Progress of In2O3-based catalysts in thermal catalytic CO2 hydrogenation reaction
-
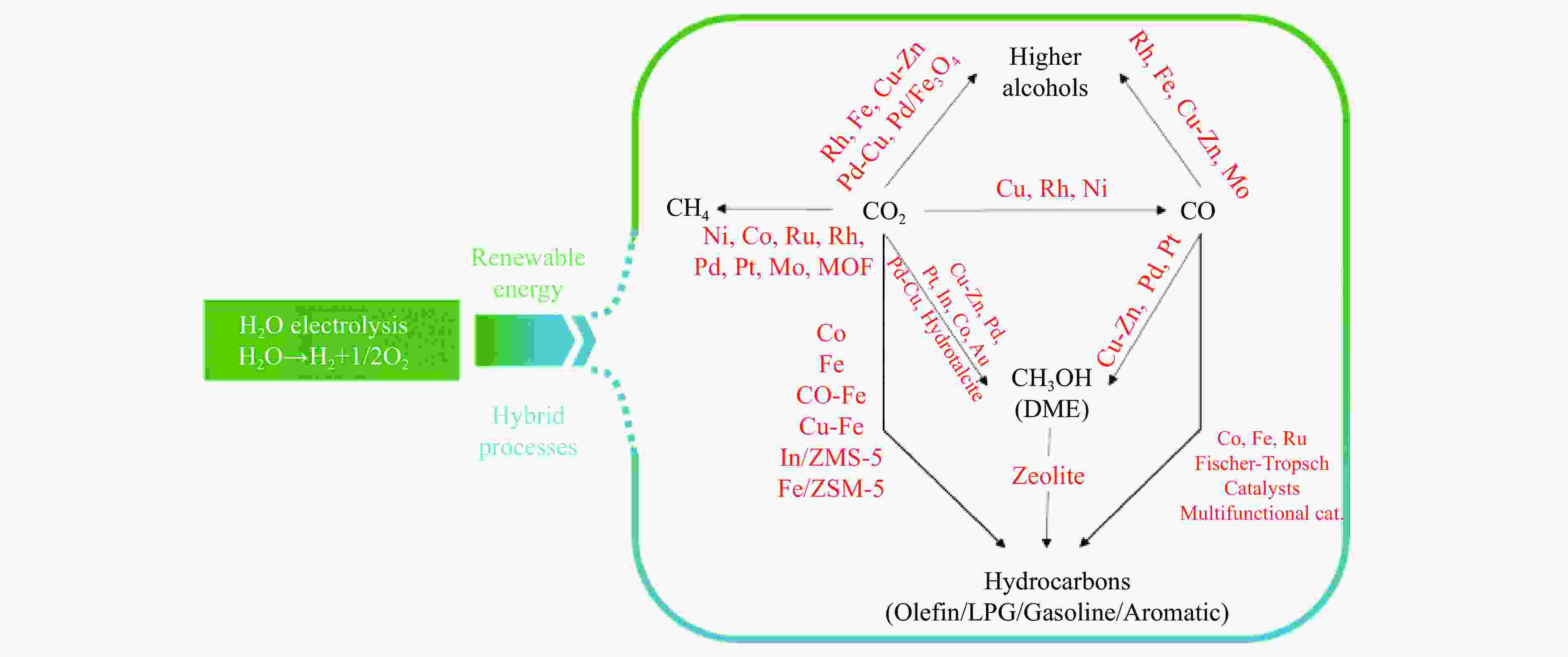
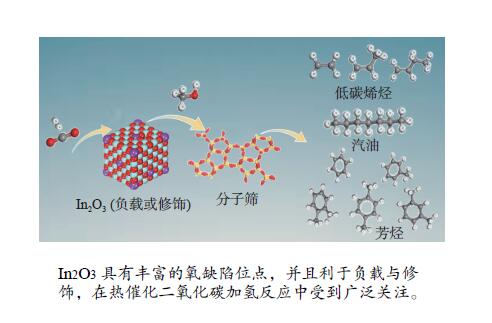
摘要: CO2催化加氢被认为是生产高附加值化学品和燃料最实用的途径之一。然而由于其化学惰性、C–C键偶联过程的高能垒和诸多的竞争反应,因此,开发高效的催化剂以促进CO2的活化并转化为多样的化工产物显得至关重要。近年来,氧化铟因具有丰富的氧缺陷位点,在催化CO2加氢方面对甲醇的高选择性以及对CO2转化的高活性引起了人们的广泛关注。本工作主要对In2O3的结构及其与氧化物负载或金属元素掺杂的复合催化剂用于催化CO2加氢制备甲醇的催化性能进行了综述。探讨了In2O3与不同类型的分子筛的接近度和元素迁移在CO2加氢制烃类产物中的影响。并对In2O3基催化剂在CO2选择性加氢方面存在的挑战和发展方向进行了总结。Abstract: Catalytic hydrogenation of CO2 is considered to be one of the most practical ways to produce value-added chemicals and fuels. However, due to the extreme chemical inertness, the high C–C coupling barrier and the many competing reactions, it is of vital important to develop the efficient catalysts for achieving the activation and transformation of CO2 into a variety of chemical products. In recent years, indium oxide has aroused great interest in CO2 hydrogenation due to its abundant oxygen vacancies, high selectivity of methanol and high activity of CO2 conversion. In this paper, the structure of In2O3 and the catalytic performance of In2O3-supported or metal-doped composite catalysts for CO2 hydrogenation to methanol are reviewed. The effects of the proximity of In2O3 to different zeolites and the migration of elements on the products of CO2 hydrogenation to hydrocarbons are also discussed. Finally, the challenges and development directions of selective hydrogenation of CO2 over In2O3-based catalysts are summarized.
-
Key words:
- carbon dioxide /
- indium oxide /
- methanol /
- hydrocarbon compound
-
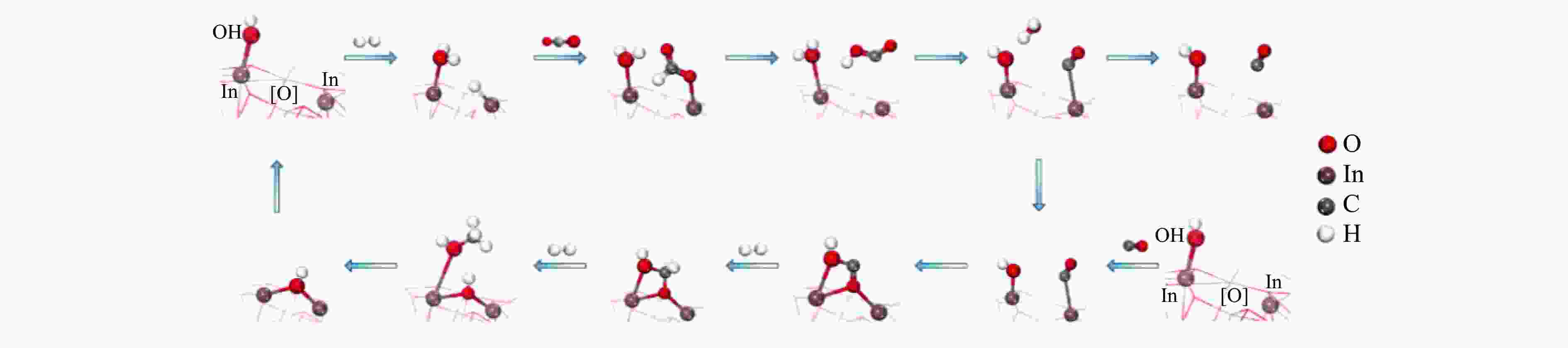
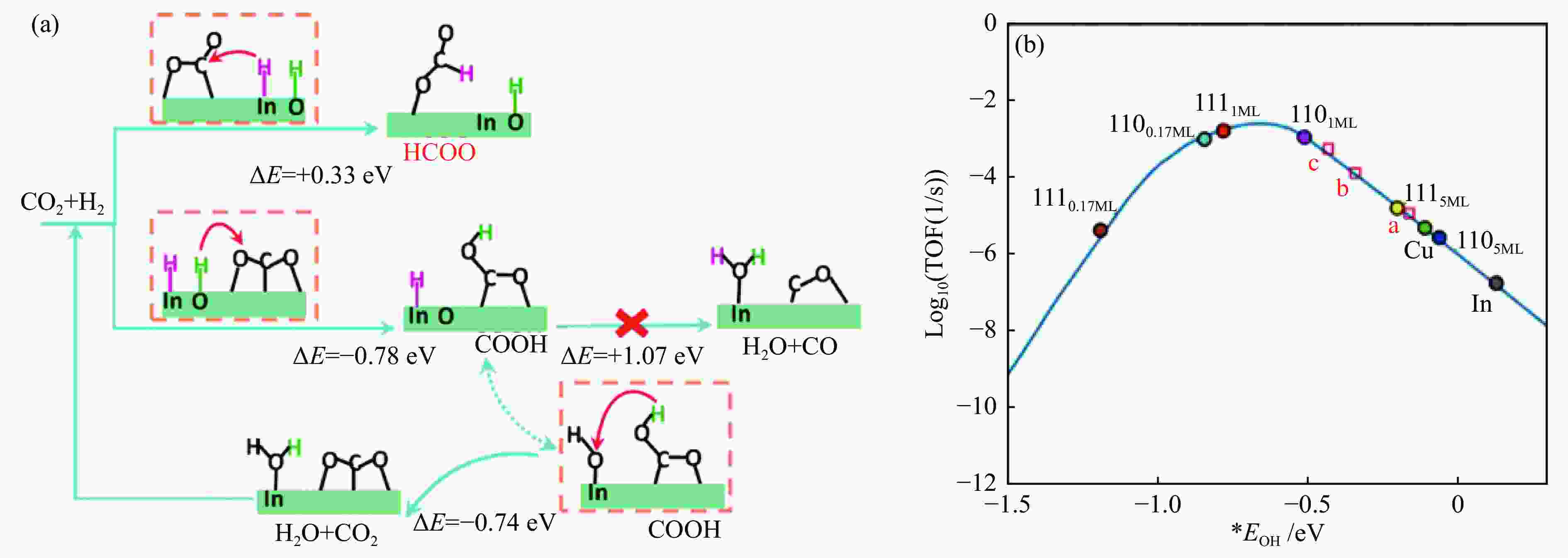
图 5 (a) In2O3上CO2加氢初始步骤的示意图,In位和O位上的H原子分别呈紫色和绿色[40];(b) 当CO的吸附能为–0.1 eV时,甲醇形成的理论活性火山与OH结合能的关系[42]
Figure 5 (a) Schematic diagram of the initial steps of CO2 hydrogenation over In2O3, with the H atoms at the In and O positions in purple and green, respectively[40]; (b) Theoretical activity volcano for methanol formation as a function of the binding energy of OH for a fixed adsorption energy of CO of –0.1 eV (Reaction conditions: 300 ℃, 0.5 MPa of CO2, and 1.5 MPa of H2)[42](with permission from American Chemical Society)
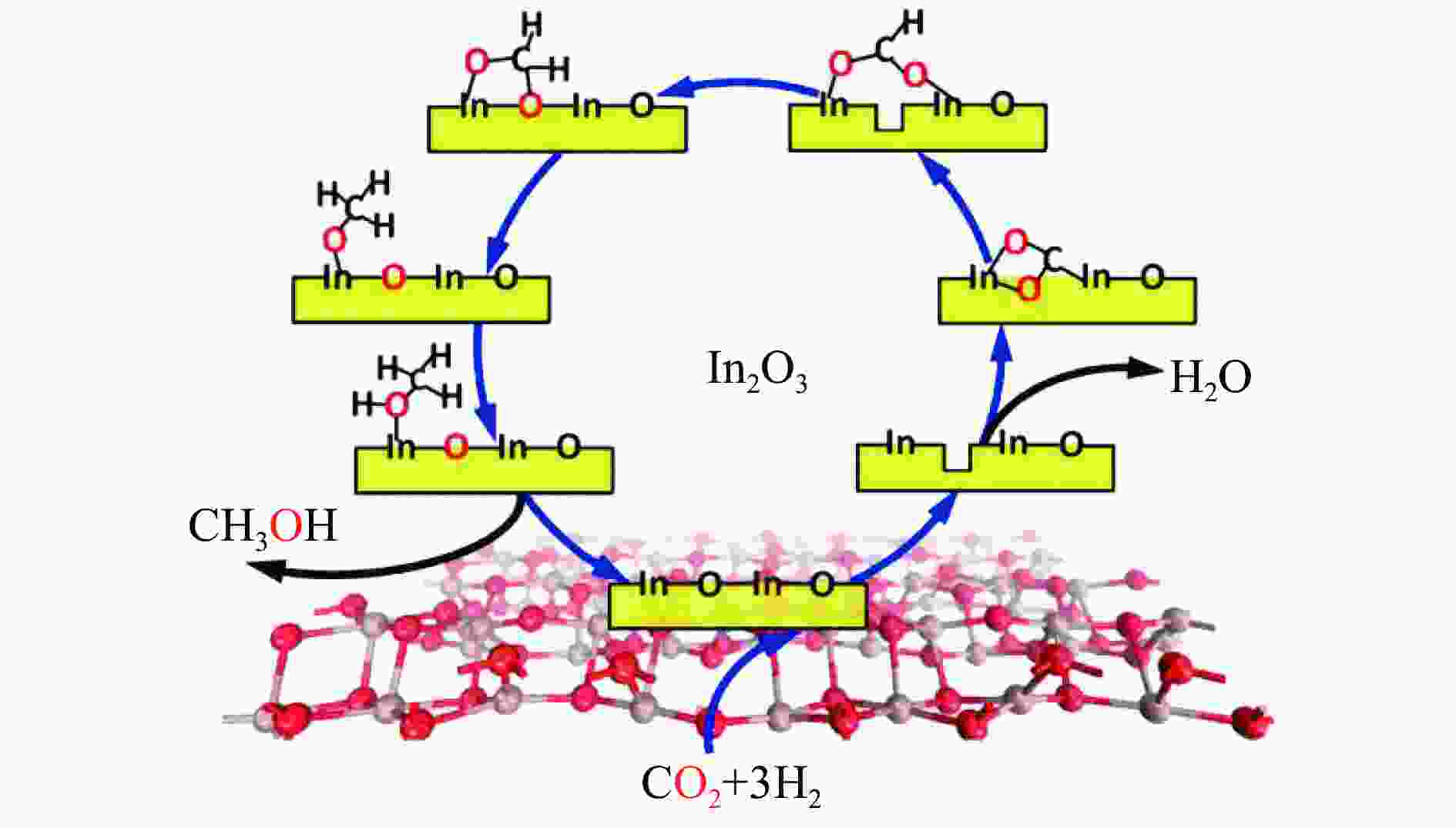
图 6 (a) In2O3表面氧空位催化CO2加氢制甲醇的基本步骤(红球: O原子,棕球: In原子,白球: H原子)[38];(b) In2O3表面氧空位催化CO2加氢过程中的吉布斯自由能变化[41];(c) In2O3(110)表面的构型[38];(d) 表面Zr掺杂浓度对In2O3(110)表面氧空位形成能的影响[43]
Figure 6 (a) Basic steps for CO2 hydrogenation to methanol catalyzed by oxygen vacancy on the surface of In2O3(red ball: O atoms, brown ball: In atoms, white ball: H atoms)[38]; (b) Gibbs free energy variation during CO2 hydrogenation catalyzed by oxygen vacancies on the surface of In2O3[41]; (c) Conformation of In2O3(110) surface[38]; (d) Effect of surface Zr doping concentration on the oxygen vacancy formation energy on the surface of In2O3(110)43](with permission from American Chemical Society, Elsevier)
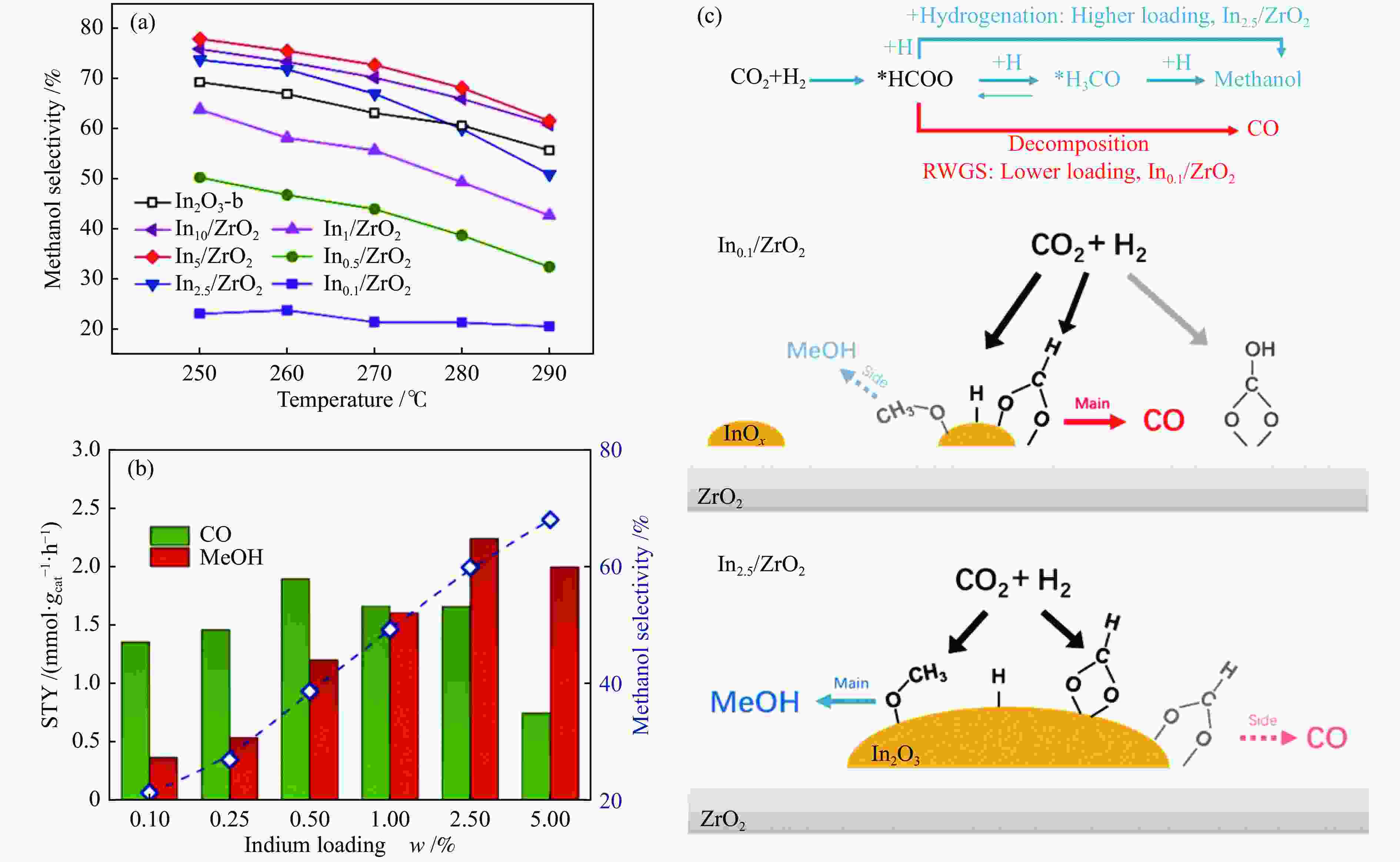
图 8 (a) 温度对甲醇选择性的影响;(b) Inx/ZrO2催化剂上CO的STY、甲醇的STY和选择性随In含量的变化;(c) In0.1/ZrO2和In2.5/ZrO2上CO2加氢路径及结构-性能关系[45]
Figure 8 (a) Effect of temperature on methanol selectivity;(b) Variation of STY of CO, STY of methanol and selectivity with In content over Inx/ZrO2 catalyst (Reaction conditions: 280 ℃, 5.0 MPa, CO2∶H2∶N2=4∶1∶1.67, GHSV=24000 h–1); (c) CO2 hydrogenation pathways and structure-property relationships on In0.1/ZrO2 and In2.5/ZrO2[45](with permission from American Chemical Society)
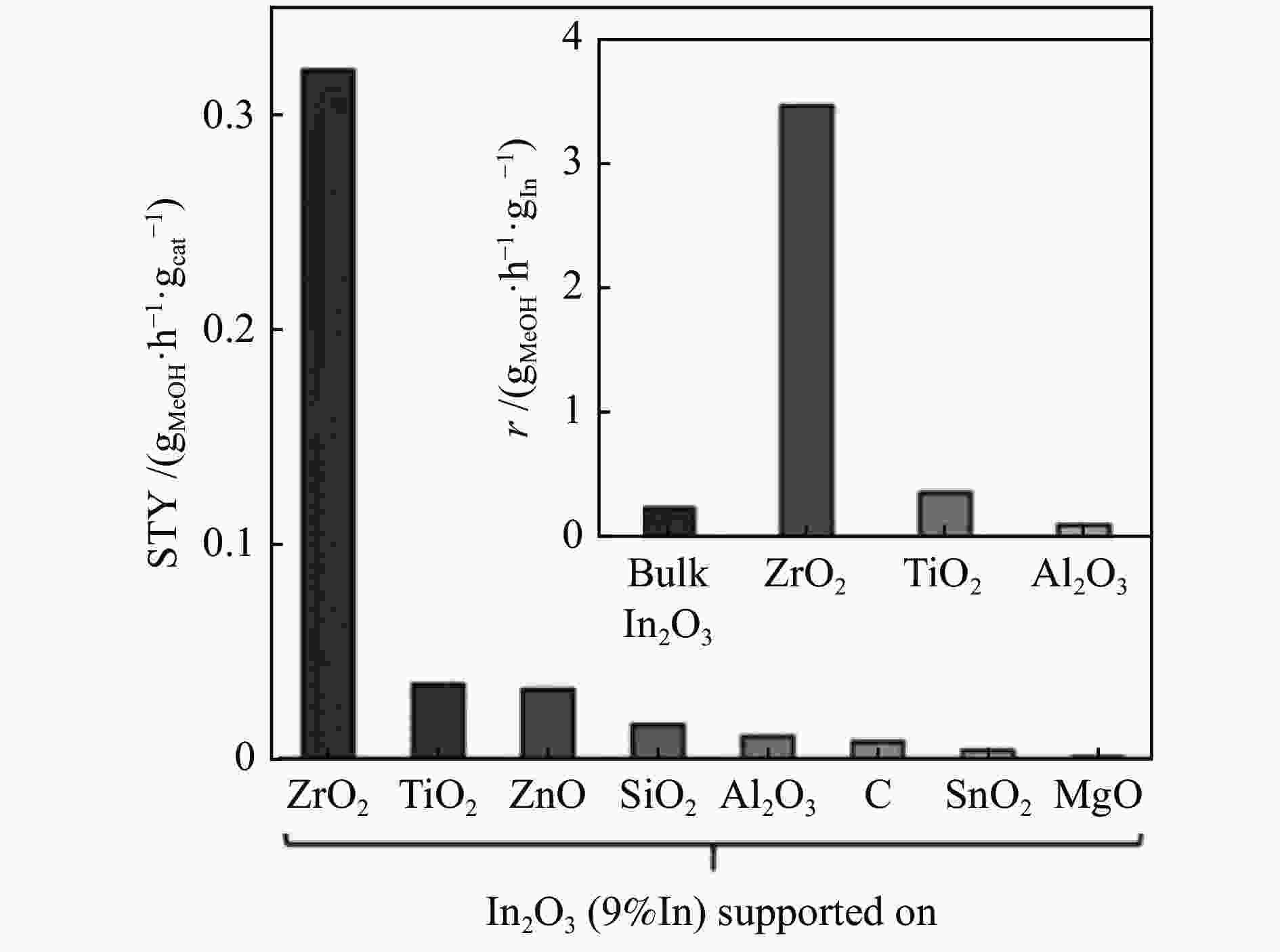
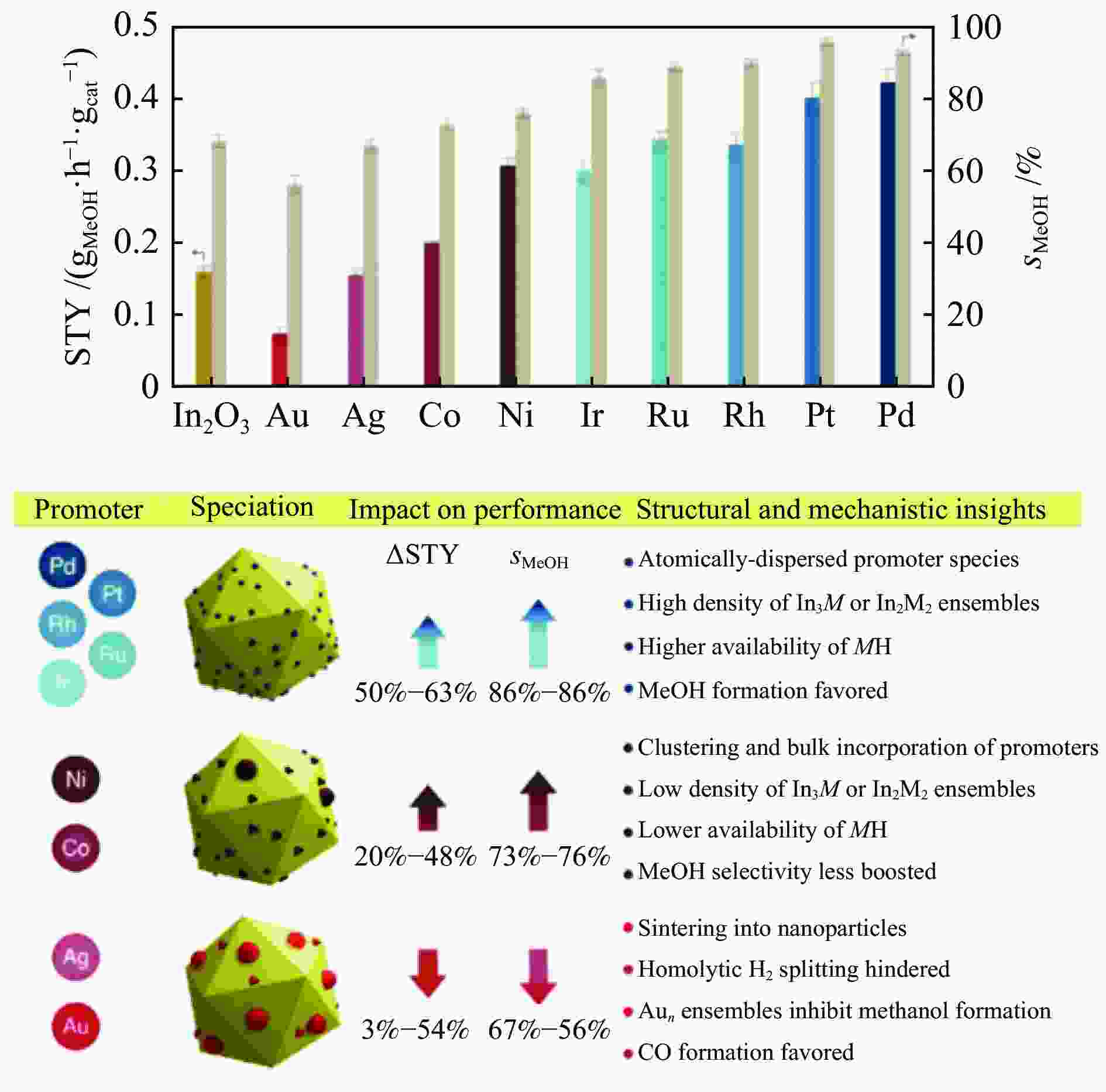
图 9 FSP法制备的纯In2O3和M-In2O3催化剂上甲醇的时空产率(STY,彩色条)和选择性(sMeOH,米色条)及M-In2O3催化剂的助剂形态及其相关结构-机理特征[61]
Figure 9 Spatiotemporal yield (STY, colored bars) and selectivity (sMeOH, beige bars) of methanol over pure In2O3 and M-In2O3 catalysts prepared by the FSP method and the auxiliary morphology of M-In2O3 catalysts and their related structure-mechanism characteristics[61] (with permission from Wiley-VCH GmbH)
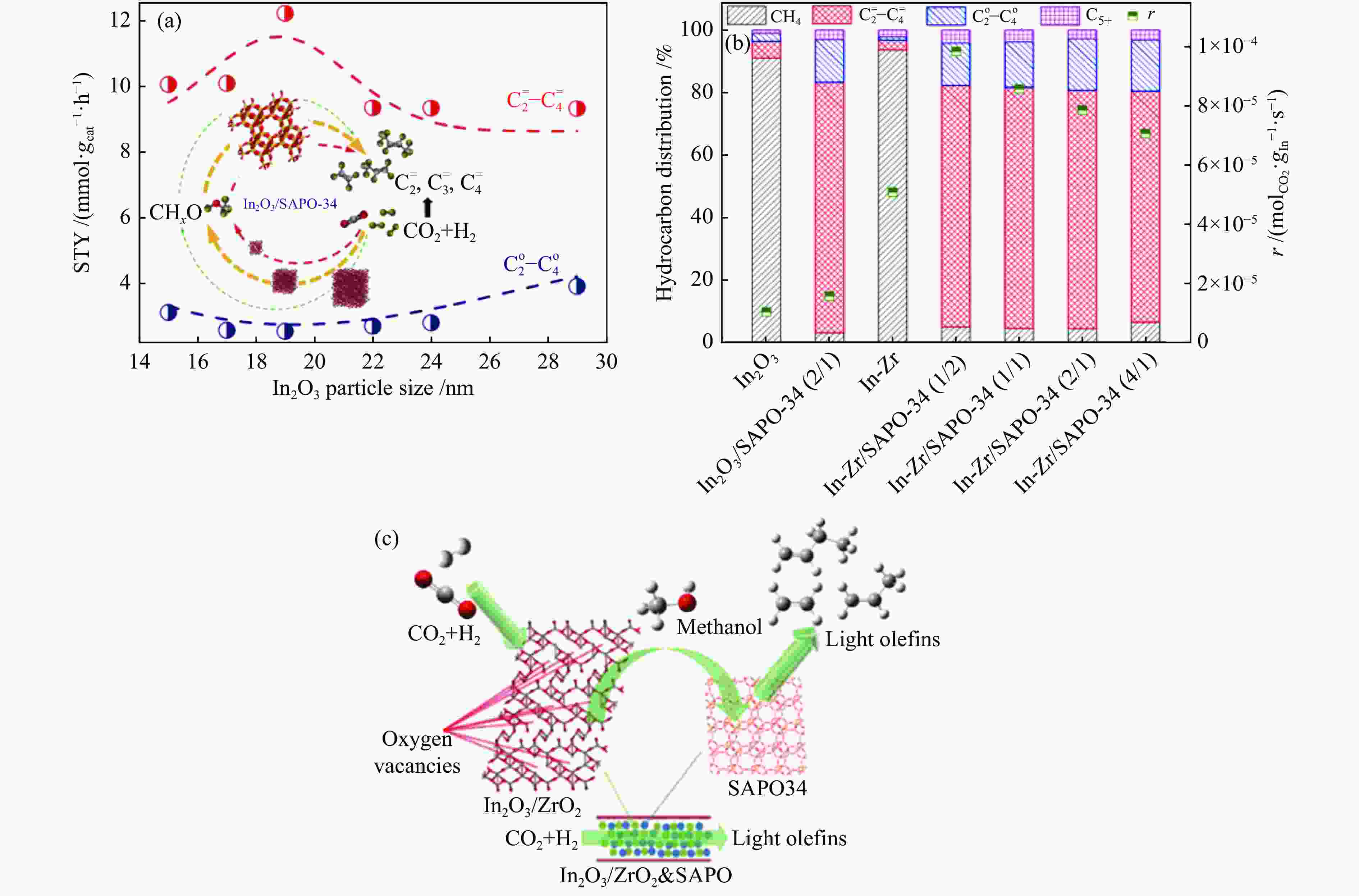
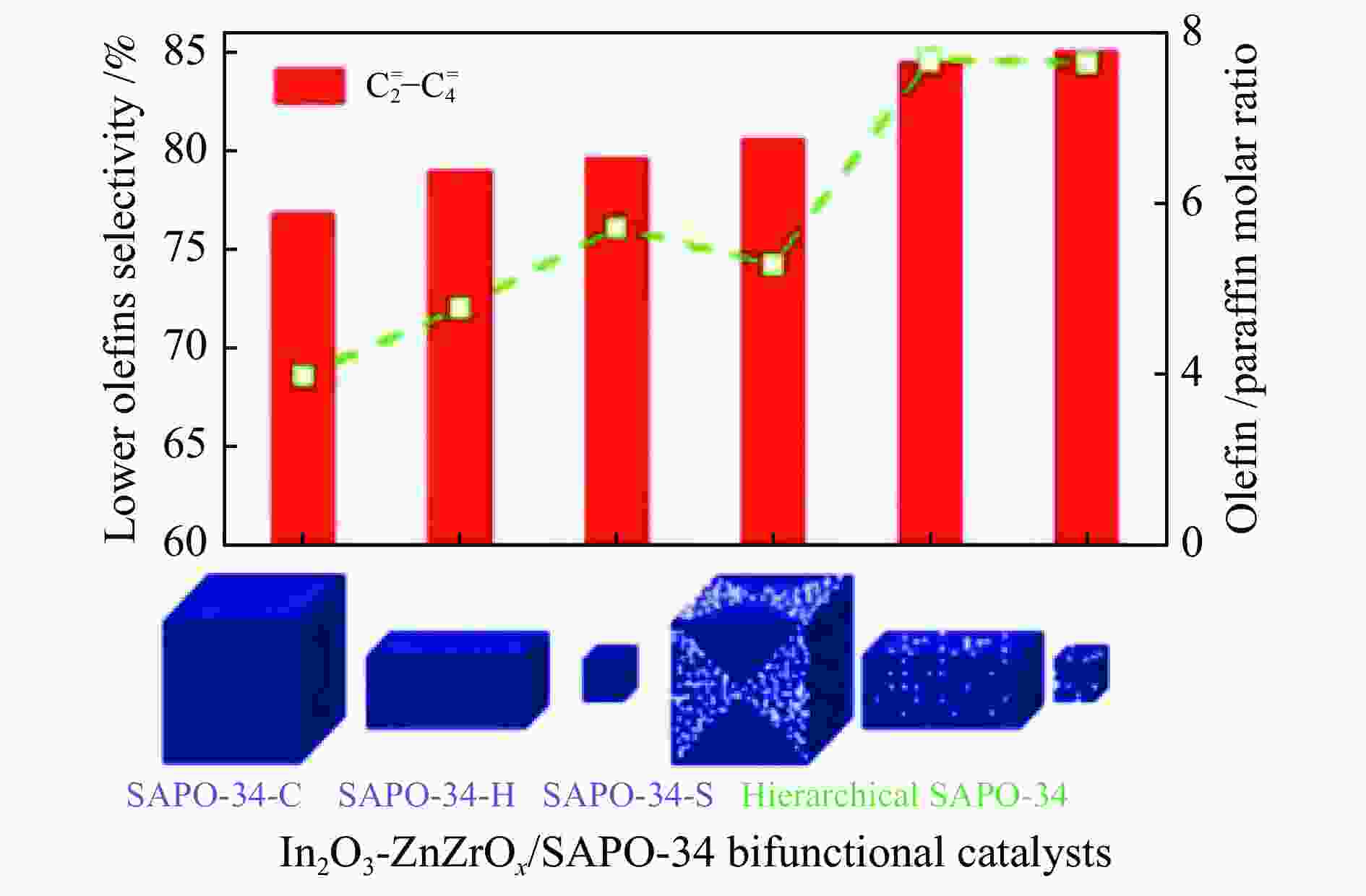
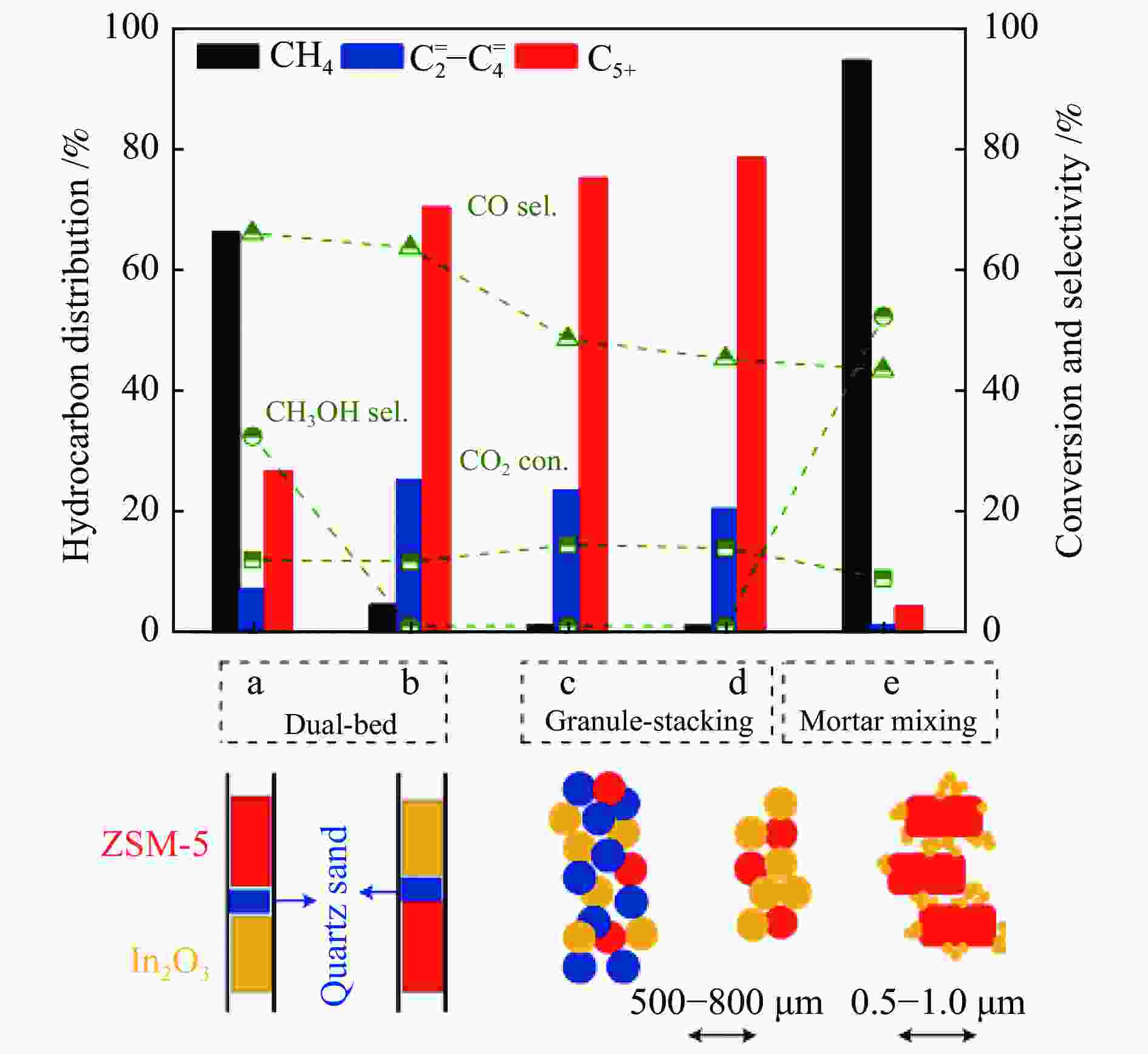
图 13 (a) In2O3晶粒尺寸对In2O3/SAPO-34催化剂催化活性的影响[74];(b) In2O3、In-Zr氧化物以及金属氧化物/SAPO-34分子筛串联催化剂上烃类产物的分布和反应速率[75];(c) In-Zr/SAPO-34体系中CO2加氢制低碳烯烃反应路线示意图[76]
Figure 13 (a) Effect of In2O3 particle size on the catalytic activity of In2O3/SAPO-34 catalyst[74]; (b) Distribution and reaction rates of hydrocarbon products over In2O3, In-Zr oxides and metal oxide/SAPO-34 tandem catalysts[75]; (c) Schematic diagram of the reaction route of CO2 hydrogenation to low carbon olefins over the In-Zr/SAPO-34 catalyst[76](with permission from Elsevier, American Chemical Society)
表 1 CO2加氢制甲醇的催化性能
Table 1 Catalytic properties of CO2 hydrogenation to methanol
Catalyst t /℃ p /MPa Space velocity H2/CO2 CO2 conv. /% MeOH sel. /% STY Ref. c-In2O3-S 300 5 9000 mL/(gcat·h) 3 12.0 71.9 8.3 mmol/(gcat·h) [34] h-In2O3-R 300 5 20000 mL/(gcat·h) 4 6.7 99.5 11.4 mmol/(gcat·h) [34] Black In2O3 250 – – 3 – 49.23 – [39] Bulk In2O3 300 5 – 4 3.4 >99.5 – [43] In2O3 300 5 21000 mL/(h·gcat) – 8.2 71.2 0.352 gMeOH/(h·gcat) [50] In2O3 400 3 9000 mL/(gcat·h) 3 31.5 1.2 – [74] 9 In2O3/ZrO2 300 5 16000 h–1 4 5.2 99.8 0.295 gMeOH/(gcat·h) [43] In5/ZrO2 250 5 24000 h–1 4 0.6 77.9 0.024 gMeOH/(gcat·h) [45] 1.5Y In2O3/ZrO2 300 4 52000 mL/(h·gcat) 4 7.6 69 0.420 gMeOH/(gcat·h) [46] 3La10In/ZrO2 300 4 52000 mL/(h·gcat) 4 7.7 66 0.420 gMeOH/(gcat·h) [46] In2O3/m-ZrO2(redox) 300 – 48000 mL/(gcat·h) 3 – 33 2.20 gMeOH/(gIn2O3·h) [47] In2O3/t-ZrO2(redox) 300 – 48000 mL/(gcat·h) 3 – 31 0.49 gMeOH/(gIn2O3·h) [47] In2O3/am-ZrO2(redox) 300 – 48000 mL/(gcat·h) 3 – 29 0.37 gMeOH/(gIn2O3·h) [47] Pd/In2O3 300 5 21000 mL/(h·gcat) – 20.5 72.1 0.885 gMeOH/(gcat·h) [50] 0.58% Pt/In2O3 300 2 24000 mL/(gcat·h) 3 6.3 56 0.482 gMeOH/(gcat·h) [54] Rh/In2O3 300 5 21000 mL/(h·gcat) 4 17.1 56.1 0.5448 gMeOH/(gcat·h) [55] Au/In2O3 300 5 21000 mL/(h·gcat) 4 11.7 67.8 0.470 gMeOH/(gcat·h) [57] In@Co-1 300 5 – 4 19 69 0.480 gMeOH/(gcat·h) [58] Ag/In2O3 300 5 21000 mL/(h·gcat) 4 13.6 58.2 0.453 gMeOH/(gcat·h) [59] CuIn@SiO2 280 3 7500 mL/(gcat·h) – 12.5 78.2 6.55 mmol/(gcat·h) [62] 表 2 不同串联催化剂在CO2加氢制烃类反应中的催化性能
Table 2 Catalytic performance of different tandem catalysts in CO2 hydrogenation to hydrocarbon reactions
Catalyst Reaction conditions Selectivity /% Hydrocarbon distribution/% Ref. t /℃ p /MPa GHSV /
(mL·gcat–1·h–1)H2/CO2 CO CH CH4 $ {\rm{C}}_2^=- {\rm{C}}_4^=$ $ {\rm{C}}_2^0- {\rm{C}}_4^0 $ C5+ In2O3/HZSM-5 340 3 9000 3 44.8 55.2 1 – 20.4 78.6 [72] InZnZrOx/NZ5 320 3 4000 3 19.8 8.0 – – – – [73] In2O3-SAPO-34 350 3 9000 3 – – 1.8 – – – [74] In2O3-SAPO-34 380 3 9000 3 68.3 31.7 2.7 81.9 13.7 1.7 [44] In-Zr(4∶1)/SAPO-34 380 3 9000 3 63.9 36.1 2.0 74.5 21.5 2.0 [44] In-Zr(1∶1)/SAPO-34 380 3 9000 3 68.6 31.4 2.9 67.2 25.0 4.9 [44] In-Zr(1∶4)/SAPO-34 380 3 9000 3 70.4 29.6 2.6 65.1 29.6 2.7 [44] In2O3-SAPO-34(2/1) 400 3 9000 3 85.9 14.1 – – – – [75] In-Zr/SAPO-34(1/2) 400 3 9000 3 84.2 15.8 – – – – [75] In-Zr/SAPO-34(1/1) 400 3 9000 3 85.3 14.7 – – – – [75] In-Zr/SAPO-34(2/1) 400 3 9000 3 85.0 15.0 4.3 76.4 16.5 2.8 [75] In-Zr/SAPO-34(4/1) 400 3 9000 3 89.2 10.6 – – – – [75] In2O3/ZnZrOx/SAPO-34 380 3 9000 3 55.8 44.2 1.6 85 11.1 2.3 [77] In2O3/ZrO2-SAPO-5 300 3 4000 3 – – 3 83 – 17 [79] -
[1] 王晗, 樊升, 王森, 董梅, 秦张峰, 樊卫斌, 王建国. 二氧化碳加氢制一些烃类化合物的研究进展[J]. 燃料化学学报,2021,49(11):1609−1619. doi: 10.1016/S1872-5813(21)60122-6WANG Han, FAN Sheng, WANG Sen, DONG Mei, QIN Zhang-feng, FAN Wei-bin, WANG Jian-guo. Research progresses in the hydrogenation of carbon dioxide to certain hydrocarbon products[J]. J Fuel Chem Technol,2021,49(11):1609−1619. doi: 10.1016/S1872-5813(21)60122-6 [2] EBI K L. Key themes in the working group II contribution to the intergovernmental panel on climate change 5th assessment report[J]. Clim Change,2012,114(3):417−426. [3] PECHENKIN A, POTEMKIN D, BADMAEV S, SMIRNOVA E, CHEREDNICHENKO K, VINOKUROV V, GLOTOV A. CO2 hydrogenation to dimethyl ether over In2O3 catalysts supported on aluminosilicate halloysite nanotubes[J]. Green Process Synth,2021,10(1):594−605. doi: 10.1515/gps-2021-0058 [4] NOCITO F, DIBENEDETTO A. Atmospheric CO2 mitigation technologies: carbon capture utilization and storage[J]. Current Opinion Green Sustainable Chem,2020,21:34−43. doi: 10.1016/j.cogsc.2019.10.002 [5] MEYLAN F D, MOREAU V, ERKMAN S. CO2 utilization in the perspective of industrial ecology, an overview[J]. J CO2 Util,2015,12:101−108. doi: 10.1016/j.jcou.2015.05.003 [6] NIE X W, LI W H, JIANG X, GUO X W, SONG C S. Recent advances in catalytic CO2 hydrogenation to alcohols and hydrocarbons[J]. Adv Catal,2019,65:121−233. [7] POROSOFF M D, YAN B, CHEN J G. Catalytic reduction of CO2 by H2 for synthesis of CO, methanol and hydrocarbons: Challenges and opportunities[J]. Energy Environ Sci,2016,9(1):62−73. doi: 10.1039/C5EE02657A [8] BANERJEE A, DICK G R, YOSHINO T, KANAN M W. Carbon dioxide utilization via carbonate-promoted C–H carboxylation[J]. Nature,2016,531(7593):215−219. doi: 10.1038/nature17185 [9] ZHOU C, SHI J Q, ZHOU W, CHENG K, ZHANG Q Q, KANG J C, WANG Y. Highly active ZnO-ZrO2 aerogels integrated with H-ZSM-5 for aromatics synthesis from carbon dioxide[J]. ACS Catal,2019,10(1):302−310. [10] POROSOFF M D, YANG X F, BOSCOBOINIK J A, CHEN J G G. Molybdenum carbide as alternative catalysts to precious metals for highly selective reduction of CO2 to CO[J]. Angew Chem Int Ed,2014,53(26):6705−6709. doi: 10.1002/anie.201404109 [11] JANKE C, DUYAR M S, HOSKINS M, FARRAUTO R. Catalytic and adsorption studies for the hydrogenation of CO2 to methane[J]. Appl Catal B: Environ,2014,152:184−191. [12] JIANG X, NIE X W, GUO X W, SONG C S, CHEN J G. Recent advances in carbon dioxide hydrogenation to methanol via heterogeneous catalysis[J]. Chem Rev,2020,120(15):7984−8034. doi: 10.1021/acs.chemrev.9b00723 [13] MENG C, ZHAO G F, SHI X R, CHEN P J, LIU Y, LU Y. Oxygen-deficient metal oxides supported nano-intermetallic InNi3C0.5 toward efficient CO2 hydrogenation to methanol[J]. Sci Adv,2021,7(32):eabi6012. doi: 10.1126/sciadv.abi6012 [14] MORET S, DYSON P J, LAURENCZY G. Direct synthesis of formic acid from carbon dioxide by hydrogenation in acidic media[J]. Nat Commun,2014,5(1):1−7. [15] ZHANG W N, WEI Y X. Regulation of product distribution in CO2 hydrogenation to light olefins[J]. Chem,2022,8(5):1170−1173. doi: 10.1016/j.chempr.2022.04.024 [16] WANG X X, YANG G H, ZHANG J F, CHEN S Y, WU Y Q, ZHANG Q D, WANG J W, HAN Y Z, TAN Y S. Synthesis of isoalkanes over a core (Fe-Zn-Zr)-shell (zeolite) catalyst by CO2 hydrogenation[J]. Chem Comm,2016,52(46):7352−7355. doi: 10.1039/C6CC01965J [17] CHOI Y H, JANG Y J, PARK H, KIM W Y, LEE Y H, CHOI S H, LEE J S. Carbon dioxide Fischer-Tropsch synthesis: A new path to carbon-neutral fuels[J]. Appl Catal B: Environ,2017,202:605−610. doi: 10.1016/j.apcatb.2016.09.072 [18] 郭晓明, 毛东森, 卢冠忠, 王嵩. CO2加氢合成甲醇催化剂的研究进展[J]. 化工进展,2012,31(3):477−488.GUO Xiao-ming, MAO Dong-sen, LU Guan-zhong, WANG Song. Progress in catalysts for methanol synthesis from CO2 hydrogenation[J]. Chem Ind Eng Prog,2012,31(3):477−488. [19] ÁLVAREZ A, BANSODE A, URAKAWA A, BAVYKINA A V, WEZENDONK T A, MAKKEE M, GASCON J, KAPTEIJN F. Challenges in the greener production of formates/formic acid, methanol, and DME by heterogeneously catalyzed CO2 hydrogenation processes[J]. Chem Rev,2017,117(14):9804−9838. doi: 10.1021/acs.chemrev.6b00816 [20] HARTADI Y, WIDMANN D, BEHM R J. Methanol formation by CO2 hydrogenation on Au/ZnO catalysts-Effect of total pressure and influence of CO on the reaction characteristics[J]. J Catal,2016,333:238−250. doi: 10.1016/j.jcat.2015.11.002 [21] TADA S, WATANABE F, KIYOTA K, SHIMODA N, HAYASHI R, TAKAHASHI M, NARIYUKI A, LGARASHI A, SATOKAWA S. Ag addition to CuO-ZrO2 catalysts promotes methanol synthesis via CO2 hydrogenation[J]. J Catal,2017,351:107−118. doi: 10.1016/j.jcat.2017.04.021 [22] SUN K H, FAN Z G, YE J Y, YAN J M, GE Q F, LI Y N, HE W J, YANG W M, LIU C J. Hydrogenation of CO2 to methanol over In2O3 catalyst[J]. J CO2 Util,2015,12:1−6. doi: 10.1016/j.jcou.2015.09.002 [23] WANG J J, LI G N, Li Z L, TANG C Z, FENG Z C, AN H Y, LIU H L, LIU T F, LI C. A highly selective and stable ZnO-ZrO2 solid solution catalyst for CO2 hydrogenation to methanol[J]. Sci Adv,2017,3(10):e1701290. doi: 10.1126/sciadv.1701290 [24] WANG J J, TANG C Z, LI G N, HAN Z, LI Z L, LIU H L, CHENG F, LI C. High-performance MaZrOx (Ma=Cd, Ga) solid solution catalysts for CO2 hydrogenation to methanol[J]. ACS Catal,2019,9(11):10253−10259. doi: 10.1021/acscatal.9b03449 [25] WANG J Y, ZHANG G H, ZHU J, ZHANG X B, DING F S, ZHANG A F, GUO X W, SONG C S. CO2 hydrogenation to methanol over In2O3-based catalysts: From mechanism to catalyst development[J]. ACS Catal,2021,11(3):1406−1423. doi: 10.1021/acscatal.0c03665 [26] KATTEL S, YAN B H, CHEN J G, LIU P. CO2 hydrogenation on Pt, Pt/SiO2 and Pt/TiO2: Importance of synergy between Pt and oxide support[J]. J Catal,2016,343:115−126. doi: 10.1016/j.jcat.2015.12.019 [27] 李龙泰, 张春杰, 罗学彬, 杨彬, 郭利民. 用于CO2催化加氢In2O3基催化剂的研究进展[J]. 材料导报,2021,35(21):21071−21078.LI Long-tai, ZHANG Chun-jie, LUO Xue-bin, YANG Bin, GUO Li-min. Recent advances in In2O3-based catalysts for CO2 hydrogenation[J]. Mater Rev,2021,35(21):21071−21078. [28] KARAZHANOV S Z, RAVINDRAN P, VAJEESTON P, ULYASHIN A, FINSTAD T G, FJELLVÅG H. Phase stability, electronic structure, and optical properties of indium oxide polytypes[J]. Phys Rev B,2007,76(7):075129. doi: 10.1103/PhysRevB.76.075129 [29] DE BOER T, BEKHEET M F, GURLO A, RIEDEL R, MOEWES A. Band gap and electronic structure of cubic, rhombohedral, and orthorhombic In2O3 polymorphs: Experiment and theory[J]. Phys Rev B,2016,93(15):155205. doi: 10.1103/PhysRevB.93.155205 [30] YANG B, LI L T, JIA Z Y, LIU X P, ZHANG C J, GUO L M. Comparative study of CO2 hydrogenation to methanol on cubic bixbyite-type and rhombohedral corundum-type indium oxide[J]. Chin Chem Lett,2020,31(10):2627−2633. doi: 10.1016/j.cclet.2020.05.031 [31] GUNJI T, NAKAMURA K, HAYAMI R, AIMI A, FUJIMOTO K, YAMAMOYO K. Synthesis of indium tin oxide films from ethyl acetoacetonato complexes at low temperatures[J]. J Solgel Sci Technol,2021,100(1):68−73. doi: 10.1007/s10971-021-05618-7 [32] QIN B, ZHOU Z M, LI S G. Reaction pathways and the role of the carbonates during CO2 hydrogenation over hexagonal In2O3 catalysts[J]. Appl Surf Sci,2021,542:148591. doi: 10.1016/j.apsusc.2020.148591 [33] NIELSEN I G, SOMMER S, IVERSEN B B. Phase control for indium oxide nanoparticles[J]. Nanoscale,2021,13(7):4038−4050. doi: 10.1039/D0NR08587A [34] DANG S S, QIN B, YANG Y, WANG H, CAI J, HAN Y, LI S G, GAO P, SUN Y H. Rationally designed indium oxide catalysts for CO2 hydrogenation to methanol with high activity and selectivity[J]. Sci Adv,2020,6(25):eaaz2060. doi: 10.1126/sciadv.aaz2060 [35] WANG J Y, LIU C Y, SENFTLE T P, ZHU J, ZHANG G H, GUO X W, SONG C S. Variation in the In2O3 crystal phase alters catalytic performance toward the reverse water gas shift reaction[J]. ACS Catal,2020,10(5):3264−3273. doi: 10.1021/acscatal.9b04239 [36] UMEGAKI T, KURATANI K, YAMADA Y, UEDA A, KURIYAMA N, KOBAYASHI T, XU Q. Hydrogen production via steam reforming of ethyl alcohol over nano-structured indium oxide catalysts[J]. J Power Sources,2008,179(2):566−570. doi: 10.1016/j.jpowsour.2008.01.010 [37] LORENZ H, JOCHUM W, KLÖTZER B, STÖGER-POLLACH M, SCHWARZ S, PFALLRE K, PENNER S. Novel methanol steam reforming activity and selectivity of pure In2O3[J]. Appl Catal A: Gen,2008,347(1):34−42. doi: 10.1016/j.apcata.2008.05.028 [38] YE J Y, LIU C J, MEI D H, GE Q F. Active oxygen vacancy site for methanol synthesis from CO2 hydrogenation on In2O3 (110): A DFT study[J]. ACS Catal,2013,3(6):1296−1306. doi: 10.1021/cs400132a [39] ZHANG Z S, MAO C L, MEIRA D M, DUCHESNE P N, TOUNTAS A A, LI Z, QIU C Y, TANG S L, SONG R, DING X, SUN J C, YU J F, HOWE J Y, TU W G, WANG L, OZIN G A. New black indium oxide-tandem photothermal CO2-H2 methanol selective catalyst[J]. Nat Commun,2022,13(1):1512. [40] YE J Y, LIU C J, GE Q F. DFT study of CO2 adsorption and hydrogenation on the In2O3 surface[J]. J Phys Chem C,2012,116(14):7817−7825. doi: 10.1021/jp3004773 [41] FREI M S, CAPDEVILA-CORTADA M, GARCÍA-MUELAS R, MONDELLI C, LÓPEZ N, STEWART J A, FERRÉ D C, PÉREZ-RAMÍREZ J. Mechanism and microkinetics of methanol synthesis via CO2 hydrogenation on indium oxide[J]. J Catal,2018,361:313−321. doi: 10.1016/j.jcat.2018.03.014 [42] CAO A, WANG Z B, LI H, NØRSKOV J K. Relations between surface oxygen vacancies and activity of methanol formation from CO2 hydrogenation over In2O3 surfaces[J]. ACS Catal,2021,11(3):1780−1786. doi: 10.1021/acscatal.0c05046 [43] DANG S S, GAO P, LIU Z Y, CHEN X Q, YANG C G, WANG H, ZHONG L S, LI S G, SUN Y H. Role of zirconium in direct CO2 hydrogenation to lower olefins on oxide/zeolite bifunctional catalysts[J]. J Catal,2018,364:382−393. doi: 10.1016/j.jcat.2018.06.010 [44] MARTIN O, MARTÍN A J, MONDELLI C, MITCHELL S, SEGAWA T F, HAUERT R, DROUILLY C, CURULLA-FERRÉ D, PÉREZ-RAMÍREZ J. Indium oxide as a superior catalyst for methanol synthesis by CO2 hydrogenation[J]. Angew Chem Int Ed,2016,55(21):6261−6265. doi: 10.1002/anie.201600943 [45] CHEN T Y, CAO C X, CHEN T B, DING X X, HUANG H, SHEN L, CAO X Y, ZHU M H, XU J, GAO J, HAN Y F. Unraveling highly tunable selectivity in CO2 hydrogenation over bimetallic In-Zr oxide catalysts[J]. ACS Catal,2019,9(9):8785−8797. doi: 10.1021/acscatal.9b01869 [46] CHOU C Y, LOBO R F. Direct conversion of CO2 into methanol over promoted indium oxide-based catalysts[J]. Appl Catal A: Gen,2019,583:117144−117153. doi: 10.1016/j.apcata.2019.117144 [47] TSOUKALOU A, SERYKH A I, WILLINGER E, KIERZKOWSKA A, ABDALA P M, FEDOROV A, MÜLLER C R. Hydrogen dissociation sites on indium-based ZrO2-supported catalysts for hydrogenation of CO2 to methanol[J]. Catal Today,2022,387:38−46. doi: 10.1016/j.cattod.2021.04.010 [48] TSOUKALOU A, BUSHKOV N S, DOCHERTY S R, MANCE D, SERYKH A I, ABDALA P M, COPÉRET C, FEDOROV A, MÜLLER C R. Surface intermediates in In-based ZrO2-supported catalysts for hydrogenation of CO2 to methanol[J]. J Phys Chem C,2022,126(4):1793−1799. doi: 10.1021/acs.jpcc.1c08814 [49] YE J Y, LIU C J, MEI D H, GE Q F. Methanol synthesis from CO2 hydrogenation over a Pd4/In2O3 model catalyst: A combined DFT and kinetic study[J]. J Catal,2014,317:44−53. doi: 10.1016/j.jcat.2014.06.002 [50] RUI N, WANG Z Y, SUN K H, YE J Y, GE Q F, LIU C J. CO2 hydrogenation to methanol over Pd/In2O3: Effects of Pd and oxygen vacancy[J]. Appl Catal B: Environ,2017,218:488−497. doi: 10.1016/j.apcatb.2017.06.069 [51] SNIDER J L, STREIBEL V, HUBERT M K A, et al. Revealing the synergy between oxide and alloy phases on the performance of bimetallic In-Pd catalysts for CO2 hydrogenation to methanol[J]. ACS Catal,2019,9(4):3399−3412. doi: 10.1021/acscatal.8b04848 [52] TIAN P, CAI Z J, ZHAN G W, HUANG J L, LI Q B. Preparation of supported In2O3/Pd nanocatalysts using natural pollen as bio-templates for CO2 hydrogenation to methanol: Effect of acid-etching on template[J]. Mol Catal,2021,516:111945. doi: 10.1016/j.mcat.2021.111945 [53] CAI Z J, HUANG M, DAI J J, ZHAN G W, SUN F L, ZHUANG G L, WANG Y Y, TIAN P, CHEN B, ULLAH S, HUANG J L, LI Q B. Fabrication of Pd/In2O3 nanocatalysts derived from MIL-68 (In) loaded with molecular metalloporphyrin (TCPP(Pd)) toward CO2 hydrogenation to methanol[J]. ACS Catal,2021,12(1):709−723. [54] HAN Z, TANG C Z, WANG J J, LI L D, LI C. Atomically dispersed Ptn + species as highly active sites in Pt/In2O3 catalysts for methanol synthesis from CO2 hydrogenation[J]. J Catal,2021,394:236−244. doi: 10.1016/j.jcat.2020.06.018 [55] WANG J, SUN K H, JIA X Y, LIU C J. CO2 hydrogenation to methanol over Rh/In2O3 catalyst[J]. Catal Today,2021,365:341−347. doi: 10.1016/j.cattod.2020.05.020 [56] ZHANG Z T, SHEN C Y, SUN K H, JIA X Y, YE J Y, LIU C J. Advances in studies of structural effect of the supported Ni catalyst for CO2 hydrogenation: from nanoparticle to single atom catalyst[J]. J Mater Chem A,2022,10:5792−5812. doi: 10.1039/D1TA09914K [57] RUI N, ZHANG F, SUN K H, LIU Z Y, XU W Q, STAVITSKI E, SENANAYAKE S D, RODRIGUEZ J A, LIU C J. Hydrogenation of CO2 to methanol on a Auδ + -In2O3-x catalyst[J]. ACS Catal,2020,10(19):11307−11317. doi: 10.1021/acscatal.0c02120 [58] LIN D F, ZHANG Z, CHEN Y Y, ZENG L X, CHEN X C, YANG X H, HUANG B Q, LUO Y J, QIAN Q R, CHEN Q H. The Co-In2O3 interaction concerning the effect of amorphous Co metal on CO2 hydrogenation to methanol[J]. J CO2 Util,2022,65:102209. doi: 10.1016/j.jcou.2022.102209 [59] SUN K H, ZHANG Z T, SHEN C Y, RUI N, LIU C J. The feasibility study of the indium oxide supported silver catalyst for selective hydrogenation of CO2 to methanol[J]. Green Energy Environ,2022,7(4):807−817. doi: 10.1016/j.gee.2021.05.004 [60] SHEN C Y, SUN K H, ZHANG Z T, RUI N, JIA X Y, MEI D H, LIU C-J. Highly active Ir/In2O3 catalysts for selective hydrogenation of CO2 to methanol: experimental and theoretical studies[J]. ACS Catal,2021,11(7):4036−4046. doi: 10.1021/acscatal.0c05628 [61] ARAÚJO T P, MORALES‐VIDAL J, ZOU T S, GARCÍA-MUELAS R, WILLI P O, ENGEL K M, SAFONOVA O V, AKL D F, KRUMEICH F, GRASS R N, MONDELLI C, LÓPEZ N, PÉREZ-RAMÍREZ J. Flame spray pyrolysis as a synthesis platform to assess metal promotion in In2O3‐catalyzed CO2 hydrogenation[J]. Adv Energy Mater,2022,12(14):2103707. doi: 10.1002/aenm.202103707 [62] SHI Z S, TAN Q Q, WU D F. A novel core-shell structured CuIn@SiO2 catalyst for CO2 hydrogenation to methanol[J]. AIChE J,2019,65(3):1047−1058. doi: 10.1002/aic.16490 [63] PUSTOVARENKO A, DIKHTIARENKO A, BAVYKINA A, GEVERS L, RAMÍREZ A, RUSSKIKH A, TELALOVIC S, AGUILAR A, HAZEMANN J L, OULD-CHIKH S, GASCON J. Metal-organic framework-derived synthesis of cobalt indium catalysts for the hydrogenation of CO2 to methanol[J]. ACS Catal,2020,10(9):5064−5076. doi: 10.1021/acscatal.0c00449 [64] WANG D, XIE Z H, POROSOFF M D, CHEN J G. Recent advances in carbon dioxide hydrogenation to produce olefins and aromatics[J]. Chem,2021,7(9):2277−2311. doi: 10.1016/j.chempr.2021.02.024 [65] WEI J, YAO R W, HAN Y, GE Q J, SUN J. Towards the development of the emerging process of CO2 heterogenous hydrogenation into high-value unsaturated heavy hydrocarbons[J]. Chem Soc Rev,2021,50(19):10764−10805. doi: 10.1039/D1CS00260K [66] YE R P, DING J, GONG W B, ARGYLE M D, ZHONG Q, WANG Y J, RUSSELL C K, XU Z H, RUSSELL A G, LI Q H, FAN M H, YAO Y G. CO2 hydrogenation to high-value products via heterogeneous catalysis[J]. Nat Commun,2019,10(1):1−15. doi: 10.1038/s41467-018-07882-8 [67] GAO P, ZHANG L N, Li S G, ZHOU Z X, SUN Y H. Novel heterogeneous catalysts for CO2 hydrogenation to liquid fuels[J]. ACS Cent Sci,2020,6(10):1657−1670. doi: 10.1021/acscentsci.0c00976 [68] GARBA M D, USMAN M, KHAN S, SHEHZAD F, GALADIMA A, EHSAN M F, GHANEM A S, HUMAYUN M. CO2 towards fuels: A review of catalytic conversion of carbon dioxide to hydrocarbons[J]. J Environ Chem Eng,2021,9(2):104756. doi: 10.1016/j.jece.2020.104756 [69] YU H L, WANG C Q, LIN T J, AN Y L, WANG Y C, CHANG Q Y, YU F, WEI Y, SUN F F, JIANG Z, LI S G, ZHONG L S. Direct production of olefins from syngas with ultrahigh carbon efficiency[J]. Nat Commun,2022,13(1):5987. [70] LI W H, ZHANG J X, JIANG X, MU M C, ZHANG A F, SONG C S, GUO X W. Co-promoted In2O3/ZrO2 integrated with ultrathin nanosheet HZSM-5 as efficient catalysts for CO2 hydrogenation to gasoline[J]. Ind Eng Chem Res,2022,61:6322−6332. doi: 10.1021/acs.iecr.2c00460 [71] 耿蕊, 董梅, 王浩, 牛宪军, 樊卫斌, 王建国, 秦张峰. 十元环分子筛在甲醇芳构化反应中催化性能的研究[J]. 燃料化学学报,2014,42(9):1119−1127. doi: 10.3969/j.issn.0253-2409.2014.09.013GENG Rui, DONG Mei, WANG Hao, NIU Xian-jun, FAN Wei-bin, WANG Jian-guo, QIN Zhang-feng. An investigation on the catalytic performance of 10 MR zeolites in methanol aromatization reaction[J]. J Fuel Chem Technol,2014,42(9):1119−1127. doi: 10.3969/j.issn.0253-2409.2014.09.013 [72] GAO P, LI S G, BU X N, DANG S S, LIU Z Y, WANG H, ZHONG L S, QIU M H, YANG C G, CAI J, WEI W, SUN Y H. Direct conversion of CO2 into liquid fuels with high selectivity over a bifunctional catalyst[J]. Nat Chem,2017,9(10):1019−1024. doi: 10.1038/nchem.2794 [73] XIN Q, GUO H Y, WANG Y C, XIAO L F, WANG W, WU W. Indium-promoted ZnZrOx/nano-ZSM-5 for efficient conversion of CO2 to aromatics with high selectivity[J]. J Environ Chem Eng,2022,10(3):108032. doi: 10.1016/j.jece.2022.108032 [74] LU S Y, YANG H Y, ZHOU Z X, ZHONG L S, LI S G, GAO P, SUN Y H. Effect of In2O3 particle size on CO2 hydrogenation to lower olefins over bifunctional catalysts[J]. Chin J Catal,2021,42(11):2038−2048. doi: 10.1016/S1872-2067(21)63851-2 [75] GAO P, DANG S S, LI S G, BU X N, LIU Z Y, QIU M H, YANG C G, WANG H, ZHONG L S, HAN Y, LIU Q, WEI W, SUN Y H. Direct production of lower olefins from CO2 conversion via bifunctional catalysis[J]. ACS Catal,2018,8(1):571−578. doi: 10.1021/acscatal.7b02649 [76] TAN L, ZHANG P P, CUI Y, SUZUKI Y, LI H J, GUO L S, YANG G H, TSUBAKI N. Direct CO2 hydrogenation to light olefins by suppressing CO by-product formation[J]. Fuel Process Technol,2019,196:106174. doi: 10.1016/j.fuproc.2019.106174 [77] DANG S S, LI S G, YANG C G, CHEN X Q, LI X P, ZHONG L S, GAO P, SUN Y H. Selective transformation of CO2 and H2 into lower olefins over In2O3-ZnZrOx/SAPO-34 bifunctional catalysts[J]. ChemSusChem,2019,12(15):3582−3591. doi: 10.1002/cssc.201900958 [78] LIU Z C, XU S M, HAO J, SONG L N, CHONG M B, CHENG D-G, CHEN F Q. Bifunctional catalysts composed of low silicon‐content SAPO‐34 nanosheets and In2O3/ZrO2 with improved performance for CO2 hydrogenation[J]. Greenhouse Gas Sci Technol,2022,12(2):305−320. doi: 10.1002/ghg.2147 [79] WANG J Y, ZHANG A F, JIANG X, SONG C S, GUO X W. Highly selective conversion of CO2 to lower hydrocarbons (C2–C4) over bifunctional catalysts composed of In2O3-ZrO2 and zeolite[J]. J CO2 Util,2018,27:81−88. doi: 10.1016/j.jcou.2018.07.006 [80] WANG Y H, WANG G Y, WAL L I V D, CHENG K, ZHANG Q H, JONG K P D, WANG Y. Visualizing element migration over bifunctional metal-zeolite catalysts and its impact on catalysis[J]. Angew Chem Int Ed,2021,60(32):17735−17743. doi: 10.1002/anie.202107264 -




 下载:
下载: