Cu-Zn-Al spinel catalyst for hydrogen production from methanol steam reforming
-
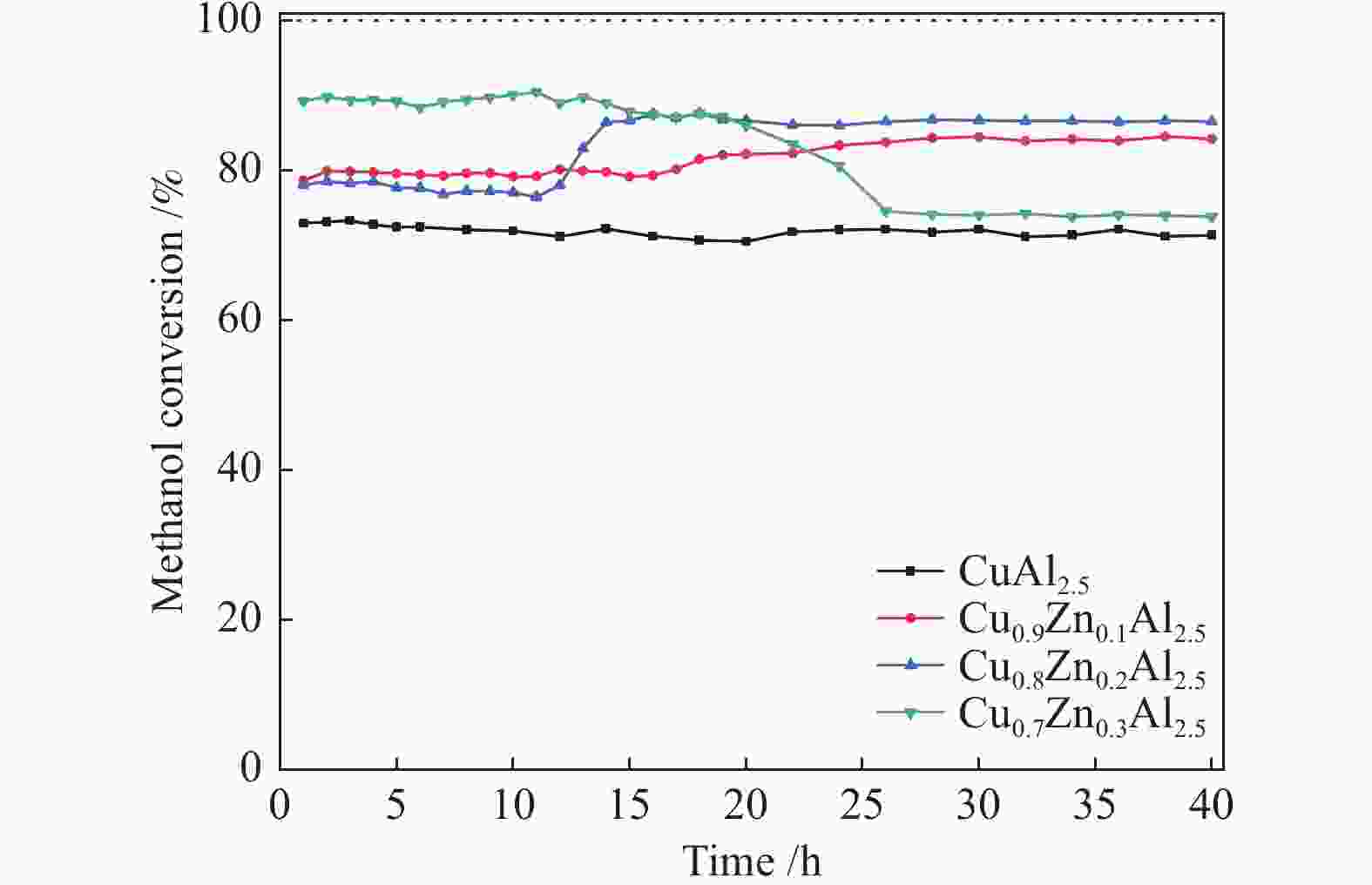
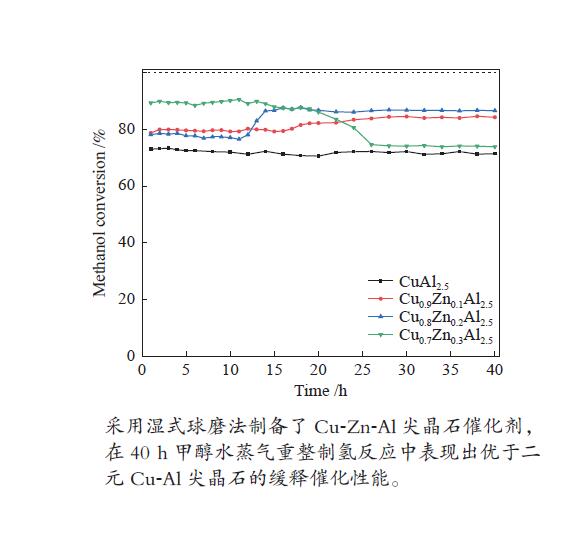
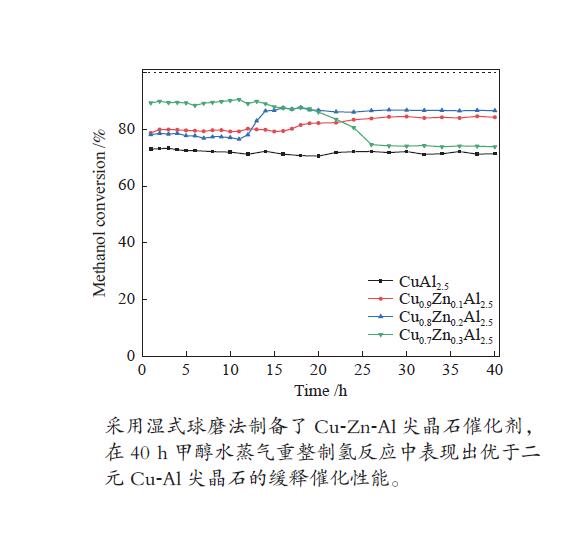
摘要: 以硝酸铜、硝酸锌、拟薄水铝石和柠檬酸为原料,采用湿式球磨法合成了Cu-Zn-Al三元尖晶石催化剂。通过TG-DTA、XRD、N2物理吸附-脱附、H2-TPR、XPS等表征手段,研究不同Cu/Zn/Al物质的量比对催化剂晶相组成、比表面积、还原性能、表面性质的影响,并通过甲醇水蒸气重整制氢反应(MSR)考察催化剂的缓释催化性能。结果表明,与Cu-Al二元尖晶石相比,Cu-Zn-Al三元尖晶石的结晶度高、比表面积大、更难还原,表现出较好的催化活性,并且其缓释催化行为大不相同。所有催化剂不经预还原处理,即可催化MSR反应,在反应40 h后趋于稳定。其中,Cu∶Zn∶Al = 0.8∶0.2∶2.5(物质的量比)的Cu-Zn-Al催化剂在反应温度265 ℃、水醇比为2、质量空速2.25 h−1的MSR反应中表现出最高的稳定活性。最后结合反应前后催化剂的表征数据,探讨了催化剂活性组分的缓释度,并基于此预测催化剂具有更长的稳定性。
-
关键词:
- 球磨法 /
- Cu-Zn-Al尖晶石 /
- 甲醇水蒸气重整 /
- 缓释催化 /
- 制氢
Abstract: The Cu-Zn-Al ternary spinel catalysts were synthesized by the wet ball milling method using copper nitrate, zinc nitrate, pseudoboehmite and citric acid as the raw materials. TG-DTA, XRD, N2 physical adsorption, H2-TPR, XPS and other characterization methods were used to study the effects of different Cu/Zn/Al molar ratios on the crystal phase composition, specific surface area, reduction performance and surface properties of the catalysts, and the catalytic performances of the catalysts were investigated by methanol steam reforming (MSR) for hydrogen production. The results indicate that comparing with the binary Cu-Al spinel, Cu-Zn-Al ternary spinel catalysts have high crystallinity, large surface area and are difficult to be reduced, which show improved catalytic performance and totally different sustained release behavior. The Cu-Zn-Al spinel catalyst with Cu∶Zn∶Al = 0.8∶0.2∶2.5 (molar ratio) exhibited the highest stable catalytic activity in MSR under a reaction temperature of 265 ℃, water/methanol ratio of 2 and mass space velocity of 2.25 h−1. The findings of this work might be served as basic data for further research of such ternary spinel catalysts. -
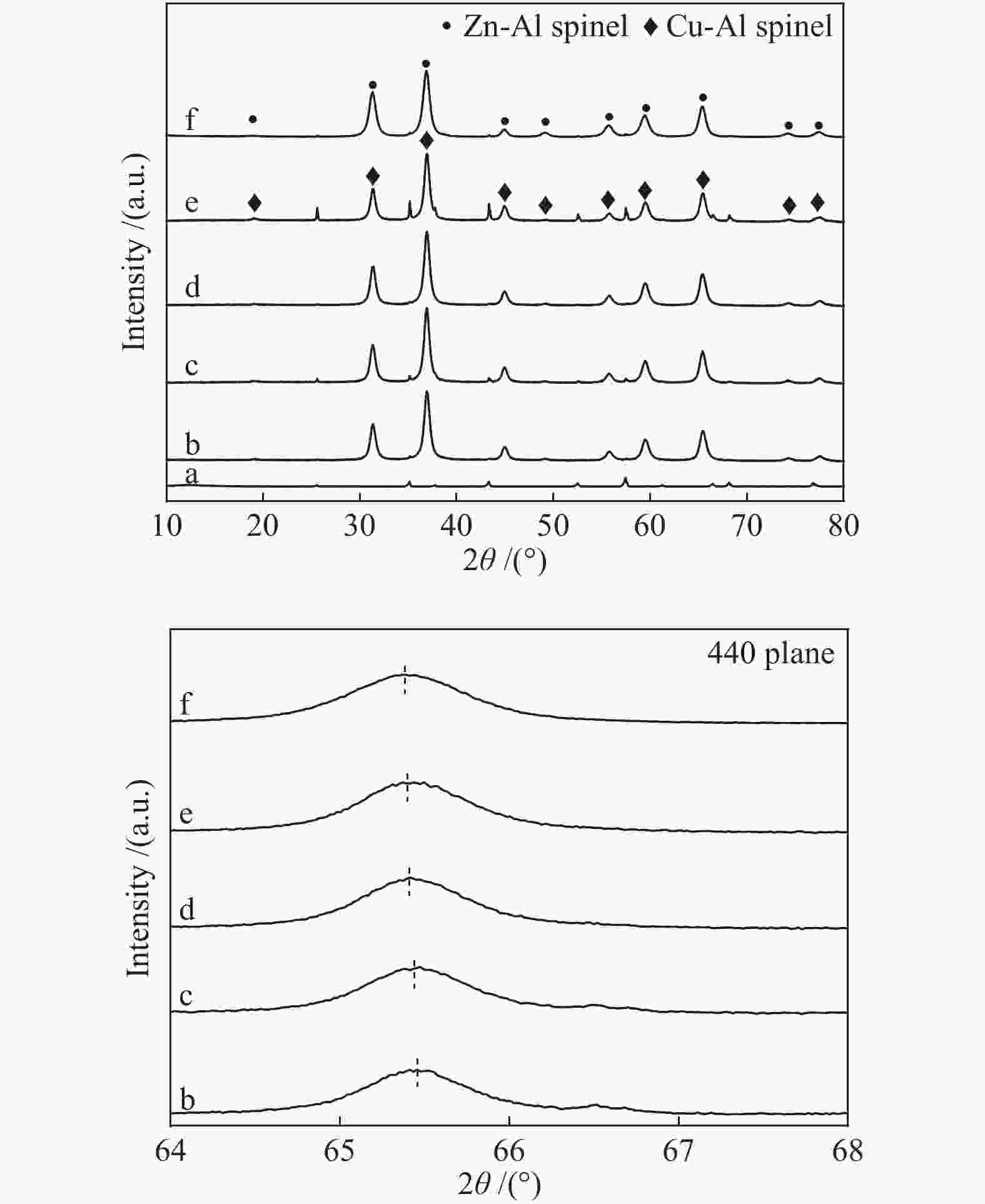
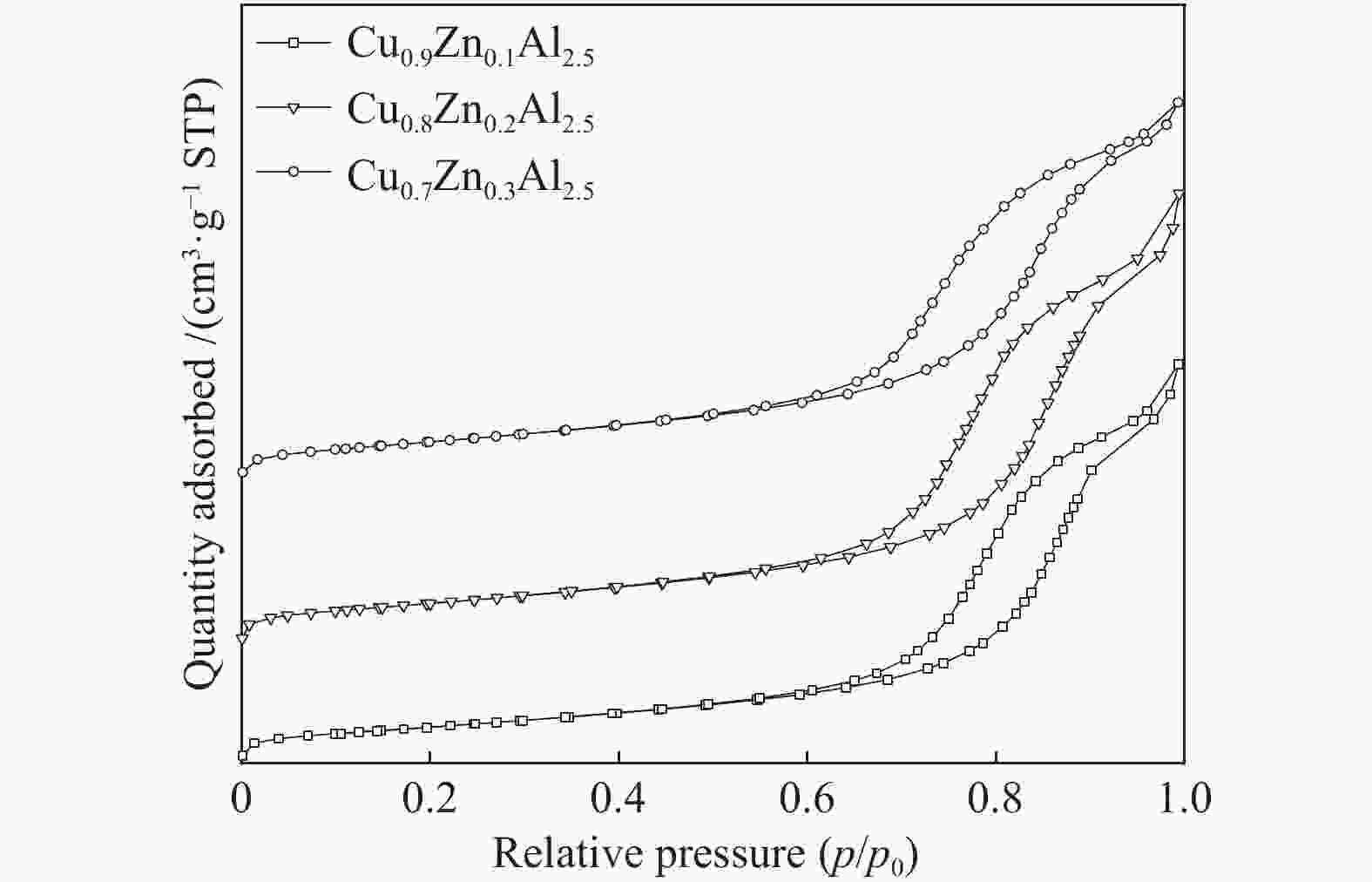
表 1 CuxZn1−xAl2.5(x = 0.9、0.8、0.7)及参比样的物化性质
Table 1 Physico-chemical property of CuxZn1−xAl2.5(x = 0.9, 0.8, 0.7) and the reference samples
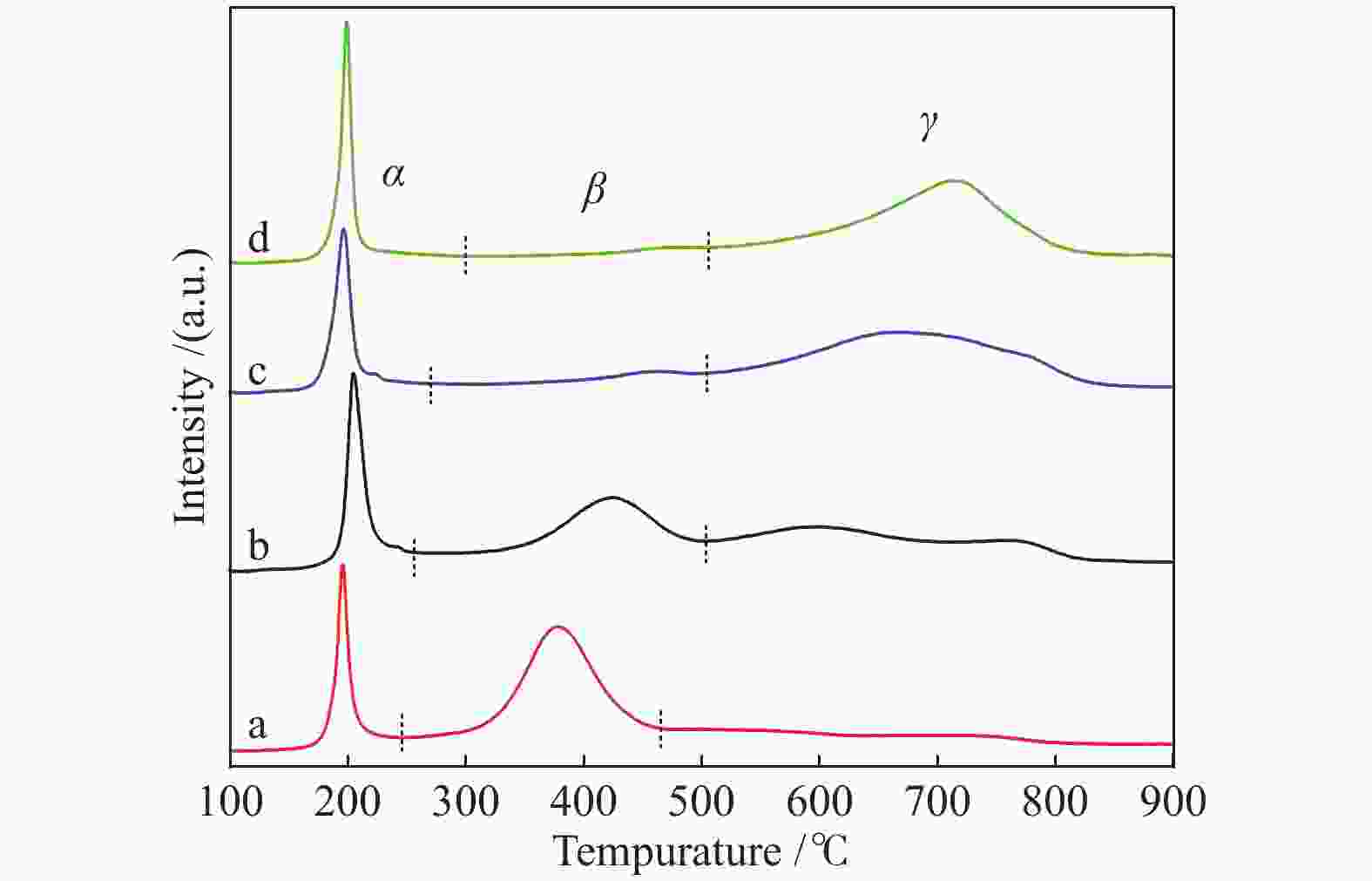
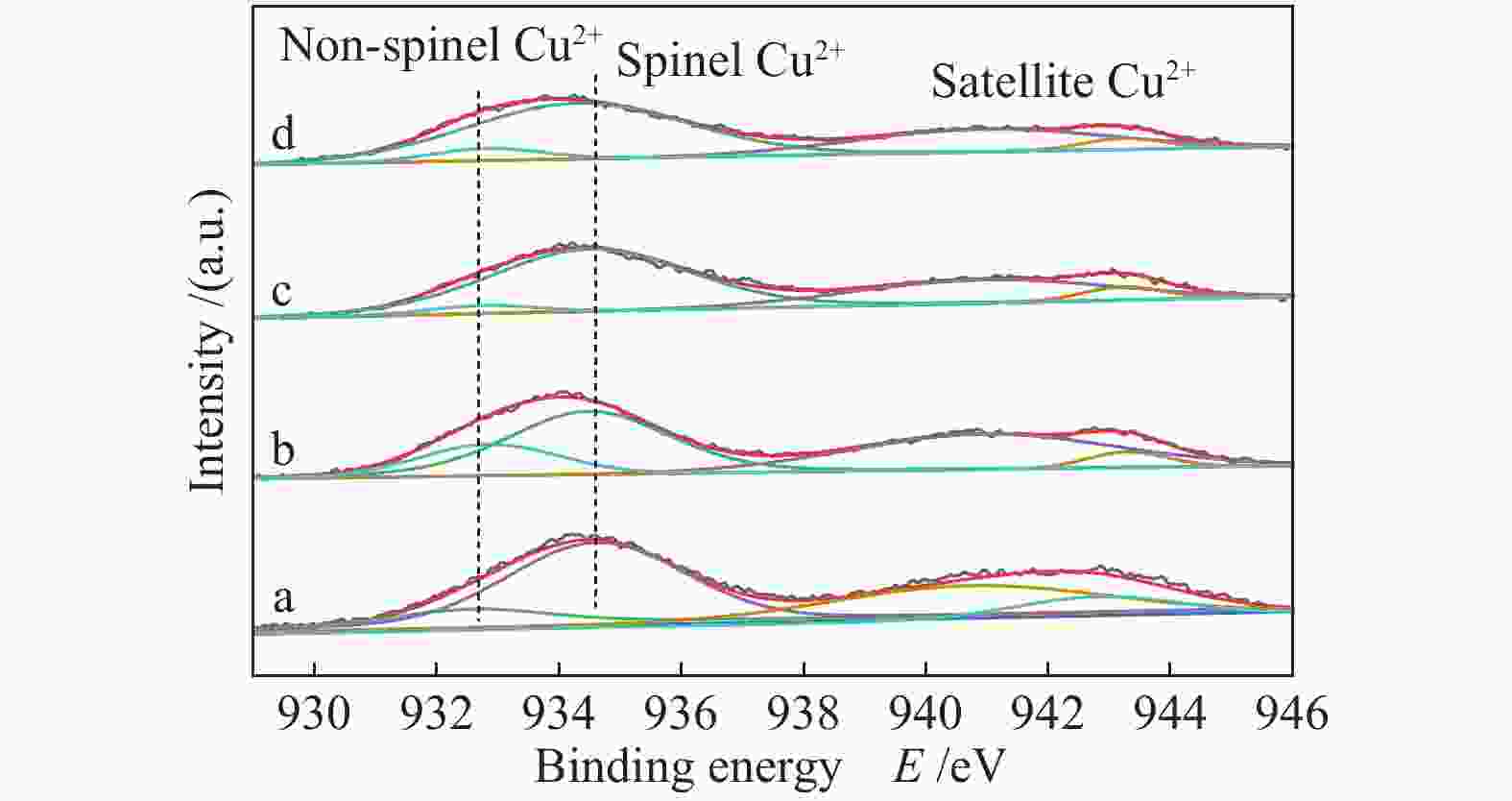
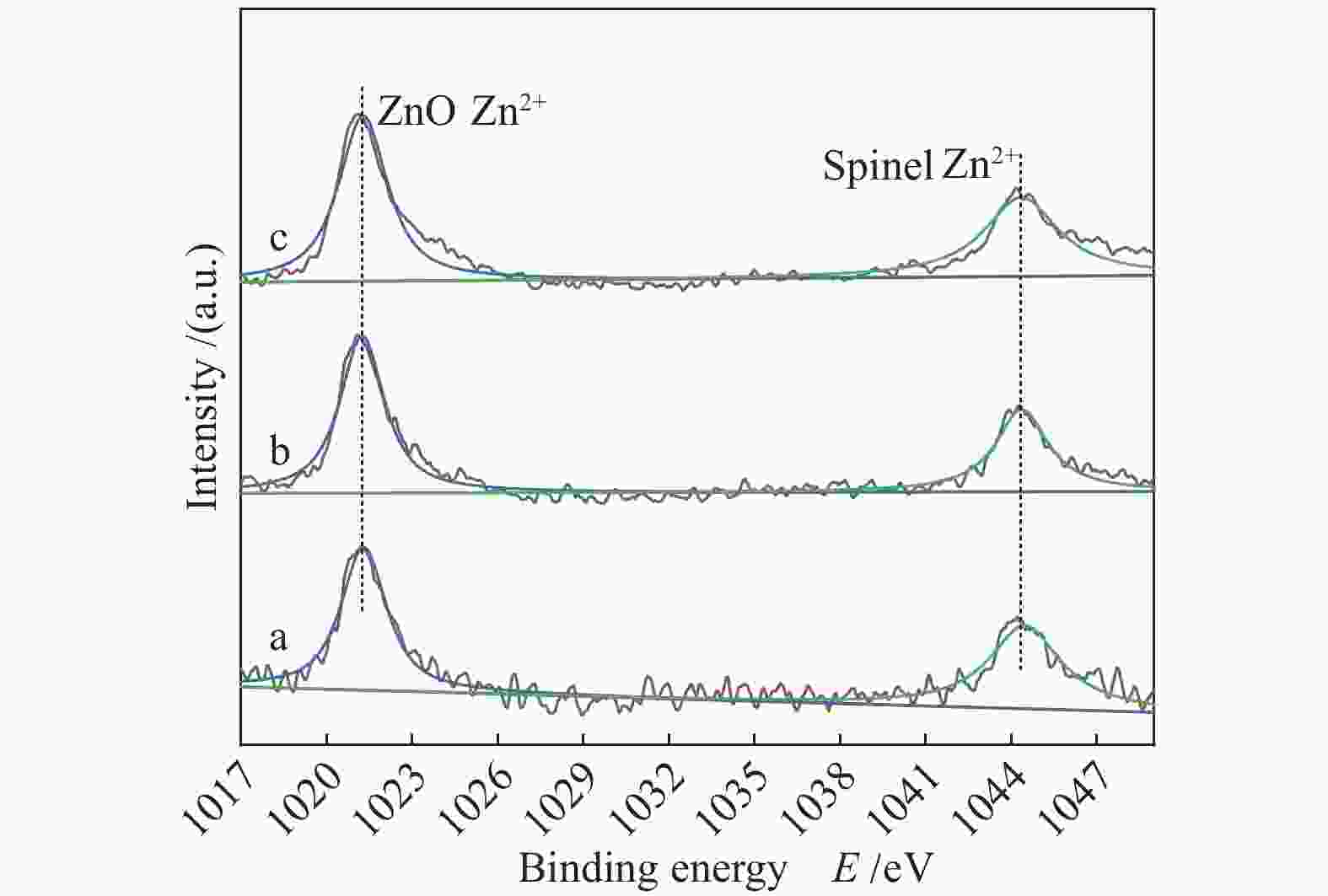
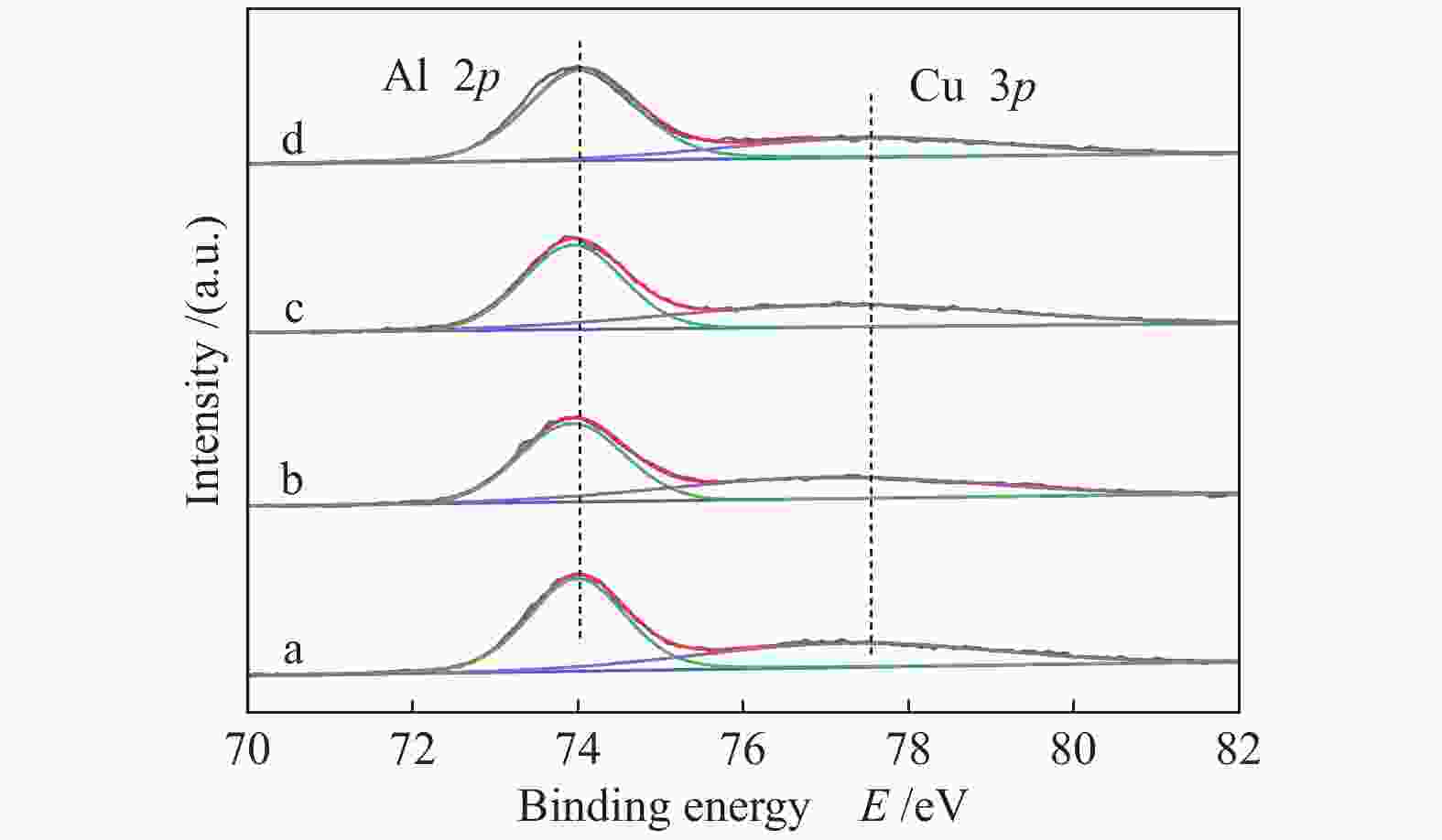
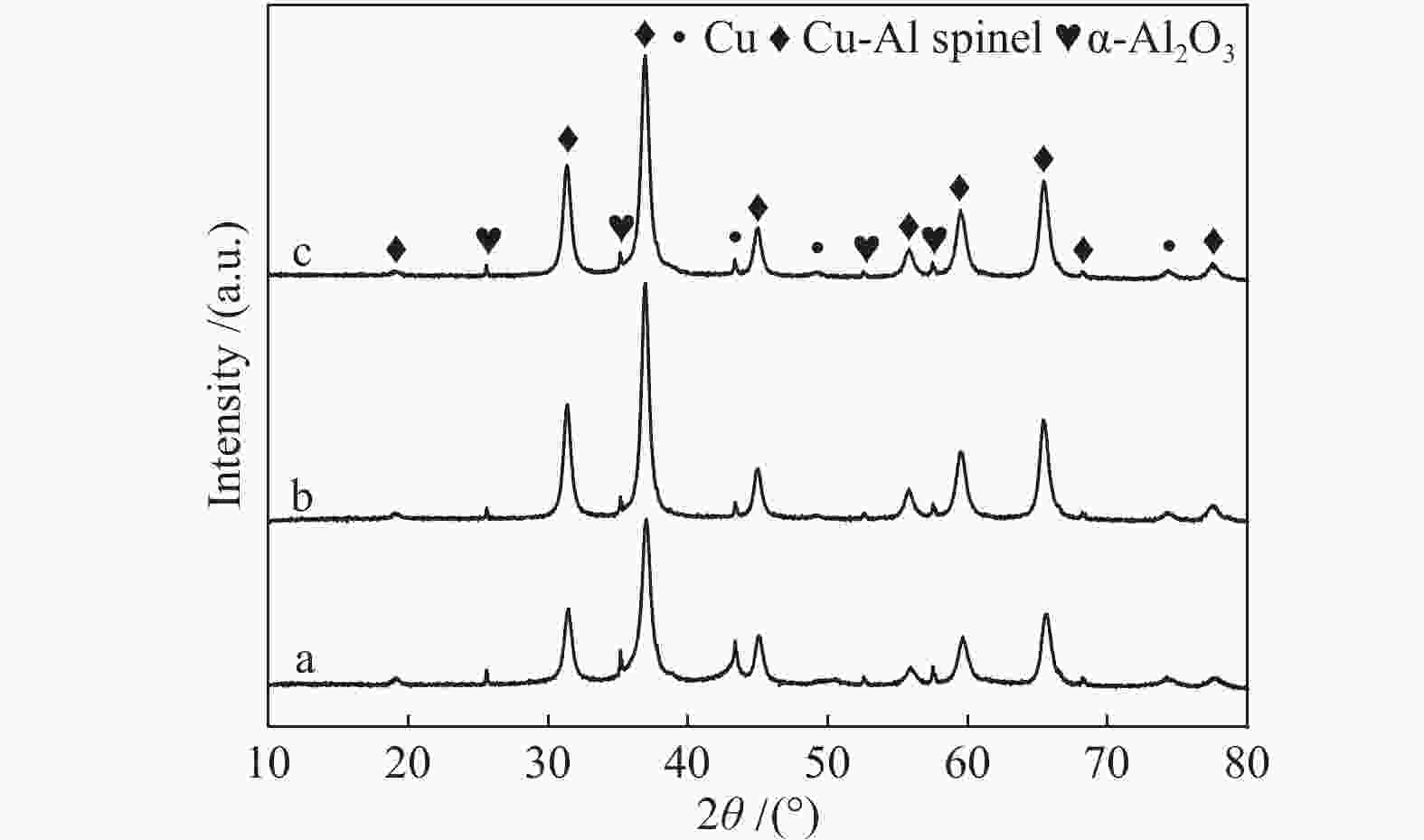
Sample CuAl2.5 Cu0.9Zn0.1Al2.5 Cu0.8Zn0.2Al2.5 Cu0.7Zn0.3Al2.5 ZnAl2.5 Sa/(m2·g−1) 40.9 41.1 45.6 48.6 62.1 vb/(mL·g−1) 0.148 0.148 0.153 0.155 0.293 dc/nm 14.5 14.4 13.4 12.8 18.9 dspineld/nm 15.65 14.00 15.31 14.71 13.64 ae/nm 0.80645 0.80666 0.80671 0.80676 0.80716 x(non-spinel Cu2+)f /% 17.3 22.4 19.0 23.2 x(easily-reducible spinel Cu2+)f /% 58.3 37.4 14.1 9.7 x(hardly-reducible spinel Cu2+)f /% 24.4 40.2 66.9 67.1 a: specific surface area; b: pore volume; c: pore size; d: the crystallite size of spinels,calculated by the Scherrer equation from the XRD patterns (Figure 2); e: cell parameter of spinel; f: calculated by the H2-TPR profiles in Figure 4 表 2 CuxZn1−xAl2.5 (x = 0.9、0.8、0.7) H2-TPR谱图还原峰含量及温度
Table 2 Reduction peak content and temperature in the H2-TPR profiles of CuxZn1−xAl2.5 (x = 0.9, 0.8, 0.7)
Sample α β γ tpeak/℃ x/% tpeak/℃ x/% tpeak/℃ x/% CuAl2.5 195 17.3 378 58.3 550 24.4 Cu0.9Zn0.1Al2.5 200 22.4 425 37.4 600 40.2 Cu0.8Zn0.2Al2.5 196 19.0 462 14.1 674 66.9 Cu0.7Zn0.3Al2.5 199 23.2 470 9.7 711 67.1 表 3 CuxZn1−xAl2.5 (x = 0.9、0.8、0.7)反应后的特性
Table 3 Characteristic data of CuxZn1−xAl2.5 (x = 0.9, 0.8, 0.7) after reaction
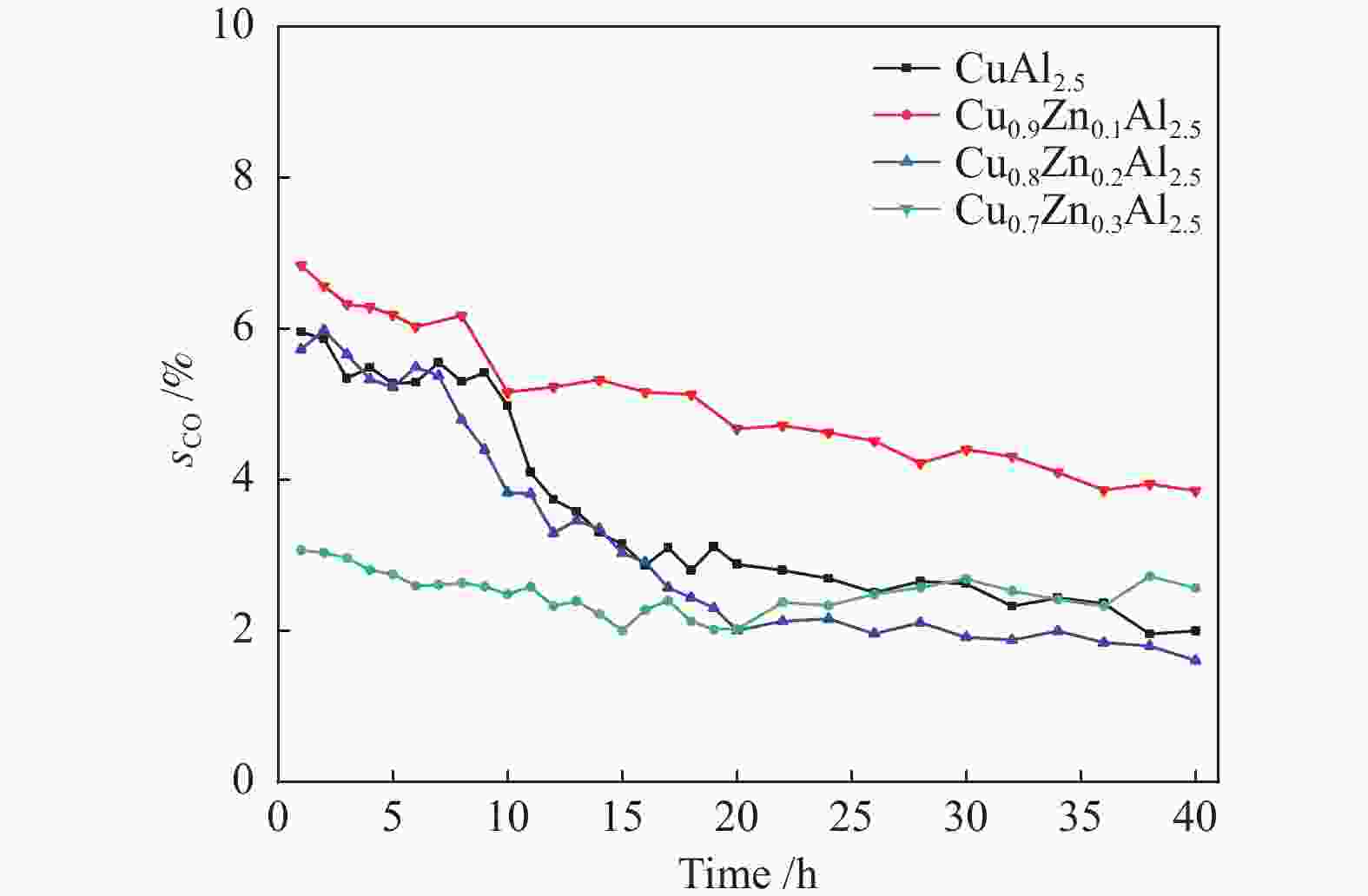
Catalyst after MSR Cu0.9Zn0.1Al2.5 Cu0.8Zn0.2Al2.5 Cu0.7Zn0.3Al2.5 dCua/nm 22.9 17.2 20.8 RDb/% 34.3 16.5 7.1 a: the crystallite size of Cu, calculated by the Scherrer equation from the XRD patterns (Figure 10); b: the release degree (RD) = ΔxCuO/xspinel -
[1] HOLM T, BORSBOOM H T, HERRERA O, MERIDA W. Hydrogen costs from water electrolysis at high temperature and pressure[J]. Energ Convers Manage,2021,237:114106−114120. doi: 10.1016/j.enconman.2021.114106 [2] KIM S H, KUMAR G, CHEN W H, KHANAL S K. Renewable hydrogen production from biomass and wastes[J]. Bioresource Technol,2021,331:125024−125029. doi: 10.1016/j.biortech.2021.125024 [3] QIAO W J, YANG S Q, ZHANG L, TIAN Y, WANG H H, ZHANG C S, GAO Z X. Performance of Cu-Ce/M-Al (M = Mg, Ni, Co, Zn) hydrotalcite derived catalysts for hydrogen production from methanol steam reforming[J]. Int J Energy Res,2021,45:12773−12784. doi: 10.1002/er.6610 [4] 庆绍军, 侯晓宁, 李林东, 张磊, 陈凯华, 高志贤, 樊卫斌. 甲醇制氢应用于氢燃料电池车的可行性及其发展前景[J]. 能源与节能,2019,2:62−65. doi: 10.3969/j.issn.2095-0802.2019.06.027QING Shao-jun, HOU Xiao-ning, LI Lin-dong, ZHANG Lei, CHEN Kai-hua, GAO Zhi-xian, FAN Wei-bin. Application feasibility and development prospect of methanol to hydrogen technology for hydrogen fuel cell vehicle[J]. Energy Energy Conser,2019,2:62−65. doi: 10.3969/j.issn.2095-0802.2019.06.027 [5] YANG S Q, ZHOU F, LIU Y J, ZHANG L, CHEN Y, WANG H H, TIAN Y, ZHANG C S, LIU D S. Morphology effect of ceria on the performance of CuO/CeO2 catalysts for hydrogen production by methanol steam reforming[J]. Int J Hydrogen Energy,2019,44:7252−7261. doi: 10.1016/j.ijhydene.2019.01.254 [6] MIERCZYNSKI P, MOSINSKA M, MANIUKIEWICZ W, NOWOSIELSKA M, CZYLKOWSKA A, SZYNKOWSKA M I. Oxy-steam reforming of methanol on copper catalysts[J]. React Kinet Mech Catal,2019,127:857−874. doi: 10.1007/s11144-019-01609-6 [7] XI H J, HOU X N, LIU Y J, QING S J, GAO Z X. Cu-Al spinel oxide as an efficient catalyst for methanol steam reforming[J]. Angew Chem Int Ed,2014,53:11886−11889. doi: 10.1002/anie.201405213 [8] 刘雅杰, 庆绍军, 侯晓宁, 张磊, 高志贤, 相宏伟. Cu-Al尖晶石的合成及非等温生成动力学分析[J]. 燃料化学学报,2020,48(3):338−348. doi: 10.3969/j.issn.0253-2409.2020.03.010LIU Ya-jie, QING Shao-jun, HOU Xiao-ning, ZHANG Lei, GAO Zhi-xian, XIANG Hong-wei. Synthesis of Cu-Al spinels and its non-isothermal formation kinetics analysis[J]. J Fuel Chem Technol,2020,48(3):338−348. doi: 10.3969/j.issn.0253-2409.2020.03.010 [9] 覃发玠, 刘雅杰, 庆绍军, 侯晓宁, 高志贤. 甲醇制氢铜铝尖晶石缓释催化剂的研究—不同铜源合成的影响[J]. 燃料化学学报,2017,45(12):1481−1488. doi: 10.3969/j.issn.0253-2409.2017.12.010QIN Fa-jie, LIU Ya-jie, QING Shao-jun, HOU Xiao-ning, GAO Zhi-xin. Cu-Al spinel as a sustained release catalyst for H2 production from methanol steam reforming: Effects of different copper sources[J]. J Fuel Chem Technol,2017,45(12):1481−1488. doi: 10.3969/j.issn.0253-2409.2017.12.010 [10] AREAN C O, VINUELA DIEZ J S, GONZALEZ J M, ARJONA A M. Crystal chemistry of CuxZn1−xAl2O4 spinels[J]. Mater Chem,1981,6:165. doi: 10.1016/0390-6035(81)90039-0 [11] NESTOUR A L, GAUDON M, VILLENEUVE G, DATURI M, ANDRIESSEN R, DEMOURGUES A. Defects in divided zinc-copper aluminate spinels: Structural features and optical absorption properties[J]. Inorg Chem,2007,46:4067−4078. doi: 10.1021/ic0624064 [12] ANAND G T, KENNEDY L J. One-pot microwave combustion synthesis of porous Zn1bzx xCuxAl2O4 (0 ≤ x ≤ 0.5) spinel nanostructures[J]. J Nanosci Nanotechnol,2013,4:3096−3103. [13] HOU X N, QING S J, LIU Y J, ZHANG L, ZHANG C S, FENG G, WANG X, GAO Z X, QIN Y. Cu1−xMgxAl3 spinel solid solution as a sustained release catalyst: One-pot green synthesis and catalytic performance in methanol steam reforming[J]. Fuel,2021,284:119041−119051. doi: 10.1016/j.fuel.2020.119041 [14] LIU Y J, QING S J, HOU X N, QIN F J, WANG X, GAO Z X, XIANG H W. Cu-Ni-Al spinel oxide as an efficient durable catalyst for methanol steam reforming[J]. ChemCatChem,2018,10:5698−5706. doi: 10.1002/cctc.201801472 [15] TIKHOV S F, VALEEV K R, SALANOV A N, CHEREPANOVA S V, BOLDYREVA N N, ZAIKOVSKII V I, SADYKOV V A, DUDINA D V, LOMOVSKY O I, ROMANENKOV V E. Phase formation during high-energy ball milling of the 33Al-45Cu-22Fe (at.%) powder mixture[J]. J Alloy Compd,2018,736:289−296. doi: 10.1016/j.jallcom.2017.11.100 [16] 闫晓峰, 高文桂, 毛文硕, 纳薇, 霍海辉, 常帅. 溶胶-凝胶法制备Cu-ZnO-ZrO2催化剂: 柠檬酸用量对催化剂性能的影响[J]. 化工进展,2020,39(10):4032−4040.YAN Xiao-feng, GAO Wen-gui, MAO Wen-shuo, NA Wei, HUO Hai-hui, CHANG Shuai. Preparation of Cu-ZnO-ZrO2 catalyst by sol-gel method: Effect of citric acid content on catalyst performance[J]. Chem Ind Eng Progress,2020,39(10):4032−4040. [17] HOU X N, QING S J, LIU Y J, LI L D, GAO Z X, QIN Y. Enhancing effect of MgO modification of Cu-Al spinel oxide catalyst for methanol steam reforming[J]. Int J Hydrogen Energy,2020,45:477−489. doi: 10.1016/j.ijhydene.2019.10.164 [18] MIERCZYNSKI P, VASILEV K, MIERCZYNSKA A, MANIUKIEWICZ W, MANIECKI T. The effect of ZnAl2O4 on the performance of Cu/ZnxAlyOx+1.5y supported catalysts in steam reforming of methanol[J]. Top Catal,2013,56:1015−1025. doi: 10.1007/s11244-013-0065-7 [19] 肖国鹏, 乔韦军, 张磊, 庆绍军, 张财顺, 高志贤. 钙钛矿型甲醇水蒸气重整制氢催化材料的研究[J]. 化学学报,2021,79(1):100−107. doi: 10.6023/A20080374XIAO Guo-peng, QIAO Wei-jun, ZHANG Lei, QING Shao-jun, ZHANG Cai-shun, GAO Zhi-xian. Study on hydrogen production catalytic materials for perovskite methanol steam reforming[J]. Acta Chim Sinica,2021,79(1):100−107. doi: 10.6023/A20080374 [20] HUANG Y H, WANG S F, TSAI A P, KAMEOKA S. Reduction behaviors and catalytic properties for methanol steam reforming of Cu-based spinel compounds CuX2O4 (X = Fe, Mn, Al, La)[J]. Ceram Int,2014,40:4541−4551. doi: 10.1016/j.ceramint.2013.08.130 [21] LI G J, GU C T, ZHU W B, WANG X F, YUAN X F, CUI Z J, WANG H L, GAO Z X. Hydrogen production from methanol decomposition using Cu-Al spinel catalysts[J]. J Clean Prod,2018,183:415−423. doi: 10.1016/j.jclepro.2018.02.088 [22] LIU Y J, QING S J, HOU X N, QIN F J, WANG X, GAO Z X. Temperature dependence of Cu-Al spinel formation and its catalytic performance in methanol steam reforming[J]. Catal Sci Technol,2017,7:5069−5078. doi: 10.1039/C7CY01236E [23] 焦桐, 许雪莲, 张磊, 翁幼云, 翁玉冰, 高志贤. CuO/CeO2-ZrO2/SiC整体催化剂催化甲醇水蒸气重整制氢的研究[J]. 化学学报,2021,79(4):513−519. doi: 10.6023/A20120562JIAO Tong, XU Xue-lian, ZHANG Lei, WENG You-yun, WENG Yu-bing, GAO Zhi-xian. Research on CuO/CeO2-ZrO2/SiC monolithic catalysts for hydrogen production by methanol steam reforming[J]. Acta Chim Sin,2021,79(4):513−519. doi: 10.6023/A20120562 [24] HOU X N, QIN F J, QING S J, LIU Y J, LI L D, GAO Z X, QIN Y. Probing the existing state of Cu(II) in Cu-Al spinel catalyst using N2O decomposition reaction with the aid of conventional characterizations[J]. Catal Sci Technol,2019,9:2993−3001. doi: 10.1039/C9CY00563C [25] AKIKA F Z, BENAMIRA M, LAHMAR H, TRARI M, AVRAMOVA I, SUZER S. Structural and optical properties of Cu-doped ZnAl2O4 and its application as photocatalyst for Cr(VI) reduction under sunlight[J]. Surf Interfaces,2020,18:100406−100416. doi: 10.1016/j.surfin.2019.100406 [26] WAGNER C D, DAVIS L E, ZELLER M V, TAYLOR J A, RAYMOND R H, GALE L H. Empirical atomic sensitivity factors for quantitative analysis by electron spectroscopy for chemical analysis[J]. Surf Interface Anal,1981,3(5):211−225. doi: 10.1002/sia.740030506 [27] 郗宏娟, 李光俊, 庆绍军, 侯晓宁, 赵金珍, 刘雅杰, 高志贤. 固相法合成铜铝尖晶石催化甲醇重整反应[J]. 燃料化学学报,2013,41(8):998−1002. doi: 10.3969/j.issn.0253-2409.2013.08.015XI Hong-juan, LI Guang-jun, QING Shao-jun, HOU Xiao-ning, ZHAO Jin-zhen, LIU Ya-jie, GAO Zhi-xian. Cu-Al spinel catalyst prepared by solid phase method for methanol steam reforming[J]. J Fuel Chem Technol,2013,41(8):998−1002. doi: 10.3969/j.issn.0253-2409.2013.08.015 [28] QING S J, HOU X N, LIU Y J, XI H J, WANG X, CHEN C M, WU Z W, GAO Z X. A novel supported Cu catalyst with highly dispersed copper nanoparticles and its remarkable catalytic performance in methanol decomposition[J]. RSC Adv,2014,4:52008−52011. doi: 10.1039/C4RA10101D [29] 庆绍军, 侯晓宁, 刘雅杰, 王磊, 李林东, 高志贤. Cu-Ni-Al尖晶石催化甲醇水蒸气重整制氢性能的研究[J]. 燃料化学学报,2018,46(10):1210−1217. doi: 10.3969/j.issn.0253-2409.2018.10.008QING Shao-jun, HOU Xiao-ning, LIU Ya-jie, WANG Lei, LI Lin-dong, GAO Zhi-xian. Catalytic performance of Cu-Ni-Al spinel for methanol steam reforming to hydrogen[J]. J Fuel Chem Technol,2018,46(10):1210−1217. doi: 10.3969/j.issn.0253-2409.2018.10.008 [30] QING S J, HOU X N, LIU Y J, LI L D, WANG X, GAO Z X, FAN W B. Strategic use of CuAlO2 as a sustained release catalyst for production of hydrogen from methanol steam reforming[J]. Chem Commun,2018,54:12242−12245. doi: 10.1039/C8CC06600K -




 下载:
下载: