Solid oxide electrolysis of carbon dioxide: Status and perspectives
-
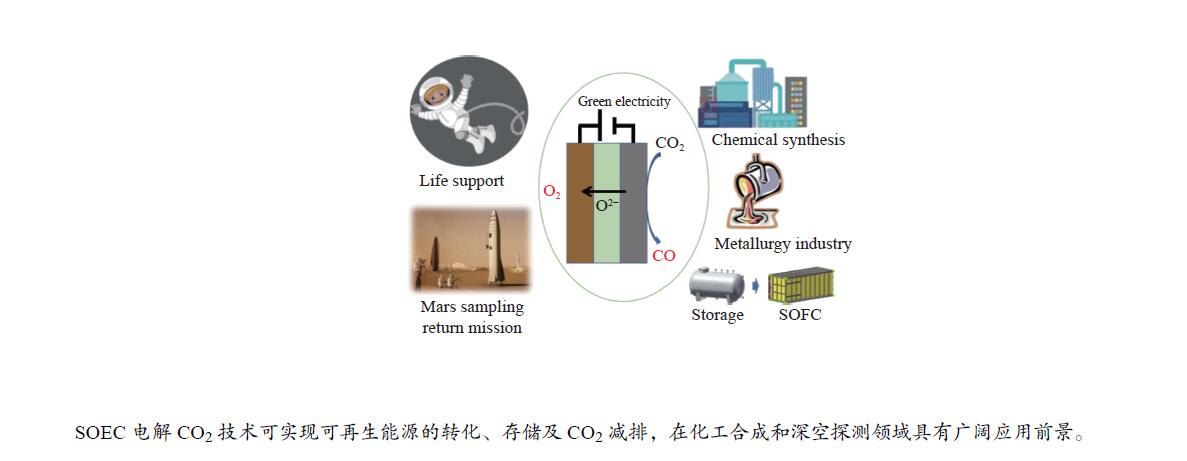
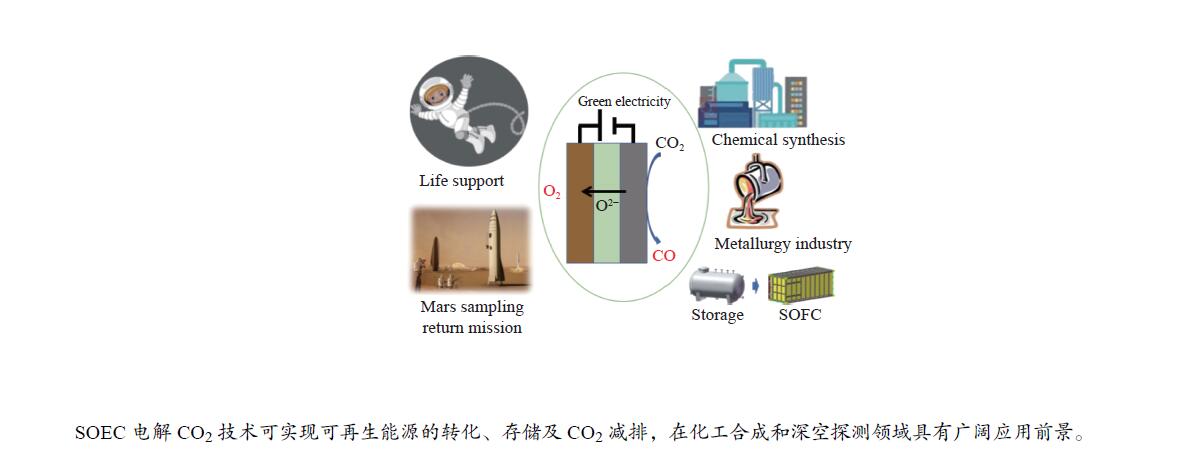
摘要: 高温固体氧化物电解CO2技术可以同时实现CO2资源化利用与可再生能源电力的转化和储存,是一种高效、绿色、灵活的CO2转化利用技术。该技术可将CO2转化为CO和O2,在化工合成和载人深空探测领域极具很好的应用前景,正逐渐成为环境与能源领域的研究热点。本综述对高温固体氧化物电解CO2技术的原理、电堆系统、应用领域、效率、经济性以及减排潜力进行了分析与总结,并就目前限制固体氧化物电解CO2技术工业化应用的关键材料、性能衰减和制约因素等问题进行了重点分析,展望了发展趋势和研究重点,以期为相关领域的学者提供参考。Abstract: High-temperature solid oxide electrolysis of CO2 is an efficient, green and flexible method of CO2 conversion and utilization, which can realize CO2 emission reduction and renewable energy power conversion and storage at the same time. It has great applied prospects in the fields of CO2 resource utilization and manned deep space exploration. And with the increasingly severe greenhouse effect and energy crisis, high-temperature solid oxide electrolysis of CO2 is gradually becoming a research hotspot in the field of international environment and energy. This review analyzes and summarizes the basic principles, key materials, performance degradation, stacks, application fields, efficiency, economy and emission reduction potential of high-temperature solid oxide electrolysis of CO2. Moreover, in view of the current problems and constraints that limit the industrial application of solid oxide electrolysis of CO2, multifaceted suggestions and strategies are put forward. This review aims to attract extensive attention in many fields and departments in China, and to promote industrial application of CO2 electrolysis in solid oxide electrolysis cell.
-
Key words:
- CO2 electrolysis /
- energy storage /
- CO and O2 production /
- solid oxide electrolysis cell
-
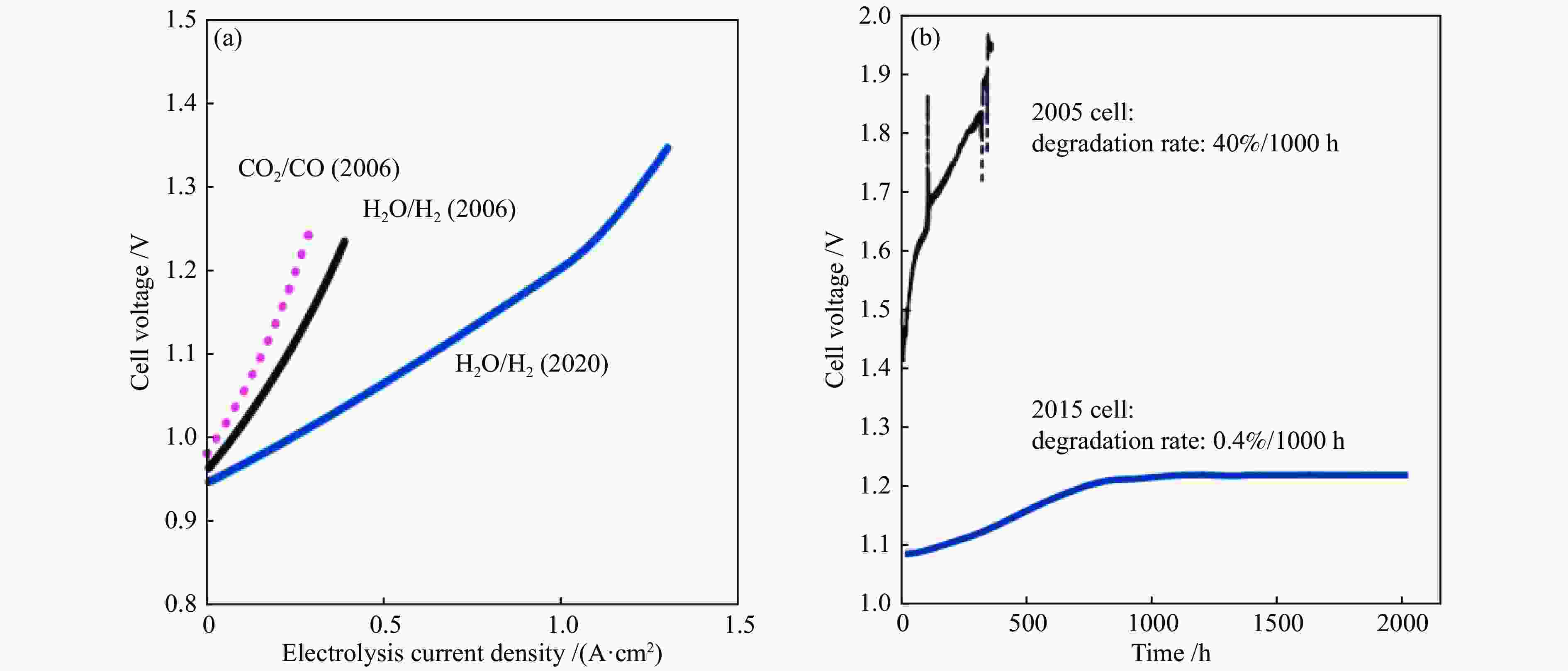
图 3 (a)电流-电压曲线,H2O/H2=1、CO2/CO=1、750 ℃;(b)稳定性测试,电流密度为1 A/cm2,温度850 ℃(2005)、800 ℃(2015)[13]
Figure 3 (a) Current-voltage curves for cells at 750 ℃, measured in H2O/H2=1 or CO2/CO=1; (b) Durability test of H2O electrolysis at 1 A/cm2 on a cell measured at 850 ℃ (2005) and 800 ℃ (2015)[13](with permission from Elsevier)
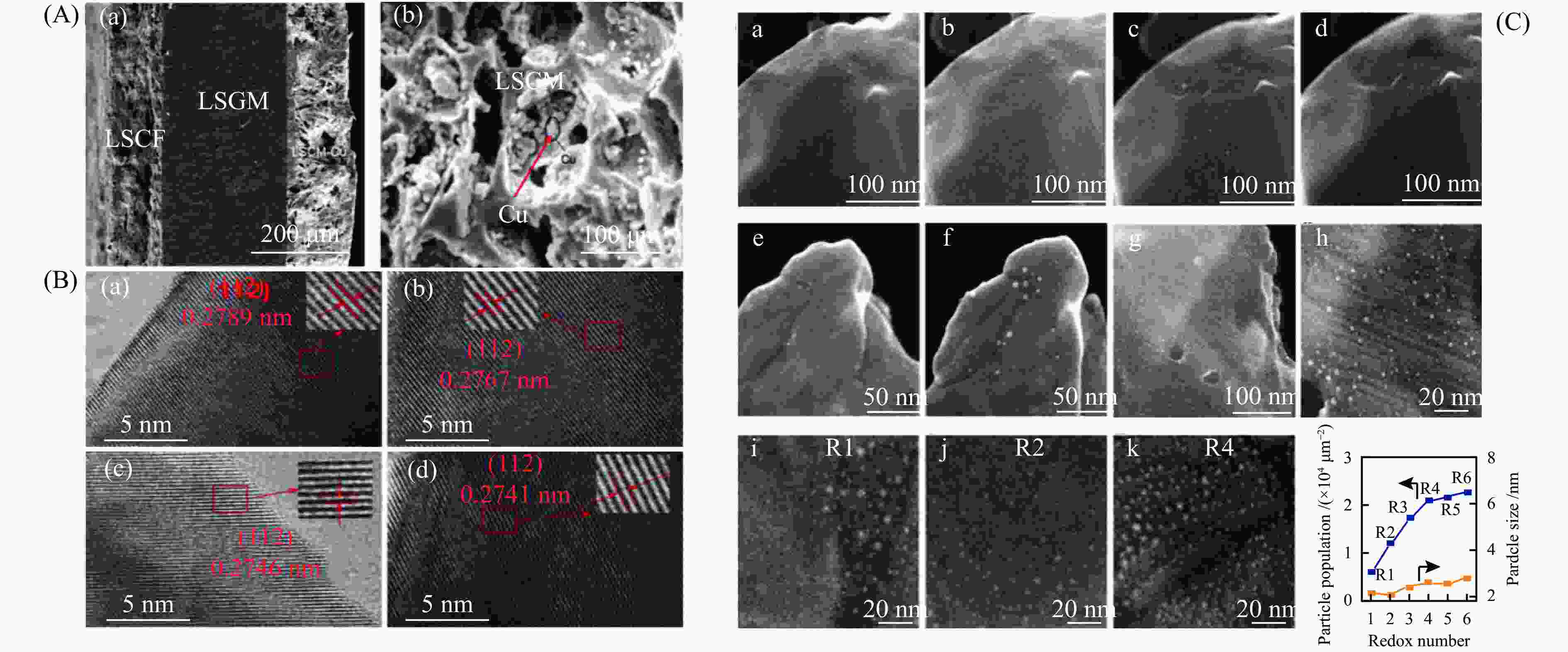
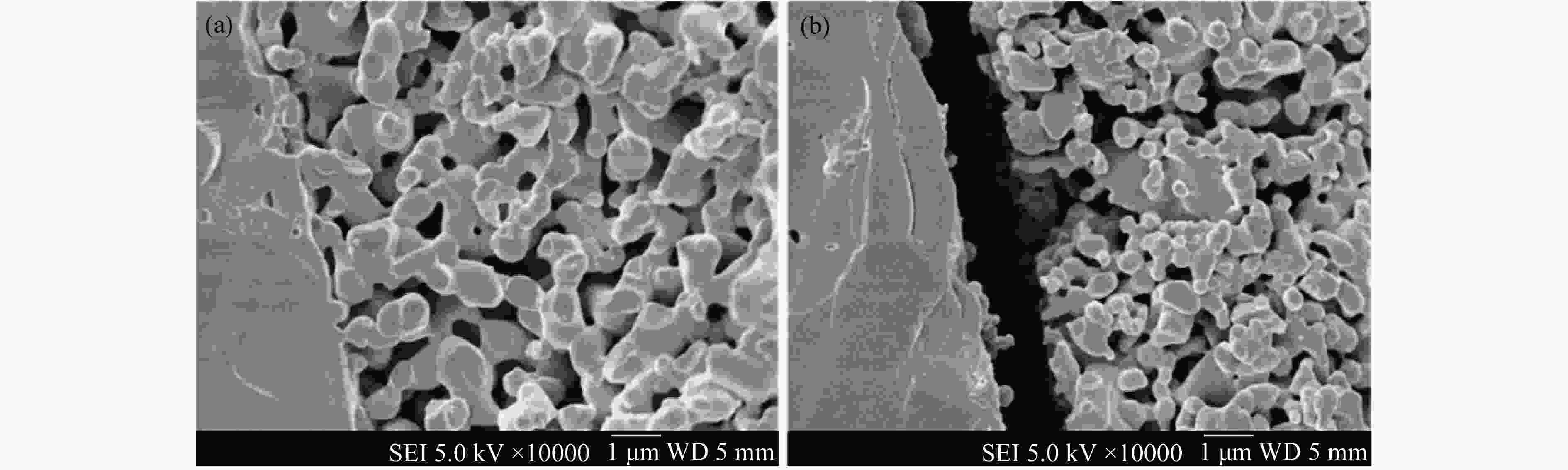
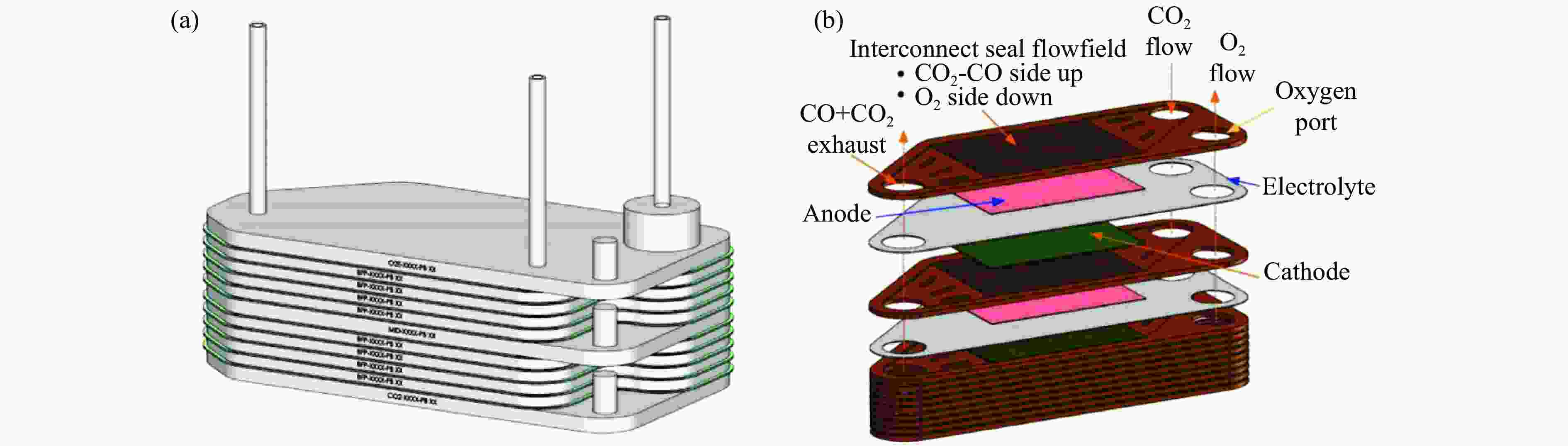
图 8 (A)LSCF|LSGM|LSCM-Cu电池的SEM剖面图(a),LSCM-Cu阴极SEM放大图(b)[73];(B)不同Bi掺杂量的La0.75–xBixSr0.25Cr0.5Fe0.5O3–δ材料的HRTEM图[74];(C)Sr2Fe1.4Ru0.1Mo0.5O6–δ在氧化还原操作过程中表面形貌和RuFe合金脱溶的动态结构演变过程的STEM图[75]
Figure 8 (A) Crosses-section SEM images of LSCF|LSGM|LSCM-Cu cell (a), enlarged SEM image of LSCM-Cu cathode (b) (with permission from Elsevier)[73]; (B) HR-TEM for the reduced La0.75–xBixSr0.25Cr0.5Fe0.5O3–δ powers (with permission from RSC)[74]; (C) In situ secondary electron (SE)-STEM images of Sr2Fe1.4Ru0.1Mo0.5O6–δ catalysts (with permission from Nature)[75]
表 1 不同陶瓷阴极的性能比较
Table 1 Performance comparison of different cermet cathodes
Cell component Feed gas Temperature/℃ Voltage/V Current/(A·cm–2) Ref. Ni-YSZ||YSZ||LSM-YSZ 30%CO/CO2 800 1.5 0.42 [59] BaCO3-Ni-YSZ||YSZ||LSM-YSZ 30%CO/CO2 800 1.5 0.42 [59] Ni-YSZ||YSZ||LSM-YSZ 10%CO/CO2 800 1.5 3.10 [19] Ni-YSZ||YSZ||LSM 30%CO/CO2 850 1.2 0.80 [54] Ni-YSZ||YSZ||LSCF 25%CO/CO2 750 1.2 0.36 [69] Ni-YSZ||GDC||GDC-PrBaCo2O5+δ 33%CO/CO2 700 1.3 1.11 [70] Ni-YSZ||YSZ||LSM-YSZ-RuO2 5%N2/CO2 800 1.4 0.93 [61] Ni-GDC||YSZ||GDC|LSCF 30%N2/CO2 1000 1.0 0.9 [58] Ag-GDC||YSZ||LSM|YSZ CO2 800 1.5 0.62 [56] Ni-Cr2O3–δ||LSGM|| 50%CO/CO2 750 2.0 0.9 [57] Ni-Cr2O3–δ||LSGM||Ba0.5Sr0.5Co0.8Fe0.2O3–δ CO/CO2/Ar 800 1.6 2.07 [71] 表 2 不同钙钛矿基复合氧化物为阴极电解CO2的性能比较
Table 2 Performance of high CO2 electrolysis with different perovskite-based cathodes
Cathode materials Feed gas Temperature/℃ U/I(V/A·cm–2) Ref. La0.75Sr0.25Cr0.5Mn0.5O3–δ-Gd0.1Ce0.9O1.95 CO2 800 1.5/0.17 [87] CeO2-La0.75Sr0.25Cr0.5Mn0.5O3–δ-Gd0.1Ce0.9O1.95 CO2 800 1.5/0.30 [87] La0.75Sr0.25Cr0.5Mn0.5O3–δ-Gd0.1Ce0.9O1.95 CO2 800 1.5/0.41 [88] Pr0.25(La0.75Sr0.25)0.75Cr0.5Mn0.5O3–δ-Gd0.1Ce0.9O1.95 CO2 800 1.5/0.67 [88] La0.6Sr0.4Fe0.8Ni0.2O3−δ CO2 850 1.55/1.21 [78] Gd0.2Ce0.8O1.9-Sr2Fe1.5Mo0.5O6–δ 5%N2/CO2 800 1.6/0.446 [89] Ni-doped La(Sr)FeO3–δ 30%CO/CO2 850 1.55/1.21 [78] NiFe-Sr1.9Fe1.5Mo0.4Ni0.1O6–δ CO2 800 1.5/2.16 [90] SrEu2Fe2O7 CO2 800 1.5/1.27 [91] La0.9Sr0.8Co0.4Mn0.6O3.9−δF0.1 30%CO/CO2 850 1.3/0.499 [92] Sr2Fe1.4Mn0.1Mo0.5O6–δ CO2 800 1.5/1.35 [93] Pt-SDC-La0.6Sr0.4Co0.2Fe0.8O3–δ CO2 800 1.6/1.42 [94] NiFe-SDC-La0.6Sr0.4Fe0.8Mn0.2O3−δ CO2 850 1.5/1.4 [95] Sr1.9La0.1Fe1.5Mo0.5O6–δ CO2 850 1.5/2.76 [96] F-doped La1.6Sr0.4Fe0.8Ni0.2O3–δ CO2 850 1.8/1.92 [97] 表 3 不同CO2电解技术的对比
Table 3 Comparison of different CO2 electrolysis technologies
SOE LTE MSE FLE Temperature/℃ 700–900 25 500–800 25 Faradaic efficiency near100% 60%–90% near100% 60%–90% Current density high low high low Energy efficiency >90% 30%–50% >70% 30%–50% -
[1] Global Monitoring Laboratory. https://gml.noaa.gov/ccgg/trends/global.html. [2] LAWA, SAUNDERS P, MIDDLETON J, MCCOY D. Global warming must stay below 1.5 ℃[J]. BMJ,2018,363:k4410. [3] 乔劲松, 韩苗苗. 多孔二元过渡金属纳米片阵列电极制备及电催化析氢研究[J]. 分子催化,2021,35(5):449−455.QIAO Jin-song, HAN Miao-miao. Preparation of porous binary transition metal nanosheets array electrode and its electrocatalytic hydrogen evolution[J]. J Mol Catal (China),2021,35(5):449−455. [4] 何玉梅, 刘冰, 李金林. CexZr1-xO2/Co/C-N催化CO2加氢性能研究[J]. 分子催化,2021,35(6):561−570.HE Yu-mei, LIU Bing, LI Jin-lin. The study of CO2 hydrogenation activity over CexZr1−xO2/Co/C-N catalysts[J]. J Mol Catal (China),2021,35(6):561−570. [5] MIKKELSEN M, JORGENSEN M. KREBS F C. The teraton challenge. A review of fixation and transformation of carbon dioxide[J]. Energy Environ Sci,2010,3(1):43−81. doi: 10.1039/B912904A [6] 潘茵茵, 宋广杰, 薛宽荣, 许胜. 非合成气法烯烃、炔烃氢甲酰化研究进展[J]. 分子催化,2021,35(2):166−177.PAN Yin-yin, SONG Guang-jie, XUE Kuan-rong, XU Sheng. The development of hydroformylation of alkenes and alkynes with syngas substitutes[J]. J Mol Catal (China),2021,35(2):166−177. [7] 张俊杰, 亚力昆江·吐尔逊, 迪丽努尔·塔力甫, 阿布力克木·阿布力孜. Ru掺杂BiOBr空心微球的原位合成及其光催化CO2还原和有机污染物降解性能研究[J]. 分子催化,2020,34(1):8−18.ZHANG Jun-jie, TURSUN Yalkunjan, TALIFU Dilinuer, ABULIZI Abulikemu. In situ synthesis of Ru doped hollow BiOBr microsphere as an efficient photocatalyst for photocatalytic CO2 reduction and organic pollutant degradation[J]. J Mol Catal (China),2020,34(1):8−18. [8] 葛建华, 章志平, 江道传, 杜平武. Ni3N修饰BiVO4用于光电催化分解水的研究[J]. 分子催化,2021,35(3):235−242.GE Jian-hua, ZHANG Zhi-ping, JIANG Dao-chuan, DU Ping-wu. Ni3N decorated BiVO4 photoanodes for solar-driven water splitting[J]. J Mol Catal(China),2021,35(3):235−242. [9] 王莎莎, 于博. 金属磷化物(NiCo)2P/NF自支撑电极制备及其电催化研究[J]. 分子催化,2020,34(1):81−86.WANG Sha-sha, YU Bo. Preparation of (NiCo)2P/NF self-supporting electrode and its electrocatalytic water splitting[J]. J Mol Catal(China),2020,34(1):81−86. [10] 梁志铭, 聂小娃, 郭新闻, 宋春山. 镍掺杂对Fe催化剂上CO2加氢制烃影响的理论计算研究[J]. 分子催化,2020,34(4):293−303.LIANG Zhi-ming, NIE Xiao-wa, GUO Xin-wen, SONG Chun-shan. DFT insight into the effect of Ni doping on hydrocarbons synthesis from CO2 hydrogenation over Fe catalyst[J]. J Mol Catal (China),2020,34(4):293−303. [11] EBBESEN S D, JENSEN S S, HAUCH A, MOGENSEN M B. High temperature electrolysis in alkaline cells, solid proton conducting cells, and solid oxide cells[J]. Chem Rev,2014,114(21):10697−10734. doi: 10.1021/cr5000865 [12] MEYEN F E, HECHT M H, HOFFMAN J A. Thermodynamic model of Mars oxygen ISRU experiment (MOXIE)[J]. Acta Astronaut,2016,129:82−87. doi: 10.1016/j.actaastro.2016.06.005 [13] HAUCH A, KUNGAS R, BLENNOW P, HANSEN A B, HANSEN J B, MATHIESEN B V, MOGENSEN M B. Recent advances in solid oxide cell technology for electrolysis [J]. Science, 2020, 370 (6513). [14] 周公文, 李宝光, 龙泽, 曾辉, 李海滨, SOFC. SOFC电解质薄膜制备技术研究进展[J]. 电源技术,2016,40(2):473−476.ZHOU Gong-wen, LI Bao-guang, LONG Ze, ZENG Hui, LI Hai-bin. Review of preparation technologies for thin electrolyte film of solid oxide fuel cells[J]. Chin J Power Sources,2016,40(2):473−476. [15] ELIKAN L, MORRIS J, WU C. Development of a solid electrolyte carbon dioxide and water reduction system for oxygen recovery[R]. NASA: 1972. [16] 葛奔, 艾德生, 林旭平, 杨志宾. 固体氧化物电解池技术应用研究进展[J]. 科技导报,2017,35(8):37−46.GE Ben, AI De-sheng, LIN Xu-ping, YANG Zhi-bin. Progress on application of solid oxide electrolysis cells[J]. Sci Technol Rev,2017,35(8):37−46. [17] 王振, 于波, 张文强, 陈靖, 徐景明. 高温共电解H2O/CO2制备清洁燃料[J]. 化学进展,2013,25(7):1229−1236.WANG Zhen, YU Bo, ZHANG Wen-qiang, CHEN Jing, XU Jing-ming. Clean fuel production through high temperature Co-electrolysis of H2O and CO2[J]. Prog Chem,2013,25(7):1229−1236. [18] 李一航, 夏长荣. 固体氧化物电解池直接电解CO2的研究进展[J]. 电化学,2020,26(2):162−174.LI Yi-hang, XIA Chang-rong. Recent advances of CO2 electrochemical reduction insolid oxide electrolysis cells[J]. J Electrochem,2020,26(2):162−174. [19] RABUNI M F, VATCHARASUWAN N, LI T, LI K. High performance micro-monolithic reversible solid oxide electrochemical reactor[J]. J Power Sources,2020,458:228026. doi: 10.1016/j.jpowsour.2020.228026 [20] Topsoe website. https://www.topsoe.com/. [21] 浙江氢邦科技有限公司官网新闻. http://www.h2-bank.com/.Zhejiang H2-Bank Technology Co.,Ltd News. http://www.h2-bank.com/. [22] NI M, LEUNG M K H, LEUNG D. Y. C. Parametric study of solid oxide steam electrolyzer for hydrogen production[J]. Int J Hydrogen Energy,2007,32(13):2305−2313. doi: 10.1016/j.ijhydene.2007.03.001 [23] SU T, LI Y, XUE S, XU Z, ZHENG M, XIA C. Kinetics of CO2 electrolysis on composite electrodes consisting of Cu and samaria-doped ceria[J]. J Mater Chem A,2019,7(4):1598−1606. doi: 10.1039/C8TA09015G [24] LI Y, YU L, YU Y, MALIUTINA K, WU Q, HE C, FAN L. Understanding CO2 electrochemical reduction kinetics of mixed-conducting cathodes by the electrical conductivity relaxation method[J]. Int J Hydrogen Energy,2021,46(15):9646−9652. doi: 10.1016/j.ijhydene.2020.07.141 [25] OPITZ A K, NENNING A, RAMESHAN C, KUBICEK M, GOTSCH T, BLUME R, HAVECKER M, KNOP-GERICKE A, RUPPURRECHTER G, KLOTZER B, FLEIG J. Surface chemistry of perovskite-type electrodes during high temperature CO2 electrolysis investigated by operando photoelectron spectroscopy[J]. ACS Appl Mater Interfaces,2017,9(41):35847−35860. doi: 10.1021/acsami.7b10673 [26] YANG Y, LI Y, JIANG Y, ZHENG M, HONG T, WU X, XIA C. The electrochemical performance and CO2 reduction mechanism on strontium doped lanthanum ferrite fuel electrode in solid oxide electrolysis cell[J]. Electrochim Acta,2018,284:159−167. doi: 10.1016/j.electacta.2018.07.187 [27] SONGY, ZHANG X, XIE K, WANG G, BAO X. High-temperature CO2 electrolysis in solid oxide electrolysis cells: developments, challenges, and prospects[J]. Adv Mater,2019,31(50):e1902033. doi: 10.1002/adma.201902033 [28] MINH N Q. Incentenary of Nernst's discovery of zirconia electrolytes - review of zirconia-based electrochemical technologies[J]. ECS Proceedings Volumes,1999,(1):127. [29] MOLENDA J, ŚWIERCZEK K, ZAJAC W. Functional materials for the IT-SOFC[J]. J Power Sources,2007,173(2):657−670. doi: 10.1016/j.jpowsour.2007.05.085 [30] ALFECHE D M, CERVERA R B. Highly conducting Sc and Y co-doped ZrO2 thin film solid electrolyte on a porous Ni/YSZ electrode prepared via simple drop-coating method[J]. Ceram Int,2020,46(8, Part A):10561−10567. [31] TEMLUXAME P, PUENGJINDA P, PENG-ONT S, NGAMPUENGPIS W, SIRIMUNGKALAKUL N, JIWANURUK T, SORNCHAMNI T, KIM-LOHSOONTORN P. Comparison of ceria and zirconia based electrolytes for solid oxide electrolysis cells[J]. Int J Hydrogen Energy,2021,46(48):24568−24580. doi: 10.1016/j.ijhydene.2020.03.121 [32] LYU Q, ZHU T, QU H, SUN Z, SUN K, ZHONG Q, HAN M. Lower down both ohmic and cathode polarization resistances of solid oxide fuel cell via hydrothermal modified gadolinia doped ceria barrier layer[J]. J Eur Ceram Soc,2021,41(12):5931−5938. doi: 10.1016/j.jeurceramsoc.2021.05.020 [33] ISHIHARA T, MATSUDA H, TAKITA Y. Doped LaGaO3 perovskite type oxide as a new oxide ionic conductor[J]. J Am Chem Soc,1994,116(9):3801−3803. doi: 10.1021/ja00088a016 [34] SHI H, SU C, RAN R, CAO J, SHAO Z. Electrolyte materials for intermediate-temperature solid oxide fuel cells[J]. Prog Nat Sci,2020,30(6):764−774. doi: 10.1016/j.pnsc.2020.09.003 [35] STEELE B C H, HEINZEL A. Materials for fuel-cell technologies[J]. Nature,2001,414(6861):345−352. doi: 10.1038/35104620 [36] DI BARTOLOMEO E, BASOLI F, LUISETTO I, TUTI S, ZURLO F, SALEHI Z, LICOCCIA S. Ni and Ni-Co La0.8Sr0.2Ga0.8Mg0.2O3−δ infiltrated cells in H2 and CH4/CO2 mixture[J]. Appl Catal B: Environ,2016,191:1−7. doi: 10.1016/j.apcatb.2016.03.010 [37] WANG L S, LI C X, LI G R, YANG G J, ZHANG S L, LI C J. Enhanced sintering behavior of LSGM electrolyte and its performance for solid oxide fuel cells deposited by vacuum cold spray[J]. J Eur Ceram Soc,2017,37(15):4751−4761. doi: 10.1016/j.jeurceramsoc.2017.06.007 [38] YU S, BI H, SUN J, ZHU L, YU H, LU C, LIU X. Effect of grain size on the electrical properties of strontium and magnesium doped lanthanum gallate electrolytes[J]. J Alloys Compd,2019,777:244−251. doi: 10.1016/j.jallcom.2018.10.257 [39] MOGENSEN M, SAMMES N M, TOMPSETT G A. Physical, chemical and electrochemical properties of pure and doped ceria[J]. Solid State Ionics,2000,129(1):63−94. [40] STEELE B C H. Appraisal of Ce1−yGdyO2−y/2 electrolytes for IT-SOFC operation at 500 ℃[J]. Solid State Ionics,2000,129(1):95−110. [41] YAHIRO H, BABA Y, EGUCHI K, ARAI H. High temperature fuel cell with ceria-yttria solid electrolyte[J]. J Electrochem Soc,1988,135(8):2077−2080. doi: 10.1149/1.2096212 [42] MADHURI C, VENKATARAMANA K, NURHAYATI A, REDDY C V. Effect of La3+ and Pr3+ co-doping on structural, thermal and electrical properties of ceria ceramics as solid electrolytes for IT-SOFC applications[J]. Curr Appl Phys,2018,18(10):1134−1142. doi: 10.1016/j.cap.2018.06.013 [43] VENKATARAMANA K, MADHURI C, MADHUSUDAN C, REDDY Y S, BHIKSHAMAIAH G, REDDY C V. Investigation on La3+ and Dy3+ co-doped ceria ceramics with an optimized average atomic number of dopants for electrolytes in IT-SOFCs[J]. Ceram Int,2018,44(6):6300−6310. doi: 10.1016/j.ceramint.2018.01.020 [44] ULLAH M K, RAZA R, ASGHAR M I, ALI A, RAFIQUE A, ABBAS G, AHMAD M A, HANIF I, AKBAR M, LUND P D. Tri-doped ceria (M0.2Ce0.8O2–δ, M= Sm0.1, Ca0.05, Gd0.05) electrolyte for hydrogen and ethanol-based fuel cells[J]. J Alloys Compd,2019,773:548−554. doi: 10.1016/j.jallcom.2018.09.201 [45] VENKATARAMANA K, MADHURI C, SURESH REDDY Y, BHIKSHAMAIAH G, VISHNUVARDHAN REDDY C. Structural, electrical and thermal expansion studies of tri-doped ceria electrolyte materials for IT-SOFCs[J]. J Alloys Compd,2017,719:97−107. doi: 10.1016/j.jallcom.2017.05.022 [46] 刘润泽, 周芬, 王青春, 郜建全, 包金小, 宋希文. 固体氧化物燃料电池用CeO2基电解质的研究进展[J]. 材料导报,2021,35(S1):29−32.LIU Run-ze, ZHOU Fen, WANG Qing-chun, GAO Jian-quan, BAO Jin-xiao, SONG Xi-wen. Research progressof CeO2-based electrolytesfor solid oxide fuel cells[J]. Materials Reports,2021,35(S1):29−32. [47] SHUK P, WIEMHOFER H D, GUTH U, GOPEL W, GREENBLATT M. Oxide ion conducting solid electrolytes based on Bi2O3[J]. Solid State Ionics,1996,89(3):179−196. [48] HUANG K, FENG M, GOODENOUGH J B. Bi2O3-Y2O3-CeO2 solid solution oxide-ion electrolyte[J]. Solid State Ionics,1996,89(1):17−24. [49] CARDENAS-TERRAZAS P S, AYALA-AYALA M T, MUNOZ-SALDANA J, FUENTES A F, LEAL-CHAVEZ D A, LEDAZMA-SILLAS J E, CARRENO-GALLARDO C, HERRERA-RAMIREZ J M. High ionic conductivity dysprosium and tantalum Co-doped bismuth oxide electrolyte for low-temperature SOFCs[J]. Ionics,2020,26(9):4579−4586. doi: 10.1007/s11581-020-03572-y [50] CHEN R, LI C X, LI C J. Plasma-sprayed (Bi2O3)0.705(Er2O3)0.245(WO3)0.05electrolyte for intermediate-temperature solid oxide fuel cells (IT-SOFCs)[J]. J Therm Spray Technol,2022,31(1):297−306. [51] ZHANG X, SONG Y, WANG G, BAO X. Co-electrolysis of CO2 and H2O in high-temperature solid oxide electrolysis cells: Recent advance in cathodes[J]. J Energy Chem,2017,26(5):839−853. doi: 10.1016/j.jechem.2017.07.003 [52] TAO G, SRIDHAR K R, CHAN C L. Study of carbon dioxide electrolysis at electrode/electrolyte interface: Part II. Pt-YSZ cermet/YSZ interface[J]. Solid State Ionics,2004,175(1):621−624. [53] TAO G, SRIDHAR K R, CHAN C L. Study of carbon dioxide electrolysis at electrode/electrolyte interface: Part I. Pt/YSZ interface[J]. Solid State Ionics,2004,175(1):615−619. [54] EBBESEN S D, MOGENSEN M. Electrolysis of carbon dioxide in solid oxide electrolysis cells[J]. J Power Sources,2009,193(1):349−358. doi: 10.1016/j.jpowsour.2009.02.093 [55] LU L, LIU W, WANG J, WANG Y, XIA C, ZHOU X D, CHEN M, GUAN W. Long-term stability of carbon dioxide electrolysis in a large-scale flat-tube solid oxide electrolysis cell based on double-sided air electrodes[J]. Appl Energy,2020,259:114130. doi: 10.1016/j.apenergy.2019.114130 [56] XIE Y, XIAO J, LIU D, LIU J, YANG C. Electrolysis of carbon dioxide in a solid oxide electrolyzer with silver-gadolinium-doped ceria cathode[J]. J Electrochem Soc,2015,162(4):F397−F402. doi: 10.1149/2.0501504jes [57] CHENG C Y, KELSALL G H, KLEIMINGER L. Reduction of CO2 to CO at Cu-ceria-gadolinia (CGO) cathode in solid oxide electrolyser[J]. J Appl Electrochem,2013,43(11):1131−1144. doi: 10.1007/s10800-013-0566-x [58] SINGH V, MUROYAMA H, MATSUI T, HASHIGAMI S, INAGAKI T, EGUCHI K. Feasibility of alternative electrode materials for high temperature CO2 reduction on solid oxide electrolysis cell[J]. J Power Sources,2015,293:642−648. doi: 10.1016/j.jpowsour.2015.05.088 [59] ZHENG M, WANG S, YANG Y, XIA C. Barium carbonate as a synergistic catalyst for the H2O/CO2 reduction reaction at Ni-yttria stabilized zirconia cathodes for solid oxide electrolysis cells[J]. J Mater Chem A,2018,6(6):2721−2729. doi: 10.1039/C7TA08249E [60] ZHAN Z, ZHAO L. Electrochemical reduction of CO2 in solid oxide electrolysis cells[J]. J Power Sources,2010,195(21):7250−7254. doi: 10.1016/j.jpowsour.2010.05.037 [61] SONG Y, ZHOU Z, ZHANG X, ZHOU Y, GONG H, LV H, LIU Q, WANG G, BAO X. Pure CO2 electrolysis over an Ni/YSZ cathode in a solid oxide electrolysis cell[J]. J Mater Chem A,2018,6(28):13661−13667. doi: 10.1039/C8TA02858C [62] WANG W, GAN L, LEMMON J P, CHEN F, IRVINE J P S, XIE K. Enhanced carbon dioxide electrolysis at redox manipulated interfaces[J]. Nat Commun,2019,10(1):1550. doi: 10.1038/s41467-019-09568-1 [63] HAUCH A, TRALSEN M L, KUNGAS R, SKAFTE T L. CO2 electrolysis-Gas impurities and electrode overpotential causing detrimental carbon deposition[J]. J Power Sources,2021,506:230108. doi: 10.1016/j.jpowsour.2021.230108 [64] DONG D, XU S, SHAO X, HUCKER L, MARIN J, PHAM T, XIE K, YE Z, YANG P, YU L, PARKINSON G, LI CZ. Hierarchically ordered porous Ni-based cathode-supported solid oxide electrolysis cells for stable CO2 electrolysis without safe gas[J]. J Mater Chem A,2017,5(46):24098−24102. doi: 10.1039/C7TA06839E [65] DIPU A L, UJISAWA Y, RYU J, KATO Y. Electrolysis of carbon dioxide for carbon monoxide production in a tubular solid oxide electrolysis cell[J]. Ann Nucl Energy,2015,81:257−262. doi: 10.1016/j.anucene.2015.02.046 [66] KAUR G, KULKARNI A P, FINI D, GIDDEY S, SEEBER A. High-performance composite cathode for electrolysis of CO2 in tubular solid oxide electrolysis cells: A pathway for efficient CO2 utilization[J]. J CO2 Util,2020,41:101271. doi: 10.1016/j.jcou.2020.101271 [67] HODJATIOPUGH O, DHIR A, STEINBERGER-WILCKENS R. The development of current collection in micro-tubular solid oxide fuel cells—A review[J]. Appl Sci,2021,11(3):1077. doi: 10.3390/app11031077 [68] SCIAZKO A, SHIMURA T, KOMATSU Y, SHIKAZONO N. Ni-GDC and Ni-YSZ electrodes operated in solid oxide electrolysis and fuel cell modes[J]. J Therm Sci Technol,2021,16(1):20−00242. [69] YAN J, CHEN H, DOGDIBEGOVIC E, STEVENSON J W, CHENG M, ZHOU XD. High-efficiency intermediate temperature solid oxide electrolyzer cells for the conversion of carbon dioxide to fuels[J]. J Power Sources,2014,252:79−84. doi: 10.1016/j.jpowsour.2013.11.047 [70] LIU T, CHEN X, WU J, SHENG Z, LIU G, WANG Y. A highly-performed, dual-layered cathode supported solid oxide electrolysis cell for efficient CO2 electrolysis fabricated by phase inversion co-tape casting method[J]. J Electrochem Soc,2017,164(12):F1130−F1135. doi: 10.1149/2.0841712jes [71] HU X, XIE K. Active and stable Ni/Cr2O3-δ cathodes for high temperature CO2 electrolysis[J]. J Power Sources,2019,430:20−24. doi: 10.1016/j.jpowsour.2019.05.014 [72] JIANG S P. Nanoscale and nano-structured electrodes of solid oxide fuel cells by infiltration: Advances and challenges[J]. Int J Hydrogen Energy,2012,37(1):449−470. doi: 10.1016/j.ijhydene.2011.09.067 [73] XING R, WANG Y, ZHU Y, LIU S, JIN C. Co-electrolysis of steam and CO2 in a solid oxide electrolysis cell with La0.75Sr0.25Cr0.5Mn0.5O3−δ-Cu ceramic composite electrode[J]. J Power Sources,2015,274:260−264. doi: 10.1016/j.jpowsour.2014.10.066 [74] QI W, GAN Y, YIN D, LI Z, WU G, XIE K, WU Y. Remarkable chemical adsorption of manganese-doped titanate for direct carbon dioxide electrolysis[J]. J Mater Chem A,2014,2(19):6904−6915. doi: 10.1039/C4TA00344F [75] LV H, LIN L, ZHANG X, LI R, SONG Y, MATSUMOTO H, TA N, ZENG C, FU Q, WANG G, BAO X. Promoting exsolution of RuFe alloy nanoparticles on Sr2Fe1.4Ru0.1Mo0.5O6-delta via repeated redox manipulations for CO2 electrolysis[J]. Nat Commun,2021,12(1):5665. doi: 10.1038/s41467-021-26001-8 [76] ZHOU Y, ZHOU Z, SONG Y, ZHANG X, GUAN F, LV H, LIU Q, MIAO S, WANG G, BAO X. Enhancing CO2 electrolysis performance with vanadium-doped perovskite cathode in solid oxide electrolysis cell[J]. Nano Energy,2018,50:43−51. doi: 10.1016/j.nanoen.2018.04.054 [77] LIU Q X, SONG Y F, LI R T, LV H F, FENG W C, SHEN Y X, ZHANG X M, WANG G X, BAO X H. A vanadium-doped BSCF perovskite for CO2 electrolysis in solid oxide electrolysis cells[J]. Int J Hydrogen Energy,2021,46(38):19814−19821. doi: 10.1016/j.ijhydene.2021.03.134 [78] LIU S, LIU Q, LUO J L. The excellence of La(Sr)Fe(Ni)O3 as an active and efficient cathode for direct CO2 electrochemical reduction at elevated temperatures[J]. J Mater Chem A,2017,5(6):2673−2680. doi: 10.1039/C6TA09151B [79] WAN Y, YANG Y, LU Y, PENG R, XIA C. A strategy to enhance the catalytic activity of electrode materials by doping bismuth for symmetrical solid oxide electrolysis cells[J]. ACS Appl Energy Mater,2022,5(2):2339−2348. doi: 10.1021/acsaem.1c03822 [80] WAN Y, XING Y, XU Z, XUE S, ZHANG S, XIA C. A-site bismuth doping, a new strategy to improve the electrocatalytic performances of lanthanum chromate anodes for solid oxide fuel cells[J]. Appl Catal B: Environ,2020,269:118809. doi: 10.1016/j.apcatb.2020.118809 [81] ANSARI H M, ADDO P K, MULMI S, YUAN H, BOTTON G A, THANGADURAI V, BIRSS V I. Deciphering the interaction of single-phase La0.3Sr0.7Fe0.7Cr0.3O3−δ with CO2/CO environments for application in reversible solid oxide cells[J]. ACS Appl Mater Interfaces,2022,14(11):13388−13399. doi: 10.1021/acsami.2c00857 [82] XI X, FAN Y, ZHANG J, LUO J L, FU X Z. In situ construction of hetero-structured perovskite composites with exsolved Fe and Cu metallic nanoparticles as efficient CO2 reduction electrocatalysts for high performance solid oxide electrolysis cells[J]. J Mater Chem A,2022,10(5):2509−2518. doi: 10.1039/D1TA07678G [83] ZHANG L, LI Y, ZHANG B, WAN Y, XU Z, ZHANG S, ZHU T, XIA C. (La, Sr)(Ti, Fe)O3−δ perovskite with in‐situ constructed FeNi3 nanoparticles as fuel electrode for reversible solid oxide cell[J]. Int J Energy Res,2021,45(15):21264−21273. doi: 10.1002/er.7177 [84] DING S, LI M, PANG W, HUA B, DUAN N, ZHANG YQ, ZHANG SN, JIN Z, LUO JL. A-site deficient perovskite with nano-socketed Ni-Fe alloy particles as highly active and durable catalyst for high-temperature CO2 electrolysis[J]. Electrochim Acta,2020,335:135683. doi: 10.1016/j.electacta.2020.135683 [85] JIANG Y, CHEN F, XIA C. A review on cathode processes and materials for electro-reduction of carbon dioxide in solid oxide electrolysis cells[J]. J Power Sources,2021,493:229713. doi: 10.1016/j.jpowsour.2021.229713 [86] YE L, XIE K. High-temperature electrocatalysis and key materials in solid oxide electrolysis cells[J]. J Energy Chem,2021,54:736−745. doi: 10.1016/j.jechem.2020.06.050 [87] ZHANG L, HU S, LI W, CAO Z, LIU H, ZHU X, YANG W. Nano-CeO2-modified cathodes for direct electrochemical CO2 reduction in solid oxide electrolysis cells[J]. ACS Sustainable Chem Eng,2019,7(10):9629−9636. doi: 10.1021/acssuschemeng.9b01183 [88] PAN Z, SHI H, WANG S, JIANG H, ZHENG Y. Highly active and stable A-site Pr-doped LaSrCrMnO-based fuel electrode for direct CO2 solid oxide electrolyzer cells[J]. Int J Hydrogen Energy,2020,45(29):14648−14659. doi: 10.1016/j.ijhydene.2020.03.224 [89] LV H, ZHOU Y, ZHANG X, SONG Y, LIU Q, WANG G, BAO X. Infiltration of Ce0.8Gd0.2O1.9 nanoparticles on Sr2Fe1.5Mo0.5O6-delta cathode for CO2 electroreduction in solid oxide electrolysis cell[J]. J Energy Chem,2019,35:71−78. doi: 10.1016/j.jechem.2018.11.002 [90] LI Y, HU B, XIA C, XU W Q. , LEMMON J P, CHEN F. A novel fuel electrode enabling direct CO2 electrolysis with excellent and stable cell performance[J]. J Mater Chem A,2017,5(39):20833−20842. doi: 10.1039/C7TA05750D [91] HUAN D, ZHANG L, ZHANG S, SHI N, LI X, ZHU K, XIA C, PENG R, LU Y. Ruddlesden-Popper oxide SrEu2Fe2O7 as a promising symmetrical electrode for pure CO2 electrolysis[J]. J Mater Chem A,2021,9(5):2706−2713. doi: 10.1039/D0TA09585K [92] PARK S, HAN H, YOON W, CHOI J, KIM Y, KIM H, KIMW B. Improving a sulfur-tolerant Ruddlesden-Popper catalyst by fluorine doping for CO2electrolysis reaction[J]. ACS Sustainable Chem Eng,2020,8(16):6564−6571. doi: 10.1021/acssuschemeng.0c01774 [93] JIANG Y, YANG Y, XIA C, BOUWMEESTER H J M. Sr2Fe1.4Mn0.1Mo0.5O6−δ perovskite cathode for highly efficient CO2 electrolysis[J]. J Mater Chem A,2019,7(40):22939−22949. doi: 10.1039/C9TA07689A [94] FENG W, SONG Y, ZHANG X, LV H, LIU Q, WANG G, BAO X. Platinum-decorated ceria enhances CO2 electroreduction in solid oxide electrolysis cells[J]. CemSusChem,2020,13(23):6290−6295. [95] DURANTI L, LUISETTO I, LICOCCIA S, D’OTTAVI C, DI BARTOLOMEO E. Novel composite fuel electrode for CO2/CO-RSOCs[J]. J Electrochem Soc,2021,168(10):104507. doi: 10.1149/1945-7111/ac2c15 [96] SUN C, BIAN L, QI J, YU W, LI S, HOU Y, WANG L, PENG J, AN S. Boosting CO2 directly electrolysis by electron doping in Sr2Fe1.5Mo0.5O6-δ double perovskite cathode[J]. J Power Sources,2022,521:230984. doi: 10.1016/j.jpowsour.2022.230984 [97] YANG C, TIAN Y, PU J, CHI B. Anion fluorine-doped La0.6Sr0.4Fe0.8Ni0.2O3−δ perovskite cathodes with enhanced electrocatalytic activity for solid oxide electrolysis cell direct CO2 electrolysis[J]. ACS Sustainable Chem Eng,2022,10(2):1047−1058. doi: 10.1021/acssuschemeng.1c07576 [98] MINHN N Q. Ceramic fuel cells[J]. J Am Ceram Soc,1993,76(3):563−588. doi: 10.1111/j.1151-2916.1993.tb03645.x [99] LION S S, WORRELL W L. Electrical properties of novel mixed-conducting oxides[J]. Appl Phys A,1989,49(1):25−31. doi: 10.1007/BF00615461 [100] SONG Y, ZHANG X, ZHOU Y, LV H, LIU Q, FENG W, WANG G, BAO X. Improving the performance of solid oxide electrolysis cell with gold nanoparticles-modified LSM-YSZ anode[J]. J Energy Chem,2019,35:181−187. doi: 10.1016/j.jechem.2019.03.013 [101] CHEN K, AI N, JIANG S P. Reasons for the high stability of nano-structured (La, Sr)MnO3 infiltrated Y2O3-ZrO2 composite oxygen electrodes of solid oxide electrolysis cells[J]. Electrochem Commun,2012,19:119−122. doi: 10.1016/j.elecom.2012.03.033 [102] JIANG W, WEI B, LV Z, WANG Z H, ZHU L, LI Y Q. Performance and stability of co-synthesized Sm0.5Sr0.5CoO3-Ce0.8Sm0.2O1.9 composite oxygen electrode for solid oxide electrolysis cells[J]. Int J Hydrogen Energy,2015,40(1):561−567. doi: 10.1016/j.ijhydene.2014.10.128 [103] GUAN F, ZHANG X, SONG Y, ZHOU Y, WANG G, BAO X. Effect of Gd0.2Ce0.8O1.9 nanoparticles on the oxygen evolution reaction of La0.6Sr0.4Co0. 2Fe0.8O3-δ anode in solid oxide electrolysis cell[J]. Chin J Catal,2018,39(9):1484−1492. doi: 10.1016/S1872-2067(18)63118-3 [104] BUCHER E, EGGER A, RIED P, SITTE W, HOLTAPPELS P. Oxygen nonstoichiometry and exchange kinetics of Ba0.5Sr0.5Co0.8Fe0.2O3−δ[J]. Solid State Ionics,2008,179(21):1032−1035. [105] BO Y, WEN Z, JING X, JING C. Microstructural characterization and electrochemical properties of Ba0.5Sr0.5Co0.8Fe0.2O3−δ and its application for anode of SOEC[J]. Int J Hydrogen Energy,2008,33(23):6873−6877. doi: 10.1016/j.ijhydene.2008.07.066 [106] KIM G, WANG S, JACOBSON A J, REIMUS L, BRODERSEN P, MIMS C A. Rapid oxygen ion diffusion and surface exchange kinetics in PrBaCo2O5+x with a perovskite related structure and ordered A cations[J]. J Mater Chem,2007,17(24):2500−2505. doi: 10.1039/b618345j [107] JENSEN S R H J, HAUCH A, HENDRIKSEN P V, MOGENSEN M, BONANOS N, JACOBSEN T. A method to separate process contributions in impedance spectra by variation of test conditions[J]. J Electrochem Soc,2007,154(12):B1325−B1330. doi: 10.1149/1.2790791 [108] WANG Y, LI W, MA L, LI W, LIU X. Degradation of solid oxide electrolysis cells: Phenomena, mechanisms, and emerging mitigation strategies—A review[J]. J Mater Sci Tech,2020,55:35−55. doi: 10.1016/j.jmst.2019.07.026 [109] HAUCH A, JENSEN S H, RAMOUSSE S, MOGENSEN M. Performance and durability of solid oxide electrolysis cells[J]. J Electrochem Soc,2006,153(9):A1741. doi: 10.1149/1.2216562 [110] JUN A, JU Y W, KIM G. Solid oxide electrolysis: Concluding remarks[J]. Faraday Discuss,2015,182:519−528. doi: 10.1039/C5FD90072G [111] CHEN K, JIANG S P. Failure mechanism of (La, Sr)MnO3 oxygen electrodes of solid oxide electrolysis cells[J]. Int J Hydrogen Energy,2011,36(17):10541−10549. doi: 10.1016/j.ijhydene.2011.05.103 [112] KEANE M, MAHAPATRA M K, VERMA A, SINGH P. LSM-YSZ interactions and anode delamination in solid oxide electrolysis cells[J]. Int J Hydrogen Energy,2012,37(22):16776−16785. doi: 10.1016/j.ijhydene.2012.08.104 [113] MOMMA A, KATO T, KAGA Y, NAGATA S. Polarization behavior of high temperature solid oxide electrolysis cells (SOEC)[J]. J Ceram Soc Jpn,1997,105(1221):369−373. doi: 10.2109/jcersj.105.369 [114] CHEN K, HYODO J, DODD A, AI N, ISHIHARA T, JIAN L, JIANG S P. Chromium deposition and poisoning of La(0.8)Sr(0.2)MnO3 oxygen electrodes of solid oxide electrolysis cells[J]. Faraday Discuss,2015,182:457−476. doi: 10.1039/C5FD00010F [115] BI J, YANG S, ZHONG S, WANG JQ, FAN C, CHEN X, LIU Y. An insight into the effects of B-site transition metals on the activity, activation effect and stability of perovskite oxygen electrodes for solid oxide electrolysis cells[J]. J Power Sources,2017,363:470−479. doi: 10.1016/j.jpowsour.2017.07.118 [116] CHEN K, HYODO J, AI N, ISHIHARA T, JIANG S P. Boron deposition and poisoning of La0.8Sr0.2MnO3 oxygen electrodes of solid oxide electrolysis cells under accelerated operation conditions[J]. Int J Hydrogen Energy,2016,41(3):1419−1431. doi: 10.1016/j.ijhydene.2015.11.013 [117] LAURENCIN J, HUBERT M, SANCHEZ D F, PYLYPKO S, MORALES M, MORATA A, MOREL B, MONTINARO D, LEFEBVRE-JOUD F, SIEBERT E. Degradation mechanism of La0.6Sr0.4Co0.2Fe0.8O3–δ/Gd0.1Ce0.9O2–δ composite electrode operated under solid oxide electrolysis and fuel cell conditions[J]. Electrochim Acta,2017,241:459−476. doi: 10.1016/j.electacta.2017.05.011 [118] DE VERO J C, DEVELOS-BAGARINAO K, KISHIMOTO H, ISHIYAMA T, YAMAJI K, HORITA T, YOKOKAWA H. Influence of La0.6Sr0.4Co0.2Fe0.8O3–δ microstructure on GDC interlayer stability and cation diffusion across the LSCF/GDC/YSZ interfaces[J]. J Electrochem Soc,2016,163(13):F1463−F1470. doi: 10.1149/2.0021614jes [119] MONACO F, FERRERIA-SANCHEZ D, HUBERT M, MOREL B, MONTINARO D, GROLIMUND D, LAURENCIN J. Oxygen electrode degradation in solid oxide cells operating in electrolysis and fuel cell modes: LSCF destabilization and interdiffusion at the electrode/electrolyte interface[J]. Int J Hydrogen Energy,2021,46(62):31533−31549. doi: 10.1016/j.ijhydene.2021.07.054 [120] MAHMOUD A, AL DAROUKH M, LIPINSKA-CHWALEK M, LUYUYSBERG M, TIETZ F, HERMANN R P. A Mössbauer spectral study of degradation in La0.58Sr0.4Fe0.5Co0.5O3−x after long-term operation in solid oxide electrolysis cells[J]. Solid State Ionics,2017,312:38−43. doi: 10.1016/j.ssi.2017.10.003 [121] FREY C E, FANG Q, SEBOLD D, BLUM L, MENZLER N H. A detailed post mortem analysis of solid oxide electrolyzer cells after long-term stack operation[J]. J Electrochem Soc,2018,165(5):F357−F364. doi: 10.1149/2.0961805jes [122] KIM-LOHSOONTORN P, BRETT D J L, LAOSIRIPOJANA N, KIM Y M, BAE J M. Performance of solid oxide electrolysis cells based on composite La0.8Sr0.2MnO3−δ-yttria stabilized zirconia and Ba0.5Sr0.5Co0.8Fe0.2O3−δ oxygen electrodes[J]. Int J Hydrogen Energy,2010,35(9):3958−3966. doi: 10.1016/j.ijhydene.2010.02.039 [123] WEI B, FENG J, ZHU L, WANG Z, ZHU X, HUANG X, ZHANG Y, XU L, GAO H, LU Z. Anodic polarization induced performance loss in GdBaCo2O5+δ oxygen electrode under solid oxide electrolysis cell conditions[J]. J Eur Ceram Soc,2018,38(5):2396−2403. doi: 10.1016/j.jeurceramsoc.2018.01.001 [124] CHATZICHRISTODOULOU C, CHEN M, HENDRIKSEN P V, JACOBSEN T, MOGENSEN M B. Understanding degradation of solid oxide electrolysis cells through modeling of electrochemical potential profiles[J]. Electrochim Acta,2016,189:265−282. doi: 10.1016/j.electacta.2015.12.067 [125] HAUCH A, JENSEN S R H J, BILDE-SORENSEN J R B, MOGENSEN M. Silica segregation in the Ni/YSZ electrode[J]. J Electrochem Soc,2007,154(7):A619. doi: 10.1149/1.2733861 [126] CHEN M, LIU YL, BENTZEN J J, ZHANG W, SUN X, HAUCH A, TAO Y, BOWEN JR, HENDRIKSEN P V. Microstructural degradation of Ni/YSZ electrodes in solid oxide electrolysis cells under high current[J]. J Electrochem Soc,2013,160(8):F883−F891. doi: 10.1149/2.098308jes [127] TIETA F, SEBOLD D, BRISSE A, SCHEFOLD J. Degradation phenomena in a solid oxide electrolysis cell after 9000h of operation[J]. J Power Sources,2013,223:129−135. doi: 10.1016/j.jpowsour.2012.09.061 [128] NUGGEHALLI SAMPATHKUMAR S, AUBIN P, COUTURIER K, SUN X, SUDIREDDY B R, DIETHELM S, PEREZ-FORTES M, VANHERLE J. Degradation study of a reversible solid oxide cell (rSOC) short stack using distribution of relaxation times (DRT) analysis[J]. Int J Hydrogen Energy,2022,47(18):10175−10193. doi: 10.1016/j.ijhydene.2022.01.104 [129] JUN A, KIM J, SHIN J, KIM G. Achieving high efficiency and eliminating degradation in solid oxide electrochemical cells using high oxygen-capacity perovskite[J]. Angew Chem Int Ed Eng,2016,55(40):12512−12515. doi: 10.1002/anie.201606972 [130] KIM J, JI HI, DASSARI H P, SHIN D, SONG H, LEE J H, KIM B K, JE H J, LEE H W, YOON K J. Degradation mechanism of electrolyte and air electrode in solid oxide electrolysis cells operating at high polarization[J]. Int J Hydrogen Energy,2013,38(3):1225−1235. doi: 10.1016/j.ijhydene.2012.10.113 [131] BADWAK S P S, CIACCHI F T, RAJENDRAN S, DRENNAN J. An investigation of conductivity, microstructure and stability of electrolyte compositions in the system 9mol% (Sc2O3-Y2O3)-ZrO2(Al2O3)[J]. Solid State Ionics,1998,109(3):167−186. [132] NOMURA K, MIZUTANI Y, KAWAI M, NAKAMURA Y, YAMAMOTO O. Aging and Raman scattering study of scandia and yttria doped zirconia[J]. Solid State Ionics,2000,132(3):235−239. [133] POLITOVA T I, IRVINE J T S. Investigation of scandia-yttria-zirconia system as an electrolyte material for intermediate temperature fuel cells—influence of yttria content in system (Y2O3)x(Sc2O3)(11−x)(ZrO2)89[J]. Solid State Ionics,2004,168(1):153−165. [134] LEE D S, KIM W S, CHOI S H, KIM J, LEE HW, LEE J H. Characterization of ZrO2 co-doped with Sc2O3 and CeO2 electrolyte for the application of intermediate temperature SOFCs[J]. Solid State Ionics,2005,176(1):33−39. [135] HUANG K, TICHY R S, GOODENOUGH J B. Superior perovskite oxide-ion conductor; strontium- and magnesium-doped LaGaO3: I, Phase relationships and electrical properties[J]. J Am Ceram Soc,1998,81(10):2565−2575. [136] HUANG K, FENG M, GOODENOUGHJ B, MILLIKEN C. Electrode performance test on single ceramic fuel cells using as electrolyte Sr‐ and Mg‐Doped LaGaO3[J]. J Electrochem Soc,1997,144(10):3620−3624. doi: 10.1149/1.1838058 [137] EGUCHI K, HATAGISHI T, ARAI H. Power generation and steam electrolysis characteristics of an electrochemical cell with a zirconia- or ceria-based electrolyte[J]. Solid State Ionics,1996,86−88:1245−1249. doi: 10.1016/0167-2738(96)00295-0 [138] MEGEL S, KUSNEZOFF M, TROFIMENKO N, SAUCHUK V, MICHAELIS A, VENSKUTONIS A, RISSBACHER K, KRAUSSLER W, BRANDNER M, BIENERT C, SIGL L S. High efficiency CFY-stack for high power applications[J]. ECS Trans,2011,35(1):269−277. doi: 10.1149/1.3570002 [139] MEGEL S, DOSCHC, ROTHE S, KUSNEZOFF M, TROFIMENKO N, SAUCHUK V, MICHAELIS A, BIENERT C, BRANDNERM, VENSKUTONISA, KRAUSSLER W, SIGL L S. CFY-stacks for use in stationary SOFC and SOEC applications[J]. ECS Trans,2013,57(1):89−98. doi: 10.1149/05701.0089ecst [140] MAHAPATRA M K, LU K. [J]. Glass-based seals for solid oxide fuel and electrolyzer cells- A review[J]. Mater Sci Eng R Rep,2010,67(5/6):65−85. [141] REDDY A A, TULYAGANOV D U, KHARTONVV, FERREIRAJMF. Development of bilayer glass-ceramic SOFC sealants via optimizing the chemical composition of glasses—a review[J]. J Solid State Electrochem,2015,19(10):2899−2916. doi: 10.1007/s10008-015-2925-5 [142] ABDOLI H, ALIZADEH P, BOCCACCINI D, AGERSTED K. Fracture toughness of glass sealants for solid oxide fuel cell application[J]. Mater Lett,2014,115:75−78. doi: 10.1016/j.matlet.2013.10.013 [143] 赵先兴, 李景云, 蔡润田, 付春才, 刘鹏翔. CHANG Jun-shi. 固体氧化物燃料电池密封材料发展现状[J]. 电池工业,2021,25(3):155−159. doi: 10.3969/j.issn.1008-7923.2021.03.009ZHAO Xian-xing, LI Jing-yun, CAI Run-tian, FU Chun-cai, LIU Peng-xiang, CHANG Jun-shi. Research progress in the properties of the sealing materials for SOFC[J]. Chin Batt Ind,2021,25(3):155−159. doi: 10.3969/j.issn.1008-7923.2021.03.009 [144] DÖNITZ W, ERDLE E. High-temperature electrolysis of water vapor—status of development and perspectives for application[J]. Int J Hydrogen Energy,1985,10(5):291−295. doi: 10.1016/0360-3199(85)90181-8 [145] STOOTS C M, O'BRIEN J E, CONDIE K G, HARTVIGSEN J J. High-temperature electrolysis for large-scale hydrogen production from nuclear energy – Experimental investigations[J]. Int J Hydrogen Energy,2010,35(10):4861−4870. doi: 10.1016/j.ijhydene.2009.10.045 [146] ZHAO C H, ZHANG W Q, YU B, WANG J C, CHEN J. Solid oxide electrolyzer cells[J]. Prog Chem,2016,28(8):1265−1288. [147] KÜNGAS R. Review—electrochemical CO2 reduction for CO production: Comparison of low- and high-temperature electrolysis technologies[J]. J Electrochem Soc,2020,167(4):044508. doi: 10.1149/1945-7111/ab7099 [148] HARTVIGSEN J, ELANGOVAN S, ELWELL J, LARSEND. Oxygen Production from Mars atmosphere carbon dioxide using solid oxide electrolysis[J]. ECS Trans,2017,78(1):2953−2963. doi: 10.1149/07801.2953ecst [149] HINTERMAN E, HOFFMAN J A. Simulating oxygen production on Mars for the Mars oxygen in-situ resource utilization experiment[J]. Acta Astronaut,2020,170:678−685. doi: 10.1016/j.actaastro.2020.02.043 [150] HECHT M, HOFFMAN J, RAPPD, MCCLEANJ, SOOHOO J, SCHAEFERR, etc. Mars oxygen ISRU experiment (MOXIE)[J]. Spcae Sci Rev,2021,217(1):9. doi: 10.1007/s11214-020-00782-8 [151] ALZAHRANI A A, DINCER I. Exergoeconomic analysis of hydrogen production using a standalone high-temperature electrolyzer[J]. Int J Hydrogen Energy,2021,46(27):13899−13907. doi: 10.1016/j.ijhydene.2020.04.055 [152] BUTTLER A, KOLTUN R, WOLF R, SPLIETHOFF H. A detailed techno-economic analysis of heat integration in high temperature electrolysis for efficient hydrogen production[J]. Int J Hydrogen Energy,2015,40(1):38−50. doi: 10.1016/j.ijhydene.2014.10.048 [153] 张玉魁, 陈换军, 孙振新, 杜昊易, 李尧, 曲宗凯. 高温固体氧化物电解水制氢效率与经济性[J]. 广东化工,2021,48(18):3−6. doi: 10.3969/j.issn.1007-1865.2021.18.003ZHANG Yu-kui, CHEN Huan-jun, SUN Zhen-xin, DU Hao-yi, LI Yao, QU Zong-kai. Efficiency and economy of hydrogen production fromhigh temperature solid oxide electrolysis of water[J]. Guangdong Chem Ind,2021,48(18):3−6. doi: 10.3969/j.issn.1007-1865.2021.18.003 [154] ANGHILANTE R, COLOMAR D, BRISSE A, MARRONY M. Bottom-up cost evaluation of SOEC systems in the range of 10–100MW[J]. Int J Hydrogen Energy,2018,43(45):20309−20322. doi: 10.1016/j.ijhydene.2018.08.161 [155] QIAN M, WU Z, YAN F, LING Z, ZHOU Y. In oxygen generation by glow discharge under simulated martian conditions[J]. Lunar Planetary Science Conference,2020,2316. [156] 张缘. 从实现碳中和目标探究碳税构建的可行性[J]. 海南金融,2022,(2):51−58. doi: 10.3969/j.issn.1003-9031.2022.02.005ZHANG Yuan. Exploring the feasibility of carbon tax construction from the realization of carbon neutrality[J]. Hainan Finance,2022,(2):51−58. doi: 10.3969/j.issn.1003-9031.2022.02.005 -




 下载:
下载: