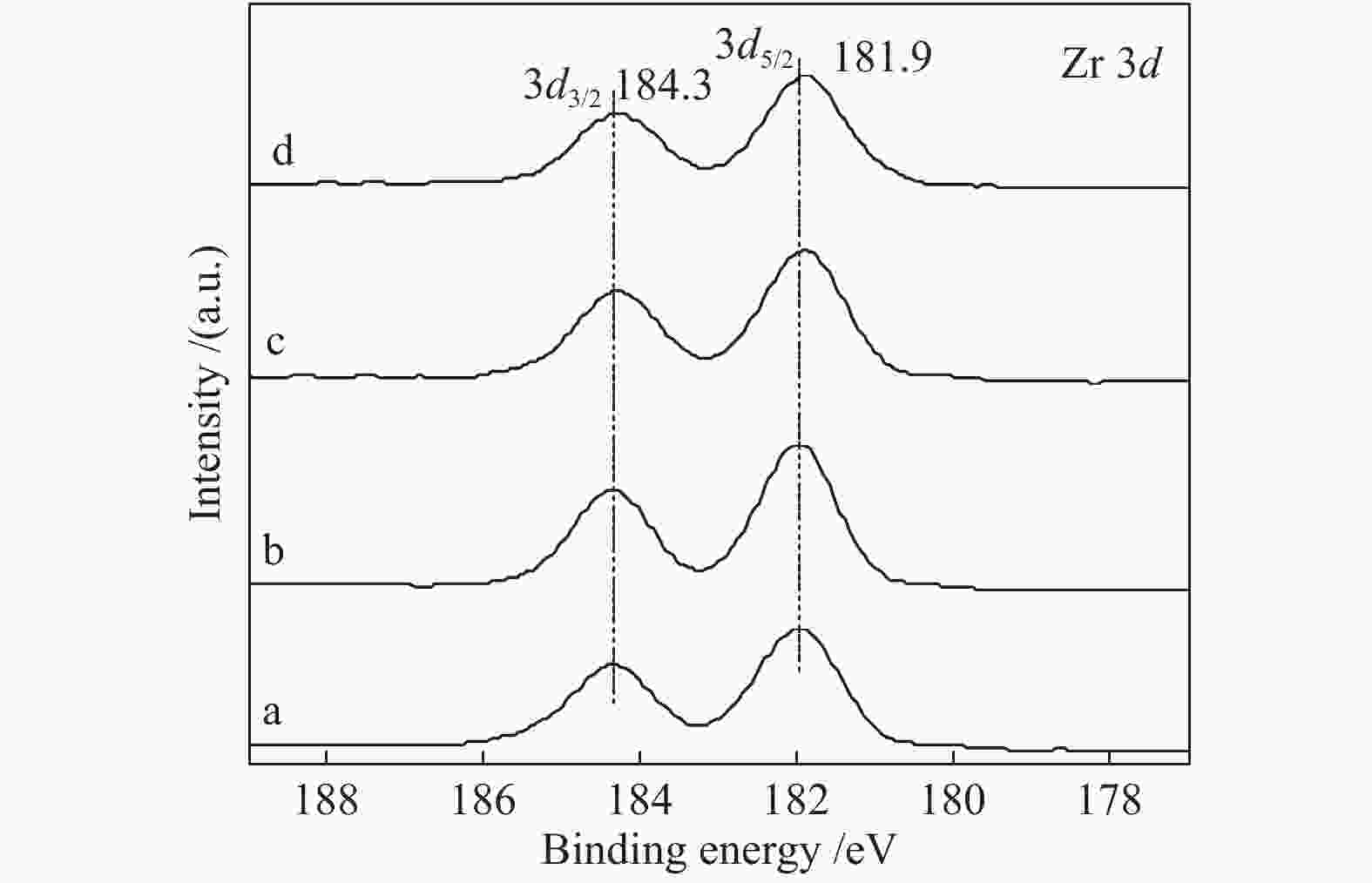
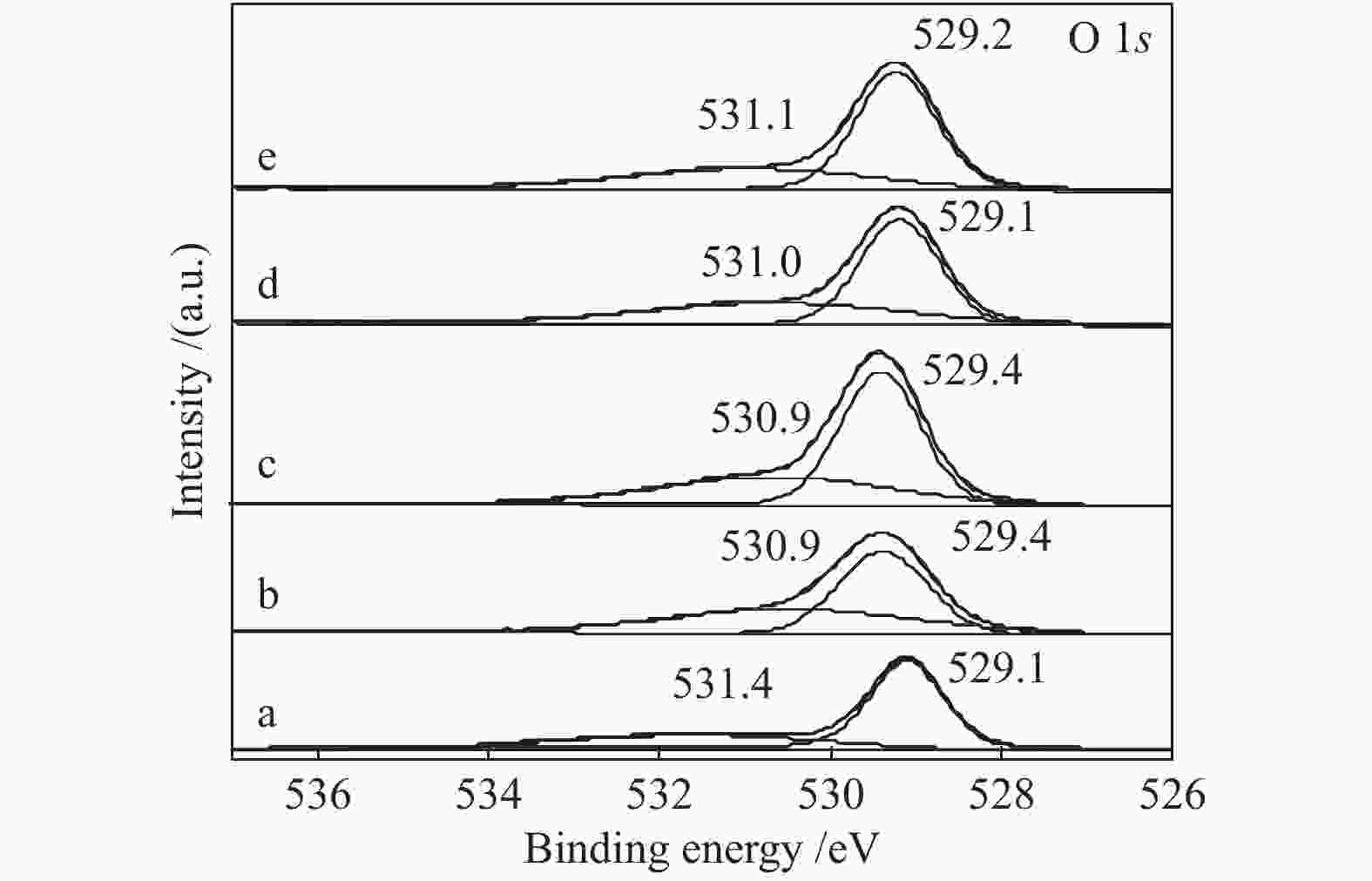
Effect of citric acid content on the hydrothermal synthesis of CuO/Ce0.8Zr0.2O2 catalytic water gas shift hydrogen production performance
-
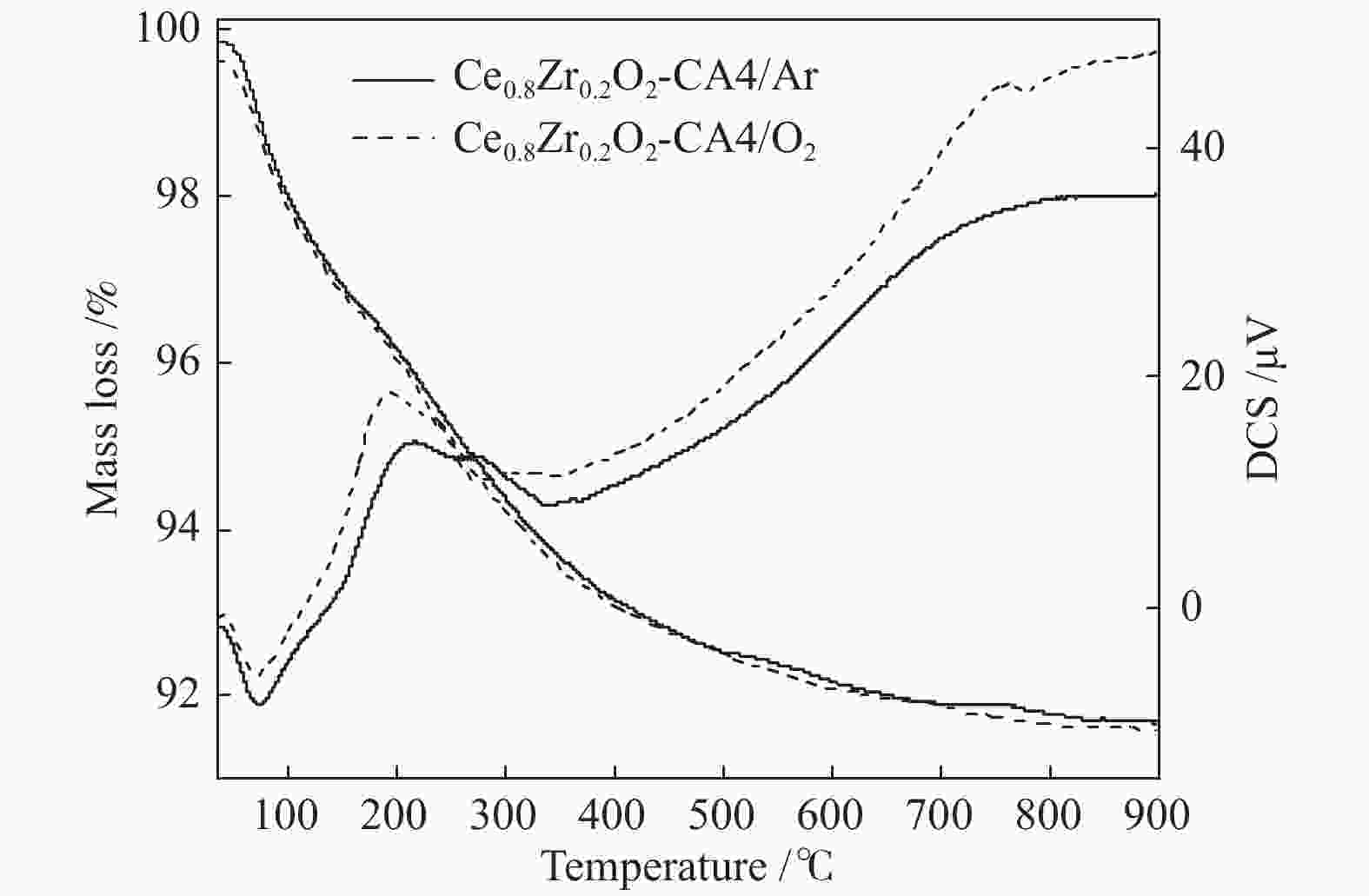
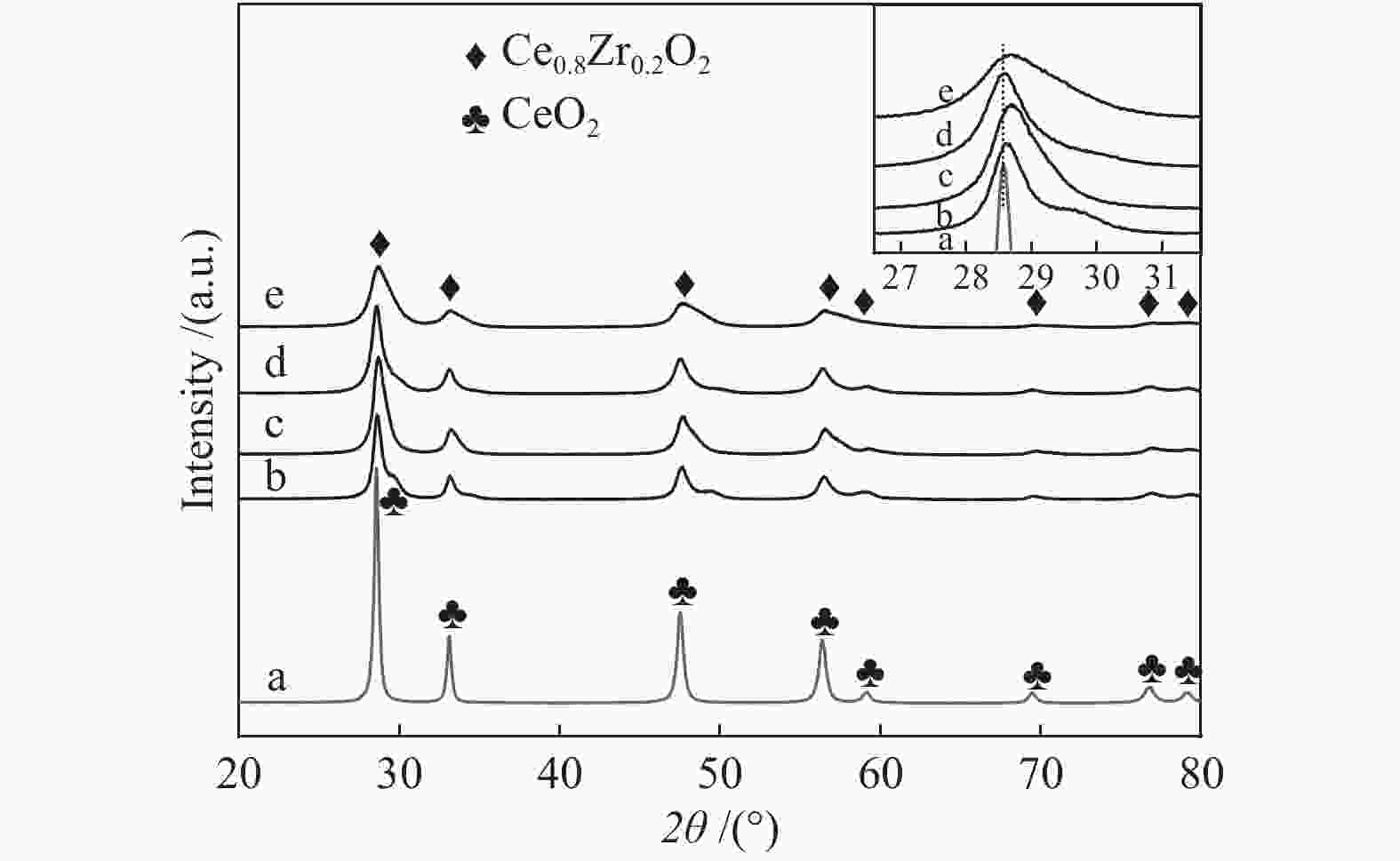
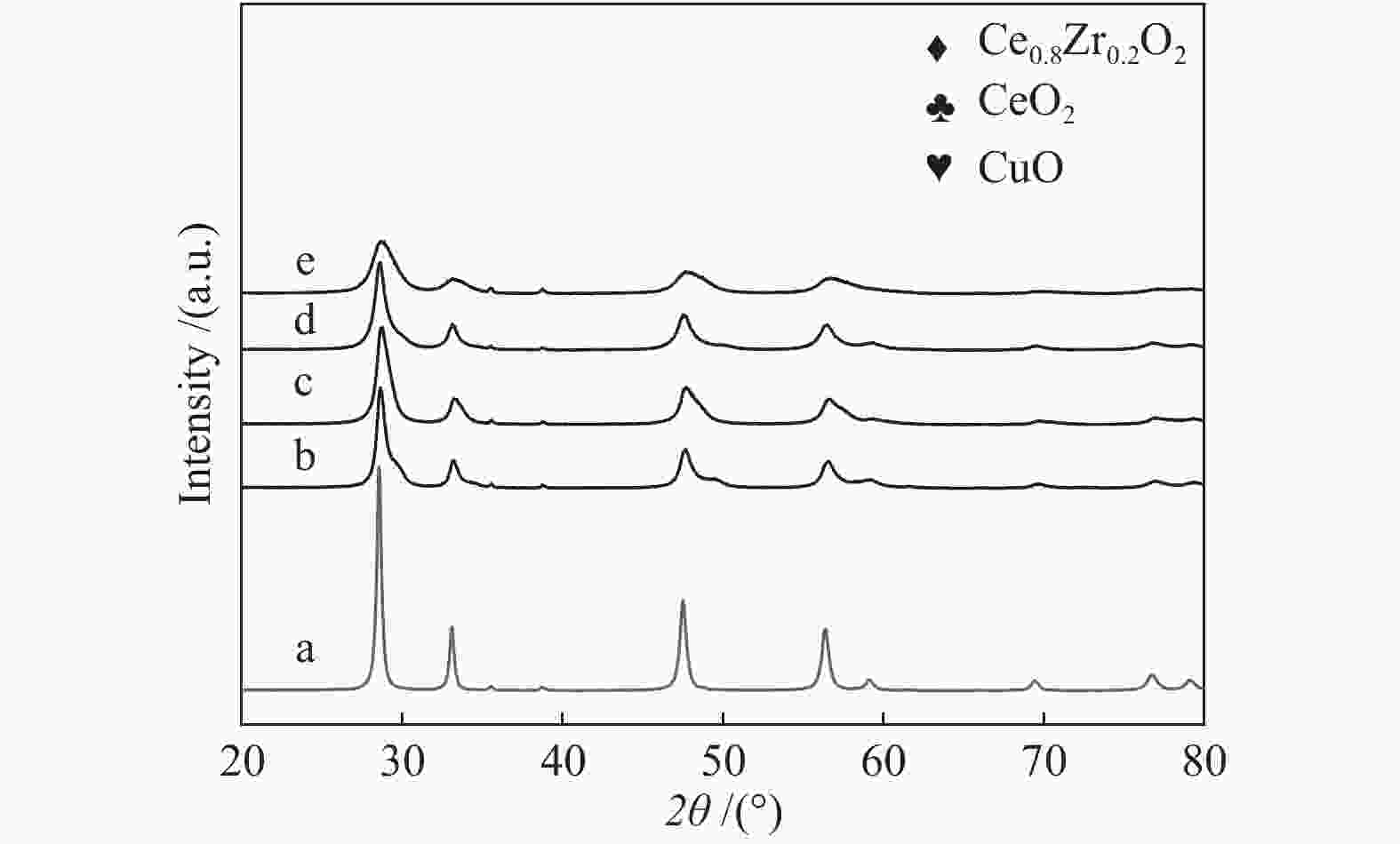
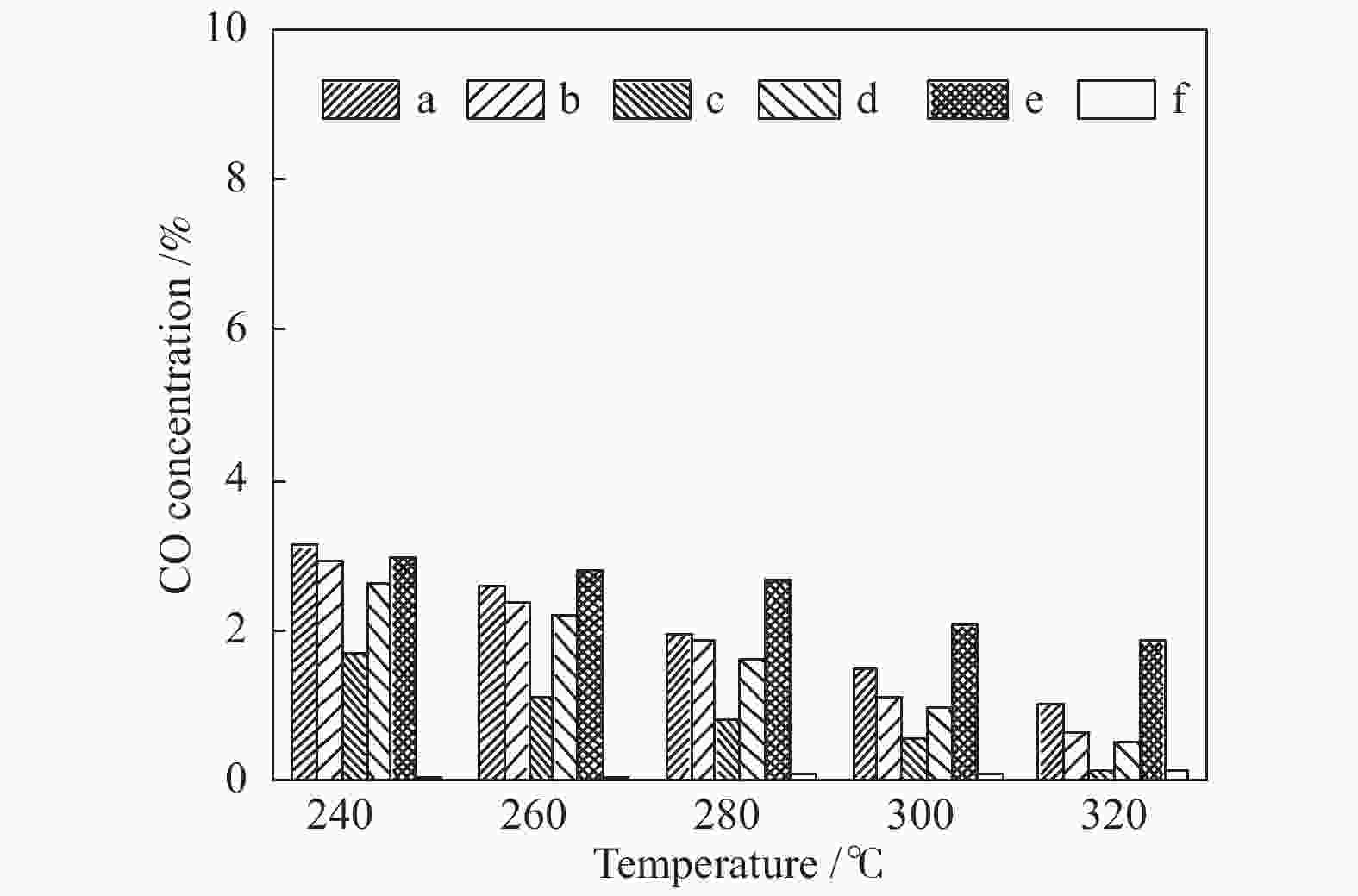
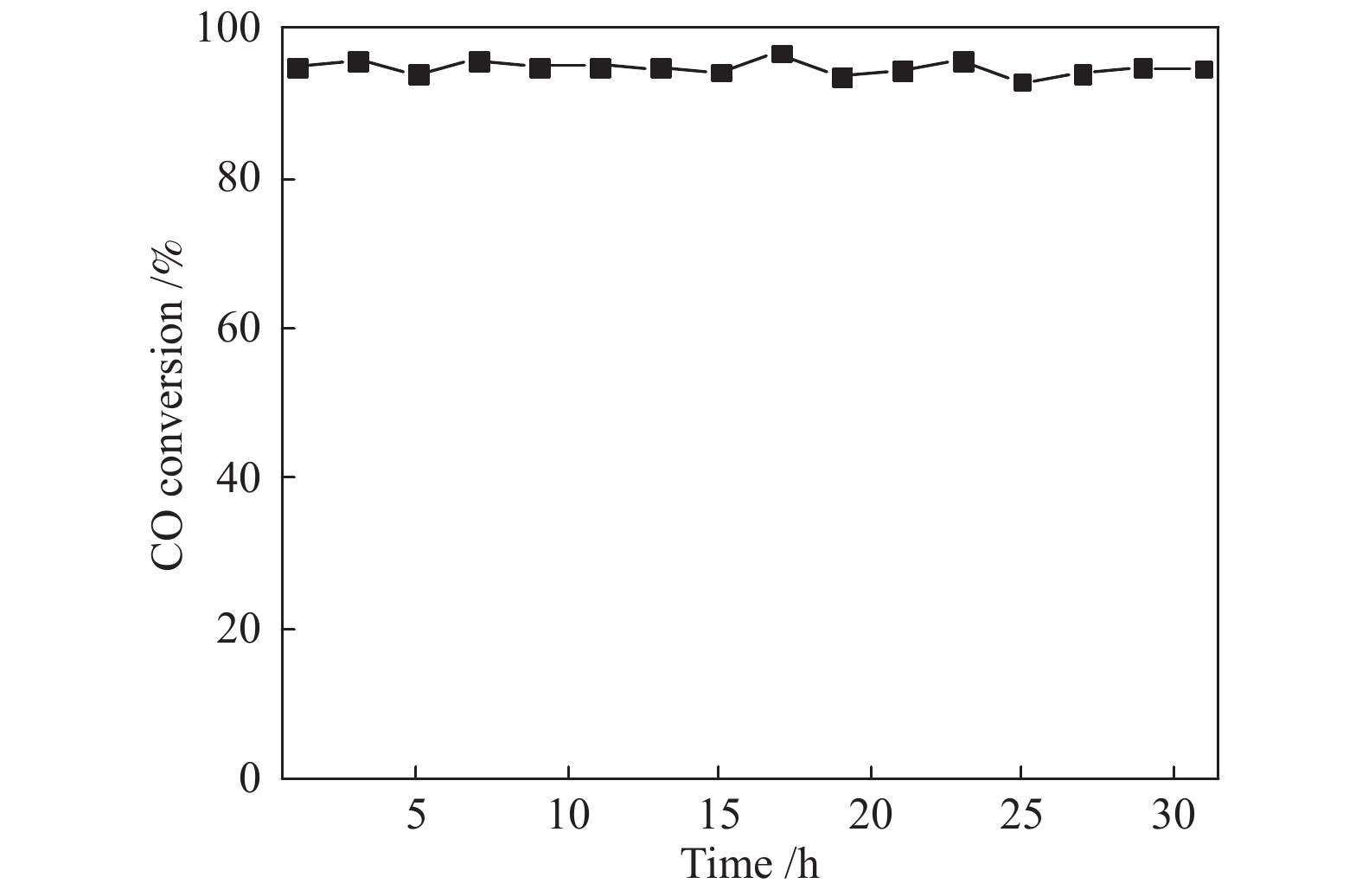
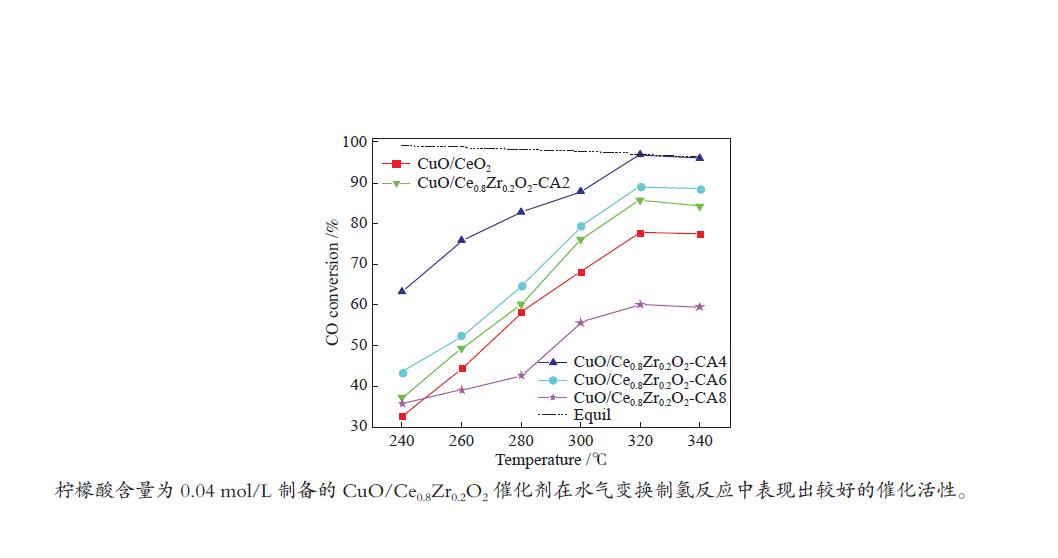
摘要: 采用水热法合成了Ce0.8Zr0.2O2固溶体,再经浸渍法负载活性组分制备了CuO/Ce0.8Zr0.2O2催化剂,考察了柠檬酸量对CuO/Ce0.8Zr0.2O2催化剂结构、性质及其催化水气变换反应制氢性能的影响。结果表明,不同柠檬酸量制备的CuO/Ce0.8Zr0.2O2催化剂的催化活性主要与Cu比表面积、还原性能及Ce0.8Zr0.2O2固溶体与CuO之间的相互作用有关。其中,柠檬酸浓度为0.04 mol/L所制备的催化剂具有较大的Cu比表面积,较低的CuO还原温度和较强的Ce0.8Zr0.2O2固溶体与CuO之间的相互作用,在水气变换制氢过程中具有较高的CO转化率,表现出了较好的催化活性。在反应温度为320 ℃,水气物质的量比n(H2O)/n(CO) = 2,总气体体积空速GHSV = 6600 h−1时,CO转化率接近热力学平衡值,为96.9%。Abstract: Ce0.8Zr0.2O2 solid solutions were synthesized by hydrothermal method, and active components were loaded by impregnation method to get CuO/Ce0.8Zr0.2O2 catalysts. Effects of citric acid content on the structure, properties and hydrogen production performance of CuO/Ce0.8Zr0.2O2 catalysts were investigated. CuO/Ce0.8Zr0.2O2 catalysts prepared with different citric acid content are distinct in Cu surface area, reduction performance and interaction between Ce0.8Zr0.2O2 solid solution and CuO. Among them, the catalyst prepared with a citric acid concentration of 0.04 mol/L has a large Cu surface area, a low CuO reduction temperature and a strong interaction between Ce0.8Zr0.2O2 solid solution and CuO, which has the best catalytic activity and the highest CO conversion in the water gas shift conversion process. At 320 ℃, water/gas molar ratio n(H2O)/n(CO) = 2, total volume velocity GHSV = 6600 h−1, its CO conversion is 96.9% close to thermodynamic equilibrium value.
-
Key words:
- hydrothermal method /
- citric acid /
- water gas shift /
- solid solution
-
表 1 不同柠檬酸量制备催化剂的元素含量
Table 1 Element content of catalysts prepared with different citric acid content
Catalyst Content of element w/% Ce/Zr (mol ratio) Cu Ce Zr O CuO/Ce0.8Zr0.2O2-CA2 4.3 66.0 13.0 16.7 3.7 CuO/Ce0.8Zr0.2O2-CA4 4.4 66.0 12.9 16.7 3.7 CuO/Ce0.8Zr0.2O2-CA6 4.3 66.4 12.7 16.6 3.8 CuO/Ce0.8Zr0.2O2-CA8 4.6 65.9 13.0 16.5 3.7 表 2 催化材料的物理化学性质
Table 2 Physicochemical properties of catalytic materials
Catalyst Surface area/(m2·g−1) Pore volume v/(cm3·g−1) Cu surface areaa/(m2·g−1) H2 production rateb/(μmol·kg−1·s−1) CeO2 23.9 0.08 − − Ce0.8Zr0.2O2-CA2 55.8 0.09 − − Ce0.8Zr0.2O2-CA4 69.2 0.15 − − Ce0.8Zr0.2O2-CA6 79.8 0.10 − − Ce0.8Zr0.2O2-CA8 47.4 0.09 − − CuO/CeO2 21.2 0.07 1.5 2959.9 CuO/ Ce0.8Zr0.2O2-CA2 37.4 0.07 2.4 3260.2 CuO/ Ce0.8Zr0.2O2-CA4 58.8 0.14 5.5 3681.9 CuO/ Ce0.8Zr0.2O2-CA6 73.1 0.09 4.8 3385.5 CuO/ Ce0.8Zr0.2O2-CA8 40.5 0.09 1.2 2283.2 a: determined by N2O experiments;
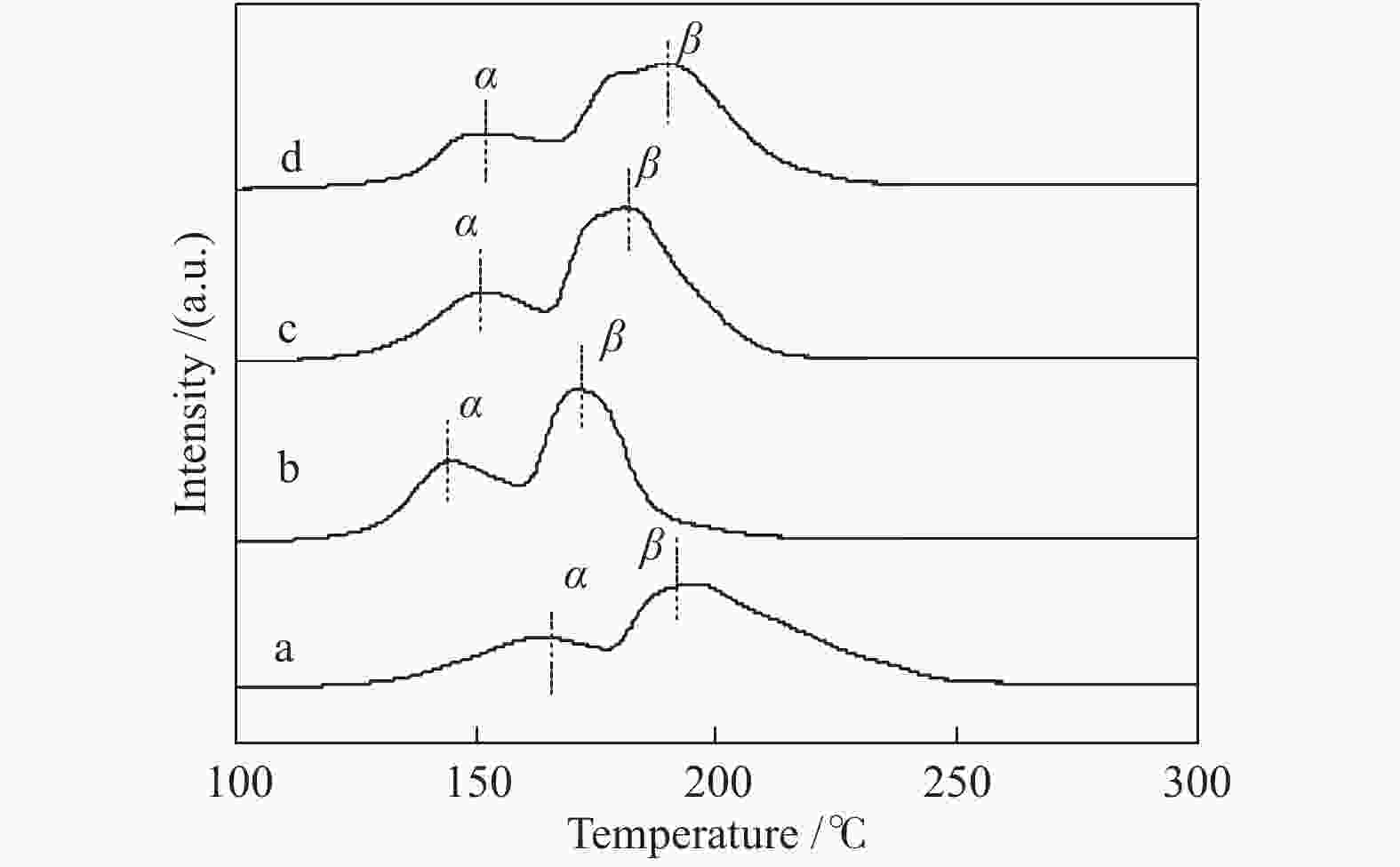
b: H2 production was calculated when temperature is 320 ℃, n(H2O)/n(CO) = 2∶1, GHSV = 6600 h−1表 3 CuO还原峰位置
Table 3 Reduction peak positions of CuO
Catalyst Peak position t/℃ peak α peak β CuO/Ce0.8Zr0.2O2-CA2 163 193 CuO/Ce0.8Zr0.2O2-CA4 144 171 CuO/Ce0.8Zr0.2O2-CA6 150 181 CuO/Ce0.8Zr0.2O2-CA8 151 189 表 4 催化剂Ce 3d的XPS曲线拟合
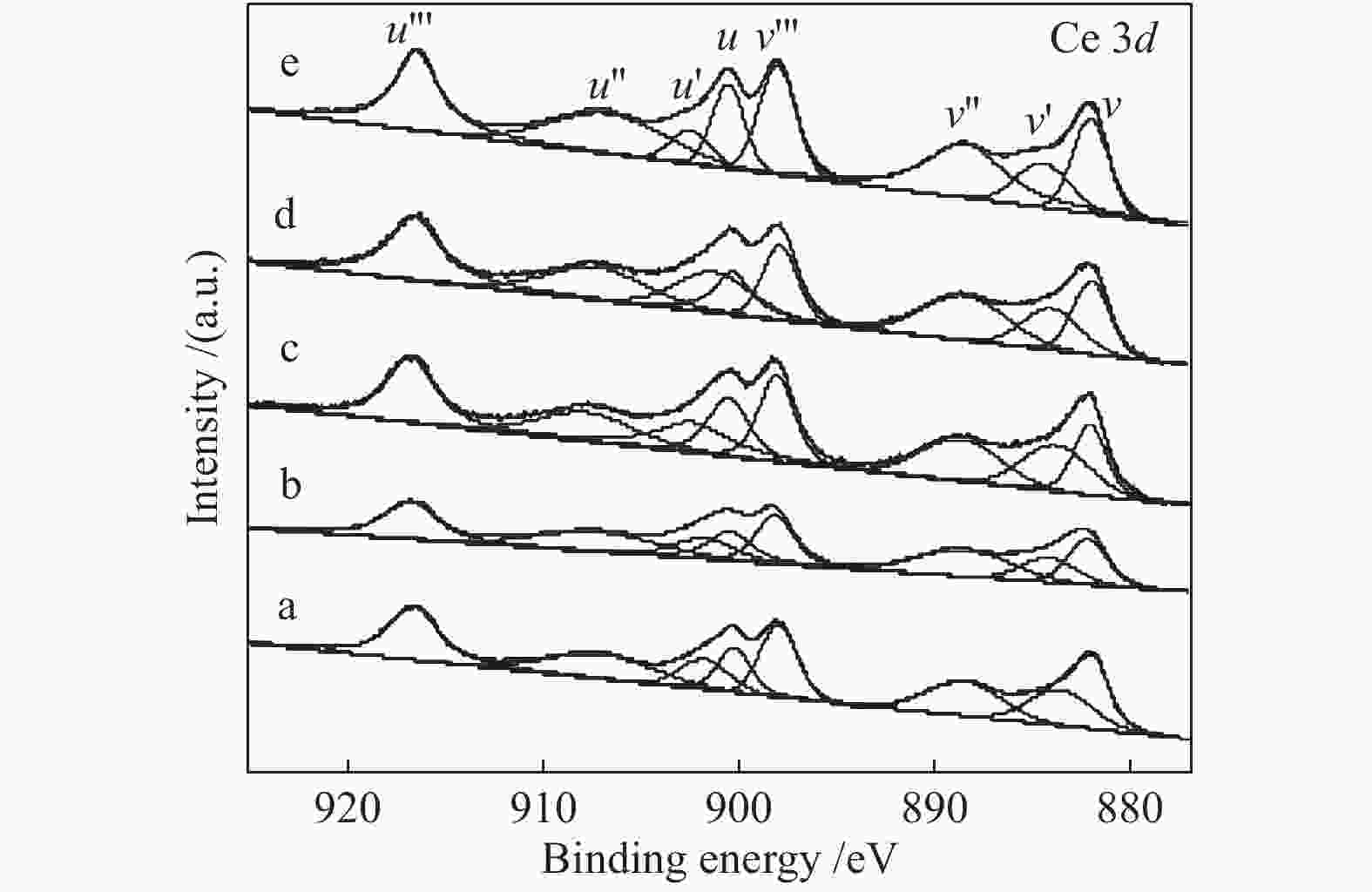
Table 4 Fitting results of Ce 3d XPS curves of catalysts
Catalyst Ce3+/(Ce3+ + Ce4+)/% CuO/CeO2 24.2 CuO/Ce0.8Zr0.2O2-CA2 25.8 CuO/Ce0.8Zr0.2O2-CA4 28.6 CuO/Ce0.8Zr0.2O2-CA6 26.8 CuO/Ce0.8Zr0.2O2-CA8 23.9 -
[1] RYAN J G, KHALID A A, WILLIAM H G. Thermochemical production of hydrogen from hydrogen sulfide with iodine thermochemical cycles[J]. Int J Hydrogen Energy,2018,43(29):12939−12947. doi: 10.1016/j.ijhydene.2018.04.217 [2] JACOBSON M Z, COLELLA W, GOLDEN D. Cleaning the air and improving health with hydrogen fuel-cell vehicles[J]. Science,2005,308(5730):1901−1905. doi: 10.1126/science.1109157 [3] MASCHARAK P K. Cobaloxime-based photocatalytic devices for hydrogen production[J]. Angew Chem Int Ed,2008,47(3):564−567. doi: 10.1002/anie.200702953 [4] 张燕杰, 陈崇启, 詹瑛瑛, 叶远松, 娄本勇, 郑国才, 林棋. CuO/ZrO2催化水煤气变换反应制氢: ZrO2载体焙烧温度的影响[J]. 燃料化学学报,2019,47(4):91−100.ZHANG Yan-jie, CHEN Chong-qi, ZHAN Ying-ying, YE Yuan-song, LOU Ben-yong, ZHENG Guo-cai, LIN Qi. CuO/ZrO2 catalysts for the production of H2 through the water-gas shift reaction: Effect of calcination temperature of ZrO2[J]. J Fuel Chem Technol,2019,47(4):91−100. [5] GOKHALE A A, DUMESIC J A, MAVRIKAKIS M. On the mechanism of low-temperature water gas shift reaction on copper[J]. J Am Chem Soc,2008,130(4):1402−1414. doi: 10.1021/ja0768237 [6] WANG X Q, RODRIGUEZ J, HANSON J, GAMARRA D. In situ studies of the active sites for the water gas shift reaction over Cu-CeO2 catalysts: Complex interaction between metallic copper and oxygen vacancies of ceria[J]. J Phys Chem B,2006,110(1):428−34. doi: 10.1021/jp055467g [7] MARONO M, SANCHEZ J M, RUIZ E. Hydrogen-rich gas production from oxygen pressurized gasification of biomass using a Fe-Cr water gas shift catalyst[J]. Int J Hydrogen Energy,2010,35(1):37−45. doi: 10.1016/j.ijhydene.2009.10.078 [8] REDDY G K, KIM S J, DONG J H, SMIRNIOTIS P G. Long-term WGS stability of Fe/Ce and Fe/Ce/Cr catalysts at high and low steam to CO ratios-XPS and mssbauer spectroscopic study[J]. Appl Catal A: Gen,2012,415:101−110. [9] REDDY G K, SMIRNIOTIS P G. Effect of copper as a dopant on the water gas shift activity of Fe/Ce and Fe/Cr modified ferrites[J]. Catal Lett,2011,141(1):27−32. doi: 10.1007/s10562-010-0465-2 [10] FU W, BAO Z H, DING W Z, CHOU K. The synergistic effect of the structural precursors of Cu/ZnO/Al2O3 catalysts for water-gas shift reaction[J]. Catal Commun,2011,12(6):505−509. doi: 10.1016/j.catcom.2010.11.017 [11] KOWALIK P, PROCHNIAK W, BOROWIECKI T. The effect of alkali metals doping on properties of Cu/ZnO/Al2O3 catalyst for water gas shift[J]. Catal Today,2011,176(1):144−148. doi: 10.1016/j.cattod.2011.01.028 [12] FIGUEIREDO R T, SANTOS M S, ANDRADE H M, FIERRO J. Effect of alkali cations on the Cu/ZnO/Al2O3 low temperature water gas-shift catalyst[J]. Catal Today,2011,172(1):166−170. doi: 10.1016/j.cattod.2011.03.073 [13] OSA A R, LUCAS A D, ROMERO A, CASERO P. High pressure water gas shift performance over a commercial non-sulfide CoMo catalyst using industrial coal-derived syngas[J]. Fuel,2012,97(1):428−434. [14] MUDIYANSELAGE K, SENANAYAKE S D, RAMIREZ P J, KUNDU S. Intermediates arising from the water-gas shift reaction over Cu surfaces: from UHV to near atmospheric pressures[J]. Top Catal,2015,58(4):271−280. [15] JEONG D W, NA H S, SHIM J O, JANG W J. Hydrogen production from low temperature WGS reaction on co-precipitated Cu-CeO2 catalysts: An optimization of Cu loading[J]. Int J Hydrogen Energy,2014,39(17):9135−9142. doi: 10.1016/j.ijhydene.2014.04.005 [16] WANG S P, WANG X Y, HUANG J, ZHANG S M. The catalytic activity for CO oxidation of CuO supported on Ce0.8Zr0.2O2 prepared via citrate method[J]. Catal Commun,2007,8(3):231−236. doi: 10.1016/j.catcom.2006.06.006 [17] JIANG L, ZHU H W, RAZZAQ R, ZHU M L. Effect of zirconium addition on the structure and properties of CuO/CeO2 catalysts for high-temperature water-gas shift in an IGCC system[J]. Int J Hydrogen Energy,2012,21(21):15914−15924. [18] JEONG D W, NA H S, SHIM J O, JANG W J, ROH H S. A crucial role for the CeO2-ZrO2 support for the low temperature water gas shift reaction over Cu-CeO2-ZrO2 catalysts[J]. Catal Sci Technol,2015,5:3706−3713. doi: 10.1039/C5CY00499C [19] 郑云弟, 林性贻, 郑起, 詹瑛瑛, 李达林, 魏可镁. ZrO2对Cu/CeO2-ZrO2水煤气变换催化剂结构、性能的影响[J]. 中国稀土学报,2005,6(8):679−683.ZHENG Yun-di, LIN Xing-yi, ZHENG Qi, ZHAN Ying-ying, LI Da-lin, WEI Ke-mei. Effects of ZrO2 content on structure and properties of Cu CeO2-ZrO2 catalysts for water-gas shift reaction[J]. J Chin Rare Earth Soc,2005,6(8):679−683. [20] GOMEZ I D, KOCEMBA I, RYNKOWSKI J M. Au/Ce1−xZrxO2 as effective catalysts for low-temperature CO oxidation[J]. App Catal B: Environ,2008,83:240−241. doi: 10.1016/j.apcatb.2008.02.012 [21] VLAIC G, FORNASIERO P, GEREMIA S, KASPAR J. Relationship between the zirconia-promoted reduction in the Rh-loaded Ce0.5Zr0.5O2 mixed oxide and the Zr-O local structure[J]. J Catal,1997,168(2):386−392. doi: 10.1006/jcat.1997.1644 [22] 张增庆, 樊君, 胡晓云, 刘恩周, 赵博, 康力敏. Ce0.5Zr0.5O2固溶体的制备、晶粒增长及应用研究[J]. 中国稀土学报,2013,31(2):217−221.ZHANG Zeng-qing, FAN Jun, HU Xiao-yun, LIU En-zhou, ZHAO Bo, KANG Li-min. Preparation, grain growth and application of Ce0.5Zr0.5O2[J]. J Chin Rare Earth Soc,2013,31(2):217−221. [23] YANG S Q, HE J P, ZHANG N, SUI X W, ZHANG L, YANG Z X. Effect of rare-earth element modification on the performance of Cu/ZnAl catalysts derived from hydrotalcite precursor in methanol steam reforming[J]. J Fuel Chem Technol,2018,46(2):179−188. doi: 10.1016/S1872-5813(18)30010-0 [24] ZHANG Y J, CHEN C Q, ZHANG Y Y, LIN Q, LOU B Y, ZHENG G C, ZHENG Q. Highly active Y-promoted CuO/ZrO2 catalysts for the production of hydrogen through water-gas shift reaction[J]. J Fuel Chem Technol,2017,45(9):1137−1149. [25] XIE H M, DU Q X, LI H, ZHOU G L, CHEN S M, JIAO Z J, REN J M. Catalytic combustion of volatile aromatic compounds over CuO-CeO2 catalyst[J]. Korean J Chem Eng,2017,34(7):1944−1951. [26] 庆绍军, 侯晓宁, 刘雅杰, 王磊, 李林东, 高志贤. Cu-Ni-Al尖晶石催化甲醇水蒸气重整制氢性能的研究[J]. 燃料化学学报,2018,46(10):69−76.QING Shao-jun, HOU Xiao-ning, LIU Ya-jie, WANG Lei, LI Lin-dong, GAO Zhi-xian. Catalytic performance of Cu-Ni-Al spine l for methanol steam reforming to hydrogen[J]. J Fuel Chem Technol,2018,46(10):69−76. [27] SHE W, JI T Q, CUI M X, YAN P F, WENG N S, LI W, LI G M. Catalytic performance of CeO2-supported Ni catalyst for hydrogenation of nitroarenes fabricated via coordination-assisted strategy[J]. ACS App Mater Interfaces,2018,10(17):14698−14707. doi: 10.1021/acsami.8b01187 [28] BENNICI S, GERVASINI A, RAVASIO N, ZACCHERIA F. Optimization of tailoring of CuOx species of silica alumina supported catalysts for the selective catalytic reduction of NOx[J]. J Phys Chem B,2003,107(22):5168−5176. doi: 10.1021/jp022064x [29] PENG X OMASTA T, ROLLER J, MUSTAIN W E. Highly active and durable Pd-Cu catalysts for oxygen reduction in alkaline exchange membrane fuel cells[J]. Front Energy,2017,11(3):299−309. doi: 10.1007/s11708-017-0495-1 -





 下载:
下载: