Effects of O2 and SO2 on As2O3 adsorption over W-Cu/γ-Al2O3 surface: An experimental combined theoretical analysis
-
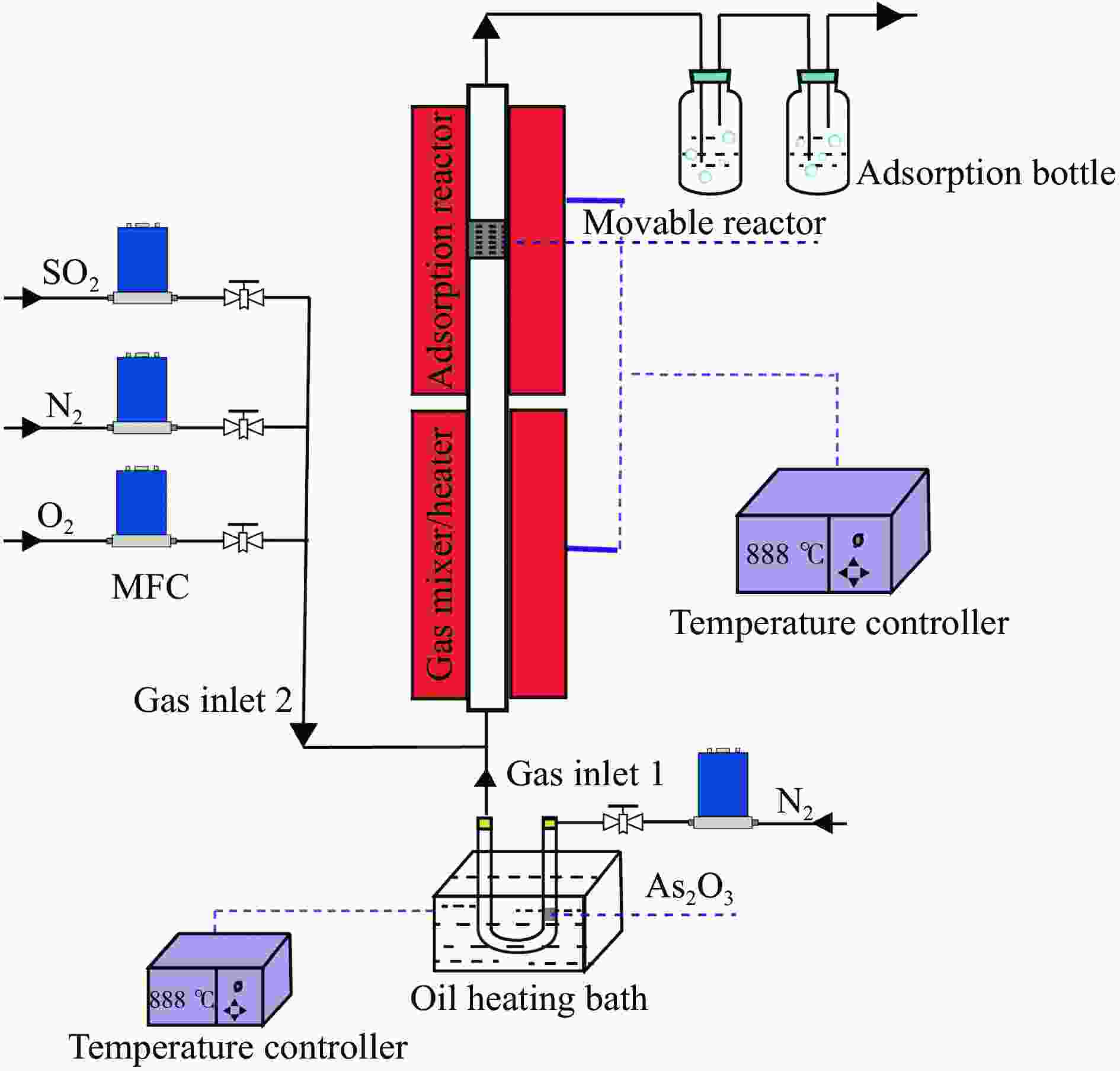
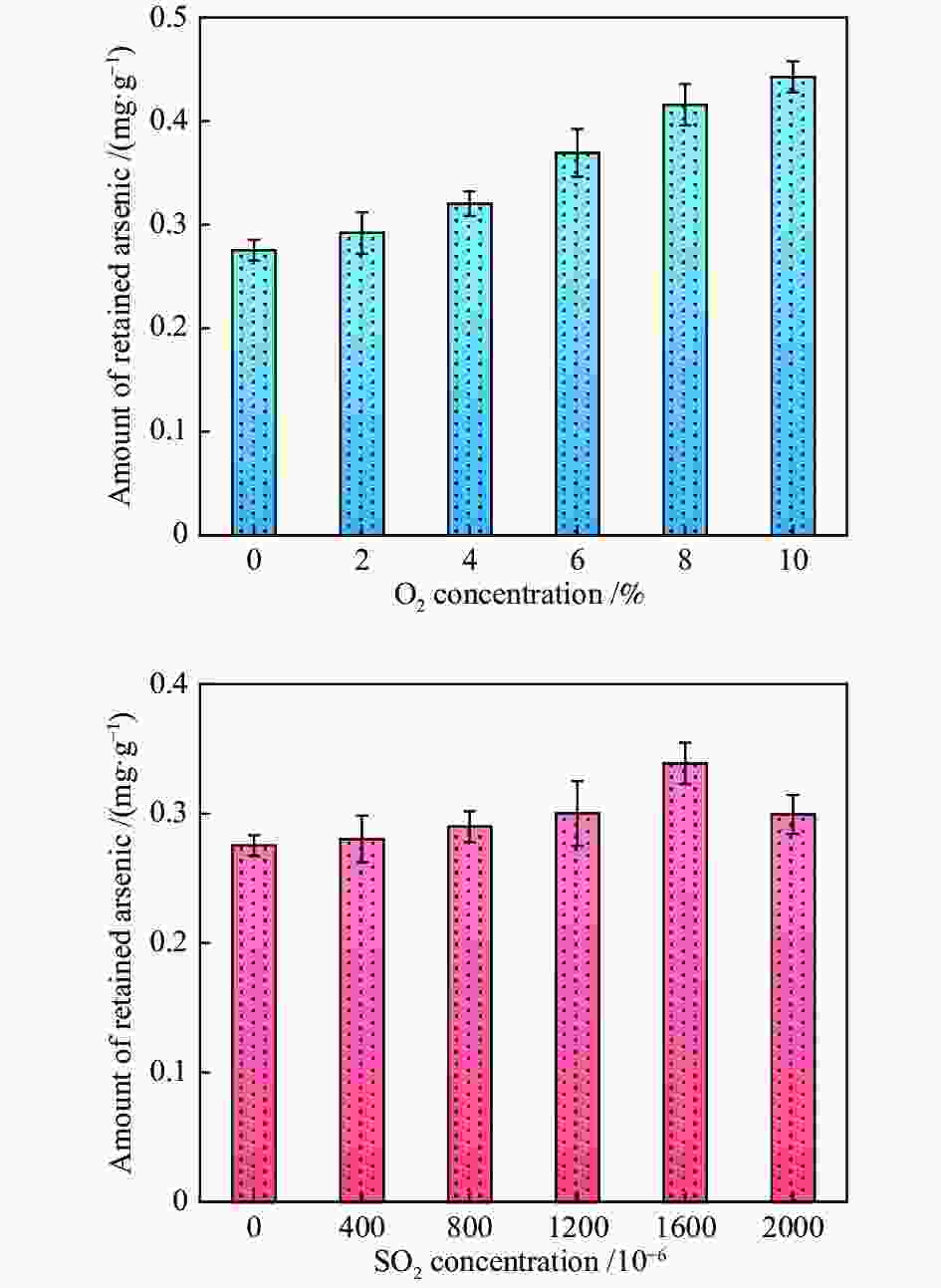
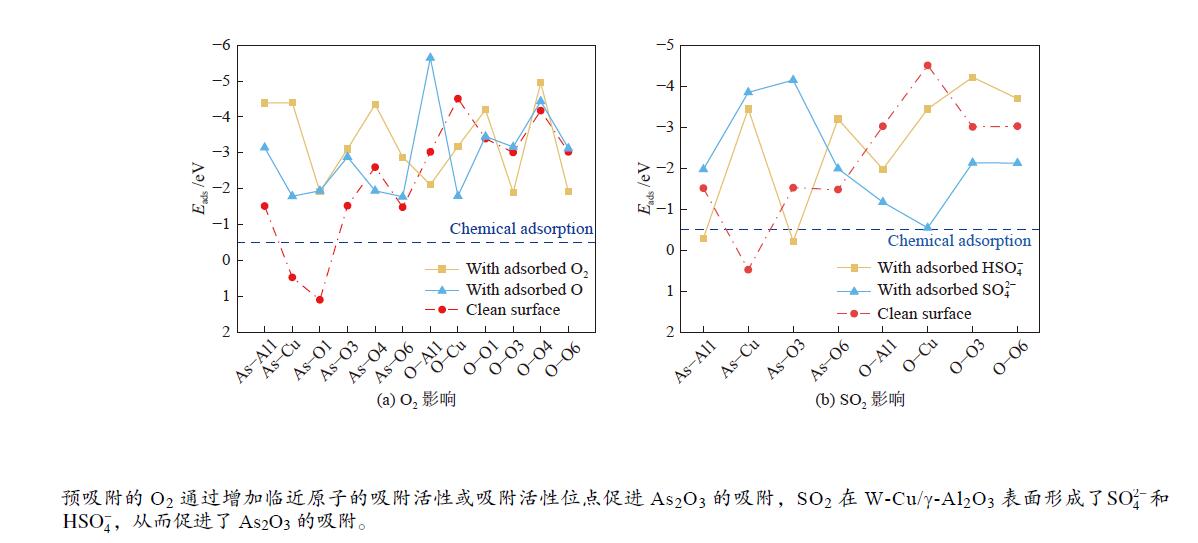
摘要: 采用实验及量子化学方法探究了O2和SO2对As2O3在W-Cu/γ-Al2O3催化剂表面吸附特性的影响。实验结果表明,O2促进了As2O3在催化剂表面的吸附,随着SO2体积分数的增加,As2O3的吸附量表现出先升高后降低的趋势。为进一步探究烟气组分对气相砷吸附的影响机理,采用密度泛函理论(DFT)方法,模拟了预吸附不同气体后催化剂表面As2O3的吸附。结果表明,O2对气相砷的促进影响主要归因于吸附氧的形成。预吸附的O原子明显增强了临近原子的吸附活性,而预吸附的O2分子则主要通过提供吸附活性位点促进As2O3的吸附。SO2在W-Cu/γ-Al2O3表面形成了
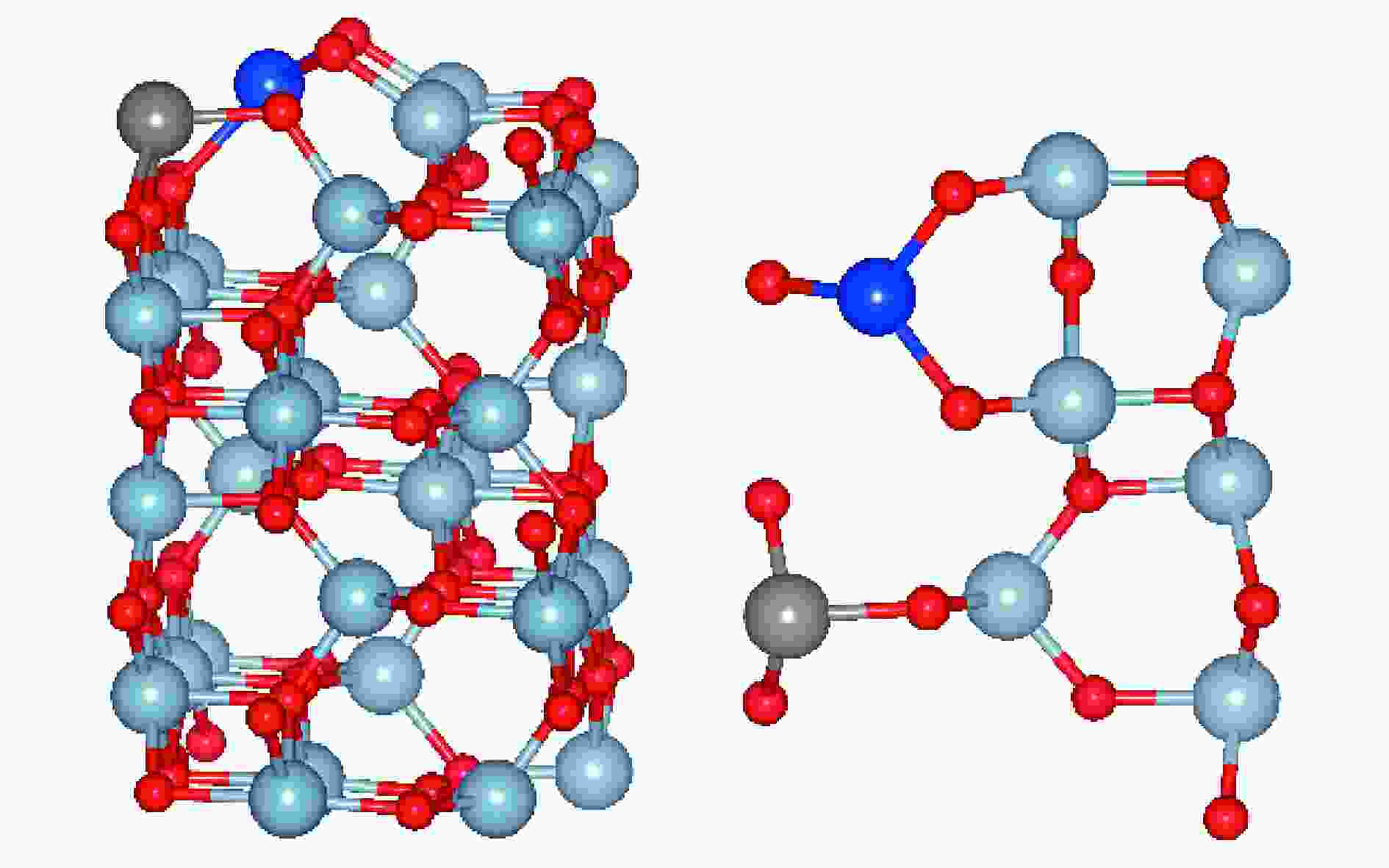
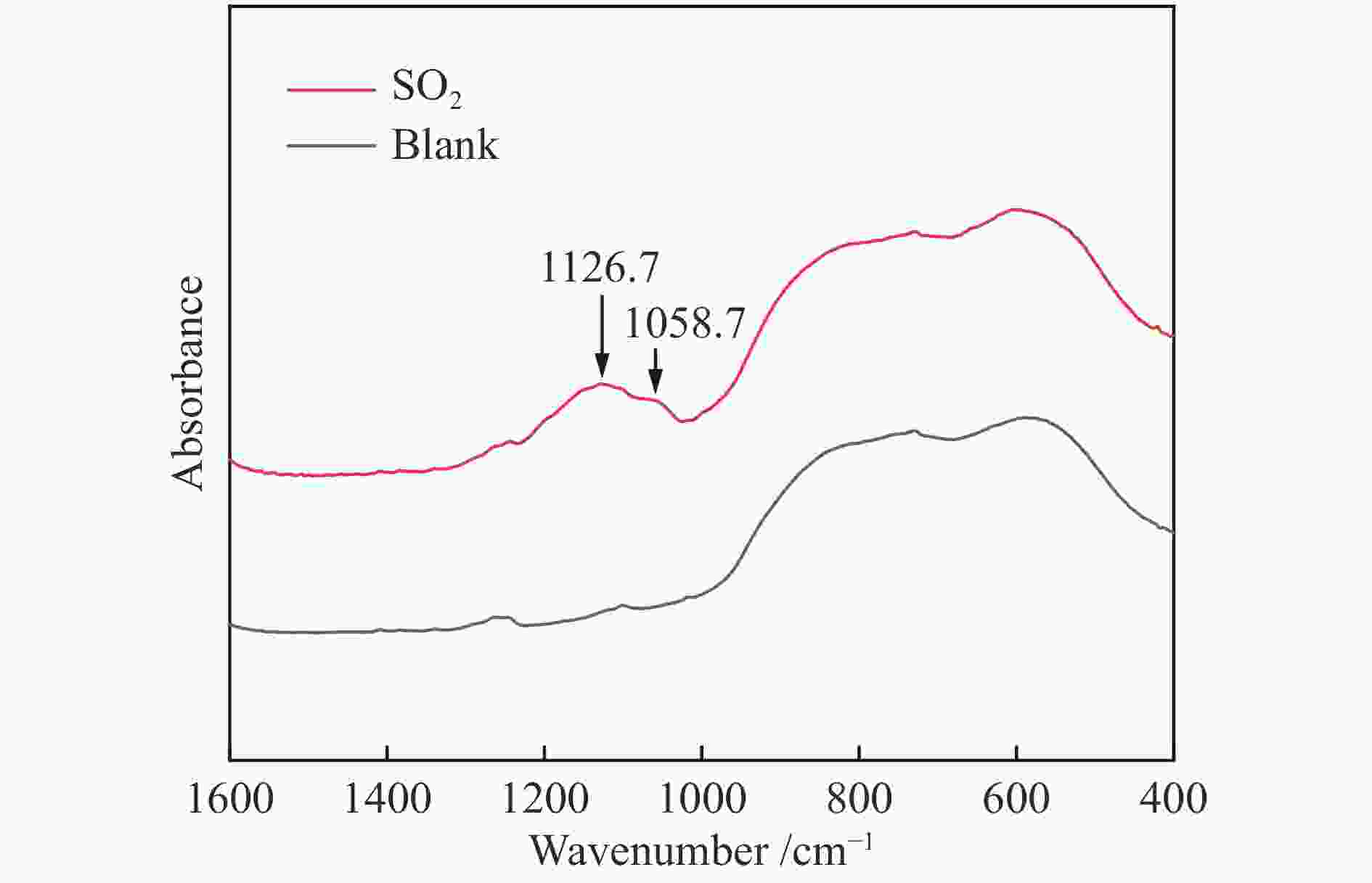
${{\rm{SO}}^{2-} _4}$ 和${\rm{HSO}}^-_4 $ ,改变了基底表面的势场,从而促进了As2O3的吸附。随体积分数的进一步增加,SO2与气相As2O3的竞争吸附作用增强,As2O3吸附量减少。Abstract: In this work, the effects of O2 and SO2 on gaseous As2O3 adsorption over W-Cu/γ-Al2O3 catalyst were investigated through adsorption experiment and density functional theory (DFT) method. Experimental results show that the As2O3 adsorption is facilitated by O2, and intensified with the increasing concentrations of SO2. However, it is slightly weakened with the SO2 concentration of 2.0×10−3. The As2O3 adsorption on W-Cu/γ-Al2O3 surface with adsorbed gas constituents was calculated by DFT simulation to reveal the effect mechanism. The promoting effect of O2 on arsenic adsorption is attributed to the formation of adsorbed oxygen. The pre-adsorbed O atom significantly enhances the adsorption activities of adjacent atoms, and the pre-adsorbed O2 molecule provides the active sites for As2O3 adsorption. When SO2 is introduced, the${{\rm{SO}}^{{2}-} _{4}}$ and${\rm{HSO}}^-_{4} $ are formed, which change the potential field of substrate surface, and further enhance the As2O3 adsorption. However, the competitive adsorption between SO2 with As2O3 is strengthened with increasing SO2 concentration, and it is the reason for the decreasing trend of As2O3 adsorption with high concentrations of SO2.-
Key words:
- O2 /
- SO2 /
- As2O3 /
- W-Cu/γ-Al2O3 /
- adsorption /
- surface /
- DFT
-
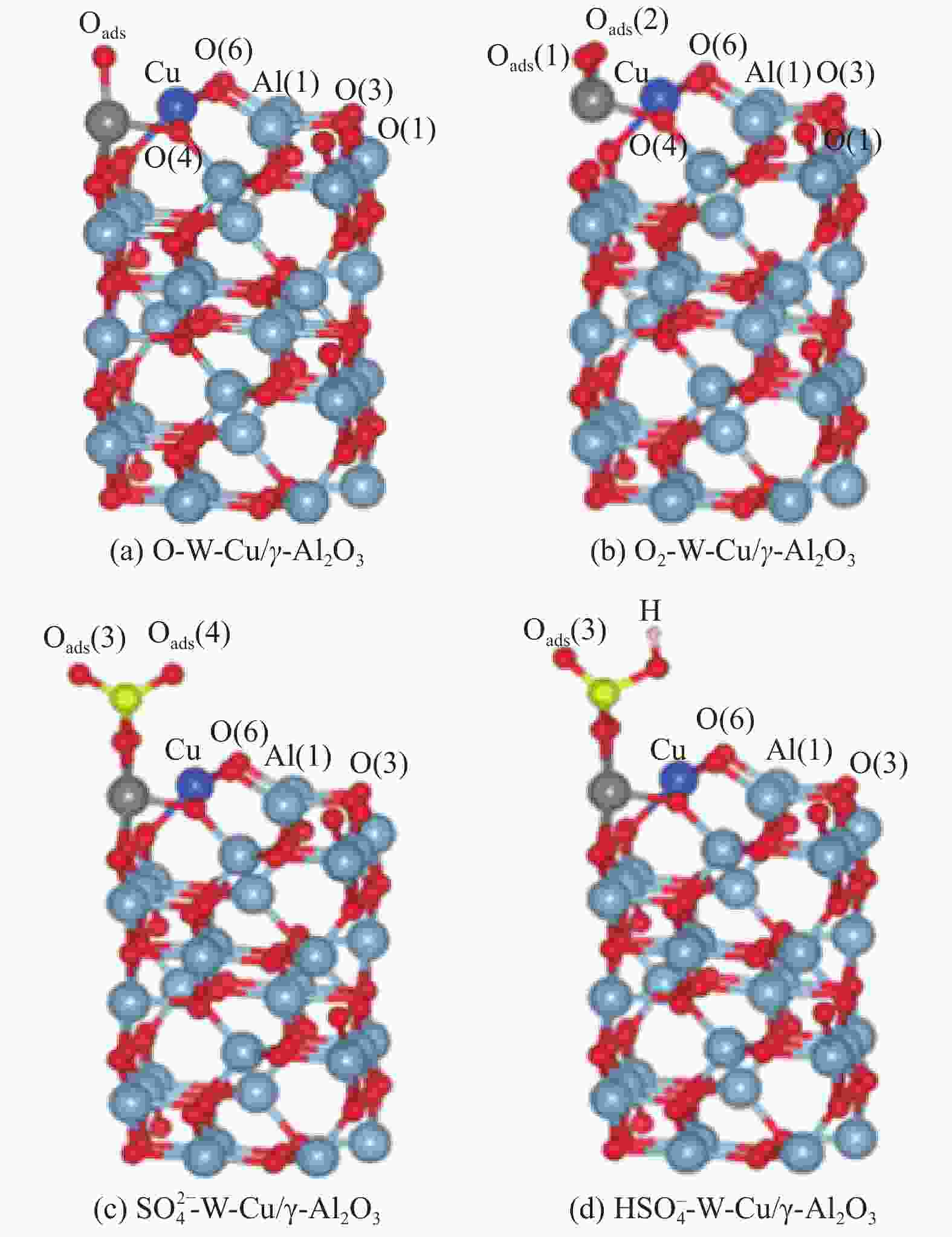
表 1 As2O3在吸附氧上的吸附能
Table 1 Adsorption energies of As2O3 on adsorbed oxygen
Configuration Eads/eV Configuration Eads /eV As−Oads −0.04 O−Oads −0.95 As−Oads1 −1.91 O−Oads1 −0.13 As−Oads2 −3.23 O−Oads2 −3.21 表 2 As2O3在
${{\bf{SO}}^{{{\boldsymbol{2}}}-} _{{\boldsymbol{4}}}}$ 和${{\bf{HSO}}^- _{{\boldsymbol{4}}}}$ 上的吸附能Table 2 Adsorption energies of As2O3 on
${{\rm{SO}}^{{2}-} _{4}} $ and${{\rm{HSO}}^{-} _{4}} $ Configuration (${{\rm{SO}}^{{2}-} _{4}} $) Eads/eV Configuration (${{\rm{HSO}}^- _{4}} $) Eads/eV As−O3ads −0.16 As−O3ads −0.67 As−O4ads −0.23 As−O4ads −0.66 O−O3ads −0.22 O−H −0.75 O−O4ads −0.18 O−H −0.57 -
[1] 杨婷婷, 白杨, 吕游, 张文广. SCR脱硝系统多目标优化控制研究[J]. 中国电机工程学报,2021,41(14):4905−4911.YANG Ting-ting, BAI Yang, LV You, ZHANG Wen-guang. Study on multi-objective optimal control of SCR denitrification system[J]. Proc CSEE,2021,41(14):4905−4911. [2] ZHOU J, GUO R T, ZHANG X F, LIU Y Z, DUAN C P, WU G L, PAN W G. Cerium oxide-based catalysts for low-temperature selective catalytic reduction of NOx with NH3: A Review[J]. Energy Fuels,2021,35(4):2981−2998. [3] 王钧伟, 徐灿, 秦伟, 张建利, 张先龙, 董彦杰, 崔晓峰. 凹凸棒石(PG)负载MnOx催化剂脱除气态Hg0的研究[J]. 燃料化学学报,2020,48(12):1442−1451. doi: 10.3969/j.issn.0253-2409.2020.12.005WANG Jun-wei, QIN Wei, ZHANG Jian-li, ZHANG Xian-long, DONG Yan-jie. CUI Xiao-feng. Hg0 removal by palygorskite ( PG) supported MnOx catalyst[J]. J Fuel Chem Technol,2020,48(12):1442−1451. doi: 10.3969/j.issn.0253-2409.2020.12.005 [4] 张霄玲, 鲍佳宁, 李运甲, 皇甫林, 李文松, 高士秋, 许光文, 李长明, 余剑. 工业MnOx颗粒催化剂的制备及其低温脱硝应用研究[J]. 化工学报,2020,71(11):5169−5177.ZHANG Xiao-ling, BAO Jia-ning, LI Yun-jia, HUANG Pu-lin, LI Wen-song, GAO Shi-qiu, XU Guang-wen, LI Chang-ming, YU Jian. Preparation and industrial application for MnOx particle catalyst for low temperature denitration[J]. J Chem Ind Eng,2020,71(11):5169−5177. [5] 黄海凤, 王庐云, 漆仲华, 卢晗锋. 柴油尾气DOC催化剂Pt-Pd/CeO2的活性和抗硫性[J]. 燃料化学学报,2013,41(11):1401−1408.HUANG Hai-feng, WANG Lu-yun, QI Zhong-hua, LU Han-feng. Activity and Sulfur resistance of Pt-Pd/CeO2 as DOC catalyst for diesel exhaust[J]. J Fuel Chem Technol,2013,41(11):1401−1408. [6] 李萍, 李长明, 段正康, 高士秋, 许光文, 余剑. 低温烟气脱硝催化剂适用条件与动力学[J]. 化工学报,2019,70(8):29681−2990.LI Ping, LI Chang-ming, DUAN Zheng-kang, GAO Shi-qiu, XU Guang-wen, YU Jian. Application conditions and kinetics simulation over SCR catalyst for flue gas denitrification under low temperature[J]. J Chem Ind Eng,2019,70(8):29681−2990. [7] UPAKUL D, AMÉLIE J, EINAR A, EILERTSEN, HERMANN E, MARK A, SATU T, BERT M, ANDREW M. Confirmation of isolated Cu2+ ions in SSZ-13 zeolite as active sites in NH3-selective catalytic reduction[J]. J Phys Chem C,2012,116(7):4809−4818. doi: 10.1021/jp212450d [8] FICKEL D, D’ADDIO E, LAUTERBACH J, LOBO R. The ammonia selective catalytic reduction activity of copper-exchanged small-pore zeolites[J]. Appl Catal B: Environ,2011,102(3/4):441−448. doi: 10.1016/j.apcatb.2010.12.022 [9] WANG H Y, WANG B D, ZHOU J L, LI G, ZHANG D J, MA Z R, XIONG R H, SUN Q, XU W Q. CuO modified vanadium-based SCR catalysts for Hg0 oxidation and NO reduction[J]. J Environ Manage,2019,239(1):17−22. [10] 赵清森. CuO/γ-Al2O3及其改性催化剂脱硫脱硝性能研究[D]. 武汉: 华中科技大学, 2009.ZHAO Qing-sen. The experimental study on desulfurization and denitrification by CuO/γ-Al2O3 and modified catalysts[D]. Wuhan: Huazhong University of Science and Technology, 2009. [11] HU J Y, LUO G Q, LI Z H, LIU, M Y, ZOU R J, LI X, YAO H. Deactivation mechanism of KCl and K2SO4 poisoned V2O5/WO3-TiO2 catalyst on gaseous elemental mercury oxidation[J]. Fuel,2020,278(9):118245. [12] ZHANG Y, ZHAO L, KANG M D, CHEN Z, GAO S J, HAO H G. Insights into high CO-SCR performance of CuCoAlO catalysts derived from LDH/MOFs composites and study of H2O/SO2 and alkali metal resistance[J]. Chem Eng J,2021,426:131873. doi: 10.1016/j.cej.2021.131873 [13] SHEN F H, LIU J, ZHANG Z, DAI J X. On-Line analysis and kinetic behavior of arsenic release during coal combustion and pyrolysis[J]. Environ Sci Technol,2015,49(22):13716−13723. doi: 10.1021/acs.est.5b03626 [14] LIU H M, PAN W P, WANG C B, SHEN F H. Volatilization of arsenic during coal combustion based on isothermal thermogravimetric analysis at 600−1500 ℃[J]. Energy Fuels,2016,30(8):6790−6798. doi: 10.1021/acs.energyfuels.6b00816 [15] LI X, LI J H, PENG Y, SI W Z, HE X, HAO J M. Regeneration of commercial SCR catalysts: Probing the existing forms of arsenic oxide[J]. Environ Sci Technol,2015,49(16):9971−9978. doi: 10.1021/acs.est.5b02257 [16] 陆强, 裴鑫琦, 徐明新, 王涵啸, 吴亚昌, 欧阳昊东. SCR脱硝催化剂抗砷中毒改性优化与再生研究进展[J]. 化工进展,2021,40(5):2365−2374.LU Qiang, PEI Xin-qi, XU Ming-xin, WANG Han-xiao, WU Ya-chang, OUYANG Hao-dong. Progress in the development and regeneration of SCR catalysts for anti-arsenic poisoning[J]. Chem Ind Eng Prog,2021,40(5):2365−2374. [17] LI X Y, J. CHEN J, XIAO Y, LU C M, YAO H. Insight into the homogenous and heterogeneous transformation behavior of arsenic on commercial V2O5-WO3-TiO2 and novel γ-Fe2O3 catalysts during selective catalytic reduction of NOx[J]. Fuel,2021,301:121051. doi: 10.1016/j.fuel.2021.121051 [18] DORIS V, HANNS H, JOSEF S, WOLFGANG G, FRANZ R. Deactivation processes on TiO2 V2O5 based denox catalysts[J]. Chem Ing Tech,1988,60(9):714−715. doi: 10.1002/cite.330600918 [19] XING J Y, WANG C B, HUANG Y L, YUE S, EDWARD J. The effect of W and Mo modification on arsenic adsorption over Cu/γ-Al2O3 catalyst: Experimental and theoretical analysis[J]. Chem Eng J,2022,432:134376. doi: 10.1016/j.cej.2021.134376 [20] XING J Y, WANG C B, ZHANG Y, SI T, LIU X. A deep insight into the role of O2 on As2O3 capture over γ-Al2O3 sorbent: Experimental and DFT study[J]. Chem Eng J,2020,410:128311. [21] ZHANG Y, WANG C B, LIU H M. Experiment and mechanism research on gas-phase As2O3 adsorption of Fe2O3/γ-Al2O3[J]. Fuel,2016,181:1034−1040. doi: 10.1016/j.fuel.2016.04.141 [22] CHEN D K, HU H Y, XU Z, LIU H, CAO J X, SHEN J H, YAO H. Findings of proper temperatures for arsenic capture by CaO in the simulated flue gas with and without SO2[J]. Chem Eng J,2015,267:201−206. doi: 10.1016/j.cej.2015.01.035 [23] HU P B, WENG Q Y, LI D L, LV T, WANG S J, ZHOU Y Q. Effects of O2, SO2, H2O and CO2 on As2O3 adsorption by γ-Al2O3 based on DFT analysis[J]. J Hazard Mater,2020,403:123866. [24] 刘翔, 张月, 邢佳颖, 郭雨生, 许桐, 王春波. Mn改性Fe2O3/γ-Al2O3脱除气相As2O3实验研究[J]. 中国电机工程学报,2021,41(15):5250−5258.LIU Xiang, ZHANG Yue, XING Jia-ying, GUO Yu-sheng, XU Tong, WANG Chun-bo. Experimental study on removal of gas-phase As2O3 by Mn modified Fe2O3/γ-Al2O3[J]. Proc CSEE,2021,41(15):5250−5258. [25] GAO Z Y, SUN Y, LI M H, YANG W J, DING X L. Adsorption sensitivity of Fe decorated different graphene supports toward toxic gas molecules (CO and NO)[J]. Appl Surf Sci,2018,456:351−359. doi: 10.1016/j.apsusc.2018.06.112 [26] HALGREN T A, LIPSCOMB W N. The synchronous-transit method for determining reaction pathways and locating molecular transition states[J]. Chem Phys Lett,1977,49(2):225−232. doi: 10.1016/0009-2614(77)80574-5 [27] CONTRERAS M L, AROSTEGUI J M, ARMESTO L. Arsenic interactions during co-combustion processes based on thermodynamic equilibrium calculations[J]. Fuel,2009,88(3):539−546. doi: 10.1016/j.fuel.2008.09.028 [28] SONG B, SONG M, CHEN D D, MENG F Y, WEI Y X. Retention of arsenic in coal combustion flue gas at high temperature in the presence of CaO[J]. Fuel,2020,259:116249. doi: 10.1016/j.fuel.2019.116249 [29] DENG X Y, MIN B K, GULOY A, CYNTHIA M. Enhancement of O2 dissociation on Au(111) by adsorbed oxygen: implications for oxidation catalysis[J]. J Am Chem Soc,2005,127(25):9267. doi: 10.1021/ja050144j [30] WANG Z, LIU J, YANG Y, LIU F, DING J Y. Heterogeneous reaction mechanism of elemental mercury oxidation by oxygen species over MnO2 catalyst[J]. Proc Combust Inst,2019,37(3):2967−2975. doi: 10.1016/j.proci.2018.06.132 [31] GAO Z Y, LI M H, SUN Y, YANG W J. Effects of oxygen functional complexes on arsenic adsorption over carbonaceous surface[J]. J Hazard Mater,2018,360(15):436−444. -
 2022-F024_补充材料修改_燃料化学学报.docx
2022-F024_补充材料修改_燃料化学学报.docx
-









 下载:
下载: