Effects of Ce0.8Cu0.2O2 oxygen carrier-coupled S-1 molecular sieve on chemical-looping performance
-
摘要: 在Ce0.8Cu0.2O2氧载体中添加不同质量S-1分子筛,并利用XRD、BET、XPS、SEM、TEM和CH4-TPR & CO2-TPO等表征对氧载体的物化特性和反应性能进行了研究。考察了S-1分子筛添加量对Ce0.8Cu0.2O2氧载体在化学链甲烷重整耦合CO2还原反应中的性能的影响。与单纯的Ce0.8Cu0.2O2氧载体相比,添加了0.3 g S-1分子筛后复合氧载体的比表面积明显增大,从15.44 m2/g提高至73.27 m2/g。同时热稳定性和结构稳定性也得到了很大的改善。添加了0.3 g S-1分子筛的复合氧载体CH4转化率由38.93%提升至56.03%,CO2还原过程中CO产率由1.18 mmol/g增加至2.16 mmol/g。Abstract: Ce0.8Cu0.2O2 oxygen carrier has excellent performance in chemical-looping reforming of methane coupled with CO2 reduction technology. Different mass of S-1 molecular sieve was added to Ce0.8Cu0.2O2 oxygen carrier. The physicochemical properties and reactivity of the carrier were characterized by XRD, BET, XPS, SEM, TEM and CH4-TPR & CO2-TPO. The effect of S-1 molecular sieve on the performance of Ce0.8Cu0.2O2 oxygen carrier in chemical-looping reforming of methane coupled with CO2 reduction was systematically investigated. Compared with Ce0.8Cu0.2O2 oxygen carrier alone, the specific surface area of the composite oxygen carrier increased from 15.44 to 73.27 m2/g after adding 0.3 g S-1 molecular sieve. At the same time, its thermal stability and structural stability were greatly improved. The CH4 conversion rate of composite oxygen carrier with 0.3 g S-1 molecular sieve increased from 38.93% to 56.03%, and the CO yield increased from 1.18 to 2.16 mmol/g during CO2 reduction.
-
Key words:
- chemical-looping /
- S-1 molecular sieves /
- reforming of methane /
- CO2 reduction /
- syngas
-
图 6 Ce0.8Cu0.2O2 (a)、Ce0.8Cu0.2O2/0.1 g S-1 (b)、Ce0.8Cu0.2O2/0.2 g S-1 (c)、Ce0.8Cu0.2O2/0.3 g S-1 (d)和Ce0.8Cu0.2O2/0.4 g S-1 (e)氧载体和S-1(f)的CH4-TPR谱图
Figure 6 CH4-TPR profiles of Ce0.8Cu0.2O2 (a), Ce0.8Cu0.2O2/0.1 g S-1 (b), Ce0.8Cu0.2O2/0.2 g S-1 (c), Ce0.8Cu0.2O2/0.3 g S-1 (d) and Ce0.8Cu0.2O2/0.4 g S-1 (e) oxygen carriers and S-1(f)
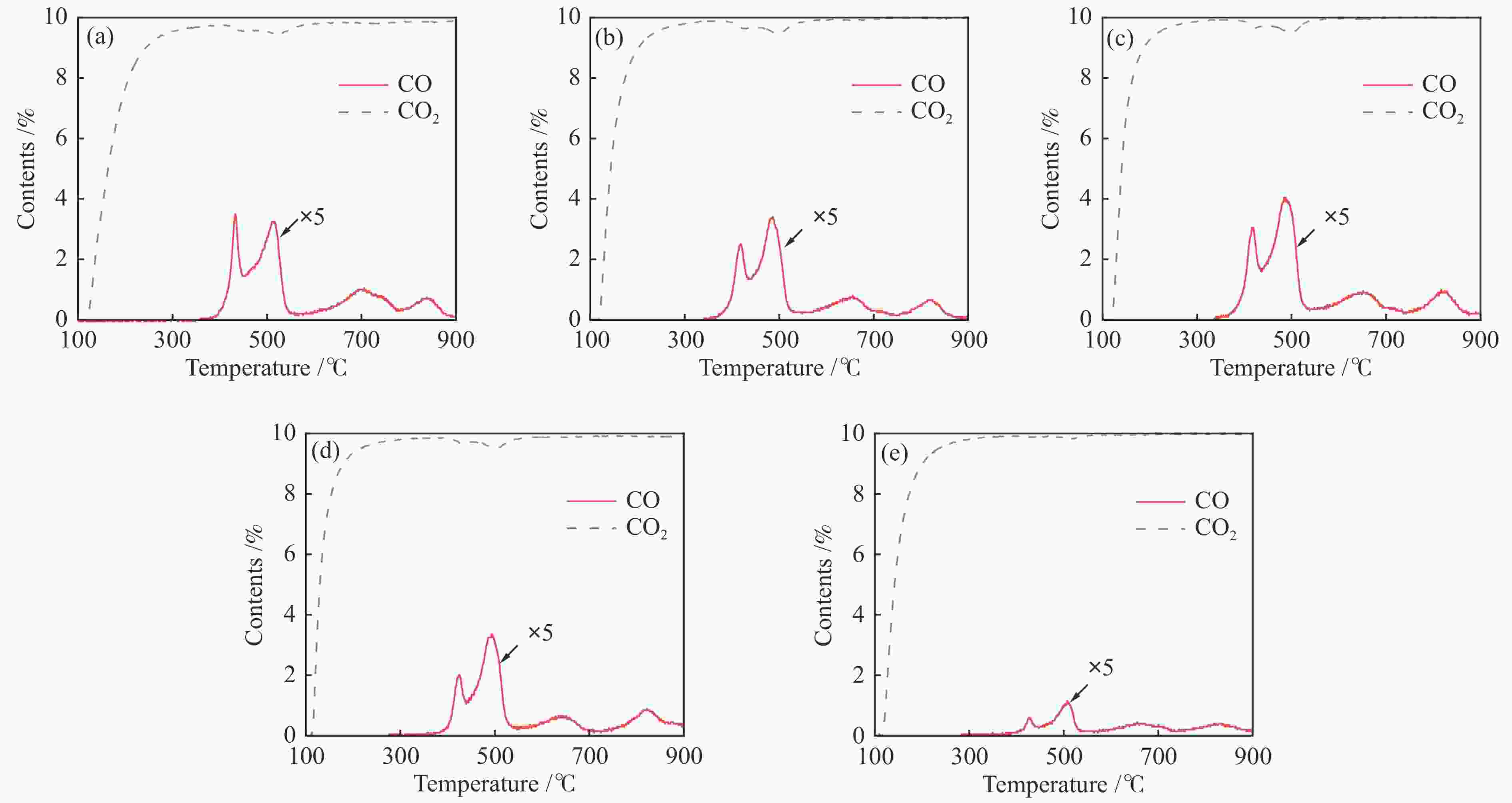
图 7 Ce0.8Cu0.2O2 (a)、Ce0.8Cu0.2O2/0.1 g S-1 (b)、Ce0.8Cu0.2O2/0.2 g S-1 (c)、Ce0.8Cu0.2O2/0.3 g S-1 (d)和Ce0.8Cu0.2O2/0.4 g S-1 (e)氧载体的CO2-TPO谱图
Figure 7 CO2-TPO curves of Ce0.8Cu0.2O2 (a), Ce0.8Cu0.2O2/0.1 g S-1 (b), Ce0.8Cu0.2O2/0.2 g S-1 (c), Ce0.8Cu0.2O2/0.3 g S-1 (d) and Ce0.8Cu0.2O2/0.4 g S-1 (e) oxygen carriers
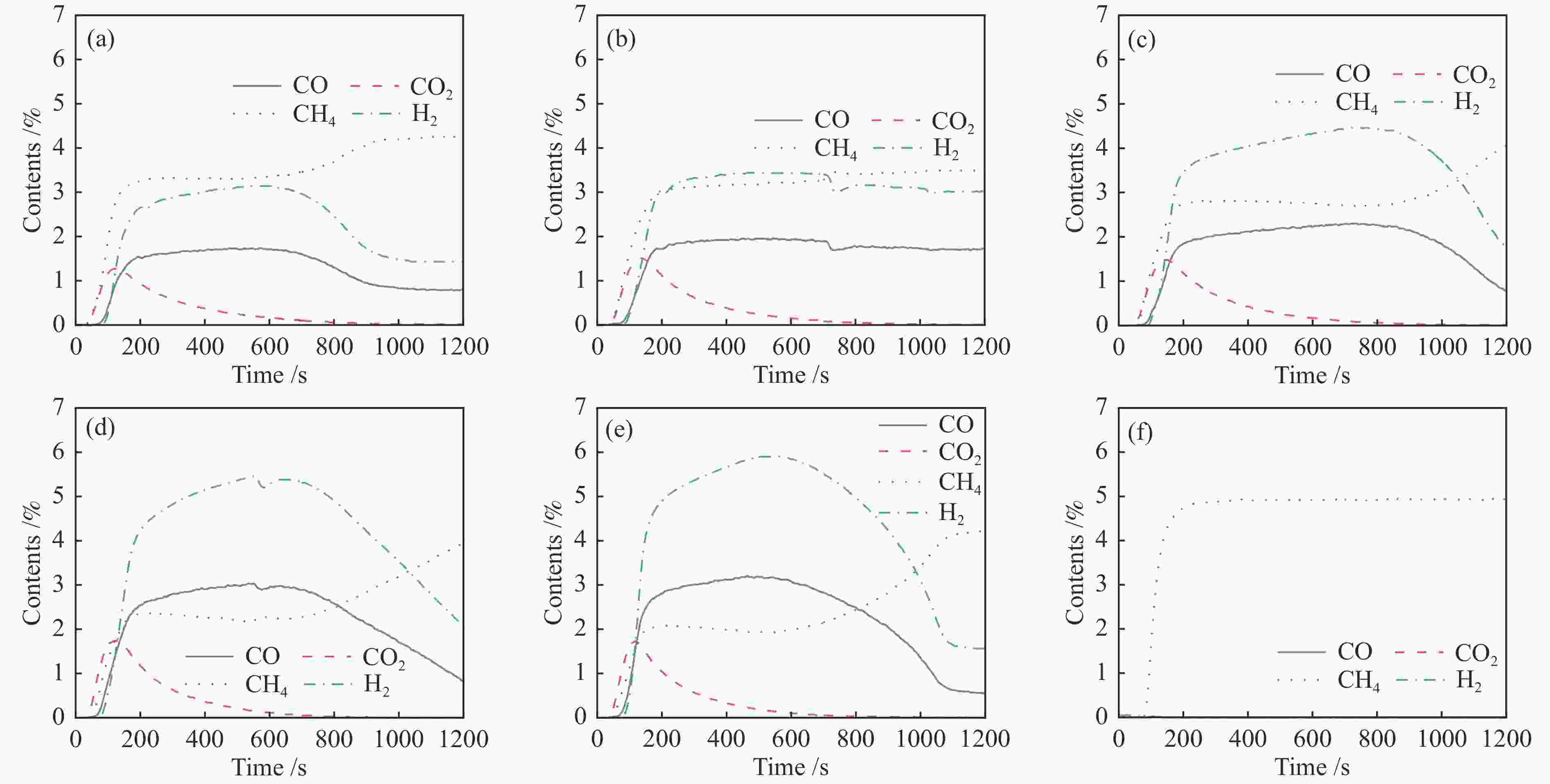
图 8 Ce0.8Cu0.2O2 (a)、Ce0.8Cu0.2O2/0.1 g S-1 (b)、Ce0.8Cu0.2O2/0.2 g S-1 (c)、Ce0.8Cu0.2O2/0.3 g S-1 (d)和Ce0.8Cu0.2O2/0.4 g S-1(e)氧载体和S-1(f)在850 ℃下甲烷部分氧化反应中气体含量的变化
Figure 8 Gas content change curve of Ce0.8Cu0.2O2 (a), Ce0.8Cu0.2O2/0.1 g S-1 (b), Ce0.8Cu0.2O2/0.2 g S-1 (c), Ce0.8Cu0.2O2/0.3 g S-1 (d), Ce0.8Cu0.2O2/0.4 g S-1 (e) oxygen carriers and S-1 (f) in the CH4 partial oxidation reactions at 850 ℃
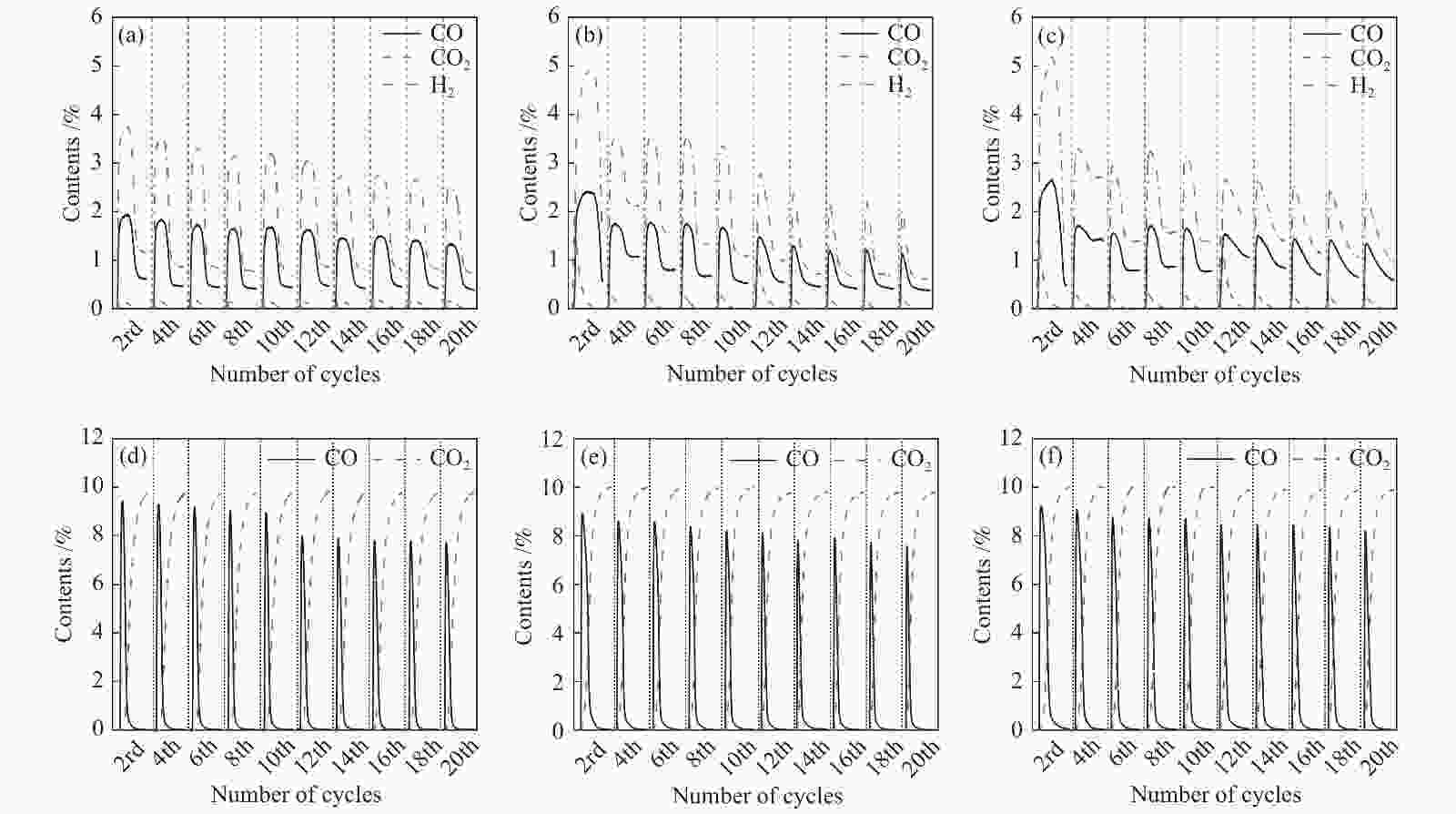
图 10 Ce0.8Cu0.2O2((a)/(d))、 Ce0.8Cu0.2O2/0.2 g S-1 ((b)/(e))和Ce0.8Cu0.2O2/0.3 g S-1 ((c)/(f))氧载体在850 ℃时20次redox循环的CH4部分氧化反应和CO2还原反应的气体含量变化
Figure 10 Gas content of Ce0.8Cu0.2O2 ((a)/(d)), Ce0.8Cu0.2O2/0.2 g S-1 ((b)/(e)) and Ce0.8Cu0.2O2/0.3 g S-1 ((c)/(f)) oxygen carriers in CH4 partial oxidation reactions and CO2 reduction reactions during 20 redox cycles at 850 ℃
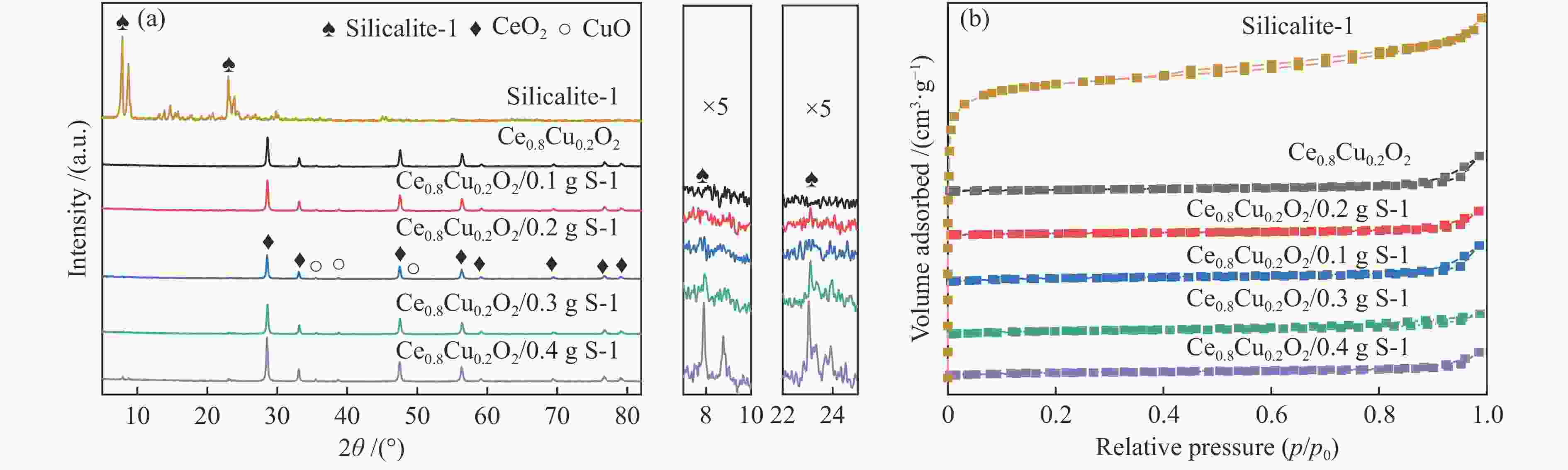
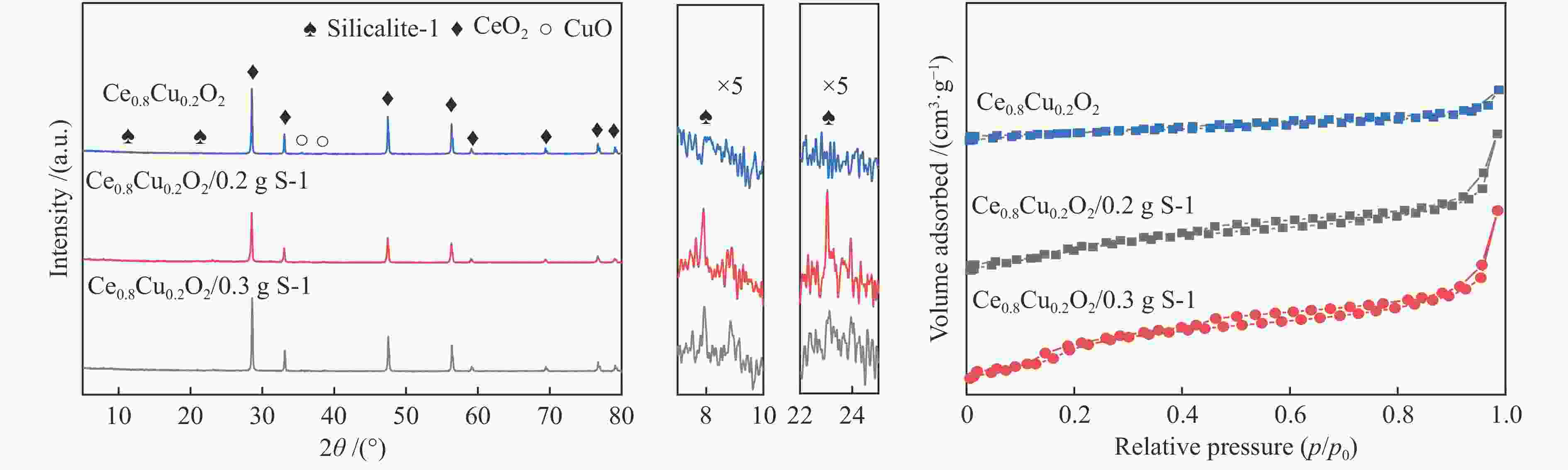
表 1 Ce0.8Cu0.2O2 样品和耦合不同比例S-1分子筛的Ce0.8Cu0.2O2 样品的平均孔径、比表面积和孔容
Table 1 Average pore diameter, BET surface area and total pore volume of Ce0.8Cu0.2O2 samples and Ce0.8Cu0.2O2 samples coupled with different proportions of S-1 zeolites
Sample Average pore diameter/nm Surface area/(m2·g−1) Total pore volume/(cm3·g−1) S-1 2.61 382.07 0.25 Ce0.8Cu0.2O2 28.38 15.44 0.11 Ce0.8Cu0.2O2/0.1 g S-1 13.79 33.21 0.11 Ce0.8Cu0.2O2/0.2 g S-1 8.15 69.40 0.14 Ce0.8Cu0.2O2/0.3 g S-1 5.11 73.27 0.09 Ce0.8Cu0.2O2/0.4 g S-1 6.62 79.00 0.13 表 2 样品的XPS特性参数
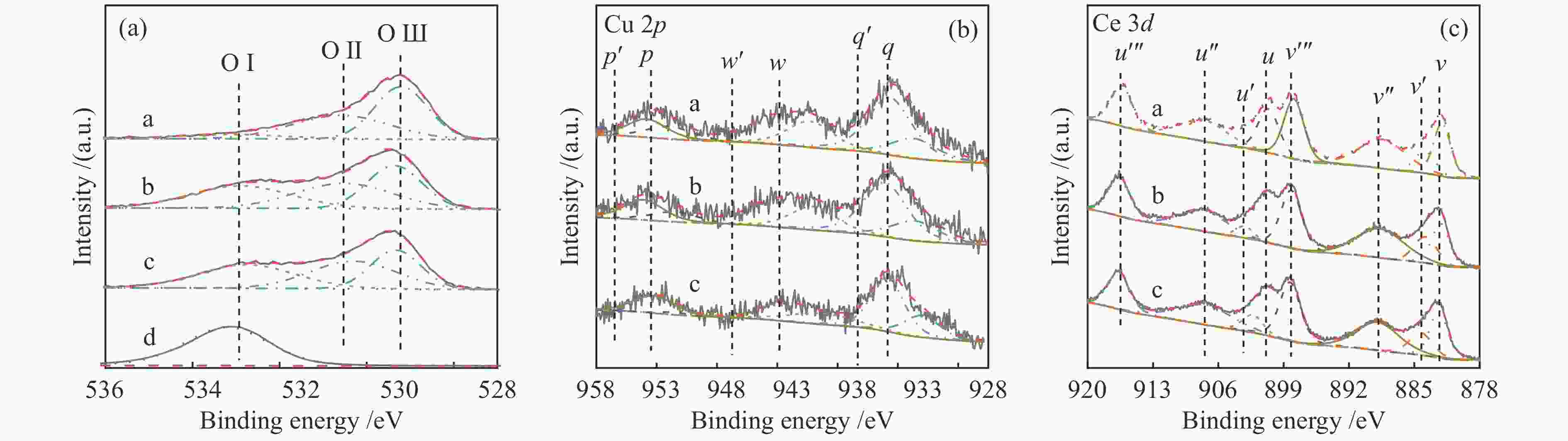
Table 2 XPS characteristic parameters of sample
Sample Surface element composition Oads / Olatt Cu + / Cu2 + Ce3 + / Ce4 + Ce0.8Cu0.2O2 1.01 0.47 0.11 Ce0.8Cu0.2O2/0.2 g S-1 2.58 0.44 0.13 Ce0.8Cu0.2O2/0.3 g S-1 2.45 0.34 0.15 表 3 样品在850 ℃下甲烷部分氧化反应中的气体含量
Table 3 Gas content of sample in the CH4 partial oxidation reactions at 850 ℃
Sample Percent conversion
of CH4/%Yield of CO /
(mmol·g−1)Yield of CO2 /
(mmol·g−1)Yield of carbon deposition /
(mmol·g−1)Ce0.8Cu0.2O2 38.93 1.25 0.19 0.014 Ce0.8Cu0.2O2/0.1 g S-1 44.87 1.68 0.20 0.015 Ce0.8Cu0.2O2/0.2 g S-1 51.07 1.83 0.21 0.033 Ce0.8Cu0.2O2/0.3 g S-1 56.03 2.24 0.19 0.031 Ce0.8Cu0.2O2/0.4 g S-1 57.30 2.20 0.18 0.034 表 4 样品在850 ℃下CO2还原反应中的气体含量
Table 4 Gas content of samples in CO2 reduction reaction at 850 ℃
Sample Yield of CO /
(mmol·g−1)Ce0.8Cu0.2O2 1.18 Ce0.8Cu0.2O2/0.1 g S-1 1.53 Ce0.8Cu0.2O2/0.2 g S-1 1.91 Ce0.8Cu0.2O2/0.3 g S-1 2.16 Ce0.8Cu0.2O2/0.4 g S-1 2.03 表 5 样品在850 ℃时20次循环过程得失氧量与积炭量变化
Table 5 Amount of oxygen gained and lost during the 20 cycles at 850 ℃ and the amount of carbon deposition variated
Sample Ce0.8Cu0.2O2/(mmol·g−1) Ce0.8Cu0.2O2/0.2 g S-1/(mmol·g−1) Ce0.8Cu0.2O2/0.3 g S-1/(mmol·g−1) oxygen
lossoxygen
gaincarbon
depositionoxygen
lossoxygen
gaincarbon
depositionoxygen
lossoxygen
gaincarbon
deposition2 2.07 1.20 0.020 2.42 2.20 0.041 2.30 2.08 0.042 4 1.23 0.71 0.051 1.33 1.27 0.049 1.56 1.57 0.043 6 0.93 0.74 0.048 1.15 1.20 0.048 1.00 1.14 0.046 8 0.90 0.81 0.052 1.00 0.97 0.054 1.17 1.18 0.044 10 0.97 0.66 0.049 0.91 0.89 0.051 1.09 1.12 0.044 12 1.93 1.52 0.016 1.26 0.82 0.028 1.75 1.36 0.017 14 0.87 1.08 0.050 0.65 0.62 0.043 1.10 1.04 0.035 16 1.02 0.95 0.048 0.57 0.54 0.043 0.98 1.04 0.035 18 0.99 0.95 0.049 0.59 0.48 0.044 0.93 0.95 0.038 20 0.87 0.88 0.049 0.48 0.39 0.044 0.88 0.86 0.037 表 6 20次redox循环后样品的平均孔径、比表面积和孔容
Table 6 Average pore diameter, BET surface area and total pore volume of samples after 20 redox cycles
Sample Average pore diameter /
nmSurface area /
(m2·g−1)Total pore volume /
(cm3·g−1)Ce0.8Cu0.2O2 10.48 3.01 0.008 Ce0.8Cu0.2O2/0.2 g S-1 4.02 33.94 0.03 Ce0.8Cu0.2O2/0.3 g S-1 3.52 51.50 0.04 -
[1] KHALILPOUR R, KARIMI I A. Evaluation of utilization alternatives for stranded natural gas[J]. Energy,2012,40(1):317−328. doi: 10.1016/j.energy.2012.01.068 [2] LI D, NAKAGAWA Y, TOMISHIGE K. Methane reforming to synthesis gas over Ni catalysts modified with noble metals[J]. Appl Catal A: Gen,2011,408(1-2):1−24. doi: 10.1016/j.apcata.2011.09.018 [3] FAROOQI A S, YUSUF M, MOHD ZABIDI N A. A comprehensive review on improving the production of rich-hydrogen via combined steam and CO2 reforming of methane over Ni-based catalysts[J]. Int J Hydrog Energy,2021,46(60):31024−31040. doi: 10.1016/j.ijhydene.2021.01.049 [4] ANTZARAS A N, HERACLEOUS E, LEMONIDOU A A. Sorption enhanced-chemical looping steam methane reforming: Optimizing the thermal coupling of regeneration in a fixed bed reactor[J]. Fuel Process Technol,2020,208:106513. [5] CHENG Z, QIN L, FAN J A. New insight into the development of oxygen carrier materials for chemical looping systems[J]. Engineering,2018,4(3):343−351. doi: 10.1016/j.eng.2018.05.002 [6] CHUAYBOON S, ABANADES S, RODAT S. Solar chemical looping reforming of methane combined with isothermal H2O/CO2 splitting using ceria oxygen carrier for syngas production[J]. J Energy Chem,2020,41:60−72. doi: 10.1016/j.jechem.2019.05.004 [7] GOLDWASSER M R, RIVAS M E, LUGO M L. Combined methane reforming in presence of CO2 and O2 over LaFe1−xCoxO3 mixed-oxide perovskites as catalysts precursors[J]. Catal Today,2005,107-108:106−113. doi: 10.1016/j.cattod.2005.07.073 [8] LIANG T-Y, LIN C-Y, CHOU F-C. Gas-phase synthesis of Ni-CeOx hybrid nanoparticles and their synergistic catalysis for simultaneous reforming of methane and carbon dioxide to syngas[J]. J Phys Chem C,2018,122(22):11789−11798. doi: 10.1021/acs.jpcc.8b00665 [9] PASHCHENKO D, MAKAROV I. Carbon deposition in steam methane reforming over a Ni-based catalyst: Experimental and thermodynamic analysis[J]. Energy,2021,222:119993. [10] RAHBARI A, RAMDIN M, VAN DEN BROEKE L J P. Combined steam reforming of methane and formic acid to produce syngas with an adjustable H2: CO ratio[J]. Ind Eng Chem Res,2018,57(31):10663−10674. doi: 10.1021/acs.iecr.8b02443 [11] WANG B, ZHANG H, XU W. Nature of active sites on Cu-CeO2 catalysts activated by high-temperature thermal aging[J]. ACS Catal,2020,10(21):12385−12392. doi: 10.1021/acscatal.0c03188 [12] ZHOU G, XIE F, DENG L. Supported mesoporous Cu/CeO2−δ catalyst for CO2 reverse water-gas shift reaction to syngas[J]. Int J Hydrog Energy,2020,45(19):11380−11393. doi: 10.1016/j.ijhydene.2020.02.058 [13] ZHOU H, YI Q, WEI G. Reaction performance and lattice oxygen migration of MnFe2O4 oxygen carrier in methane-carbon dioxide reaction system[J]. Int J Hydrog Energy,2020,45(55):30254−30266. doi: 10.1016/j.ijhydene.2020.08.103 [14] GAO N, CHENG M, QUAN C. Syngas production via combined dry and steam reforming of methane over Ni-Ce/ZSM-5 catalyst[J]. Fuel,2020,273:117702. [15] RAFIEE A, KHALILPOUR K R, MILANI D. CO2 conversion and utilization pathways[M]. Polygeneration with Polystorage for Chemical and Energy Hubs. New York: Academic Press, 2019: 213−245. [16] ZHANG B, HUYAN Y, WANG J. Synthesis of CeO2 nanoparticles with different morphologies and their properties as peroxidase mimic[J]. J Am Ceram Soc,2019,102(4):2218−2227. [17] SASTRE D, SERRANO D P, PIZARRO P. Chemical insights on the activity of La1−xSrxFeO3 perovskites for chemical looping reforming of methane coupled with CO2-splitting[J]. J CO2 Util,2019,31:16−26. doi: 10.1016/j.jcou.2019.02.013 [18] WANG C, WANG Y, CHEN M. Recent advances during CH4 dry reforming for syngas production: A mini review[J]. Int J Hydrog Energy,2021,46(7):5852−5874. doi: 10.1016/j.ijhydene.2020.10.240 [19] WEI Q, GAO X, WANG L. Rational design of nickel-based catalyst coupling with combined methane reforming to steadily produce syngas[J]. Fuel,2020,271:117631. [20] ZHAO K, HE F, HUANG Z. Perovskite-type oxides LaFe1−xCox O3 for chemical looping steam methane reforming to syngas and hydrogen co-production[J]. Appl Energy,2016,168:193−203. doi: 10.1016/j.apenergy.2016.01.052 [21] WEISSENBERGER T, LEONHARDT R, ZUBIRI B A. Synthesis and characterisation of hierarchically structured titanium silicalite-1 zeolites with large intracrystalline macropores[J]. Chemistry,2019,25(63):14430−14440. doi: 10.1002/chem.201903287 [22] YAO X, CHEN L, KONG T. Support effect of the supported ceria-based catalysts during NH3 -SCR reaction[J]. Chin J Catal,2017,38(8):1423−1430. doi: 10.1016/S1872-2067(17)62868-7 [23] 伊志豪, 孙杰, 李吉刚. 花球状 CuO / CeO2 材料上 HCN 的催化消除[J]. 材料导报,2021,35(8):8017−8022. doi: 10.11896/cldb.20010081YI Zhi-hao, SUN Jie, LI Ji-gang. Catalytic elimination of HCN on curd-like CuO/CeO2 materials[J]. Materials Reports,2021,35(8):8017−8022. doi: 10.11896/cldb.20010081 [24] MATEOS-PEDRERO C, SILVA H, PACHECO TANAKA D A. CuO/ZnO catalysts for methanol steam reforming: The role of the support polarity ratio and surface area[J]. Appl Catal B: Environ,2015,174−175:67−76. doi: 10.1016/j.apcatb.2015.02.039 [25] YANG J, DING H, ZHU Z. Surface modification of CeO2 nanoflakes by low temperature plasma treatment to enhance imine yield: Influences of different plasma atmospheres[J]. Appl Surf Sci,2018,454:173−180. doi: 10.1016/j.apsusc.2018.05.135 [26] ZHANG J, YANG J, WANG J. Surface oxygen vacancies dominated CeO2 as efficient catalyst for imine synthesis: Influences of different cerium precursors[J]. Mol Catal,2017,443:131−138. doi: 10.1016/j.mcat.2017.09.030 [27] XIE Y, WU J, JING G. Structural origin of high catalytic activity for preferential CO oxidation over CuO/CeO2 nanocatalysts with different shapes[J]. Appl Catal B: Environ,2018,239:665−676. doi: 10.1016/j.apcatb.2018.08.066 [28] WNAG X, RODRIGUE Z, JOSE A. In situ studies of the active sites for the water gas shift reaction over Cu-CeO2 catalysts: complex interaction between metallic copper and oxygen vacancies of ceria[J]. J Phys Chem B,2006,110(1):428−34. doi: 10.1021/jp055467g [29] ZHU Y Y, LIU R L, MA X X. Metal modified hexaaluminates for syngas generation and CO2 utilization via chemical looping[J]. Int J Hydrog Energy,2019,44(21):10218−10231. [30] SUN Y, YAN N, LI J. The effect of calcination temperature on the electrochemical properties of La0.3Sr0.7Fe0.7Cr0.3O3−x (LSFC) perovskite oxide anode of solid oxide fuel cells (SOFCs)[J]. Sustain Energy Techno,2014,8:92−98. doi: 10.1016/j.seta.2014.08.001 -




 下载:
下载: