Study of grain size effect of lanthanum oxide catalyzed methane oxidation coupling reaction
-
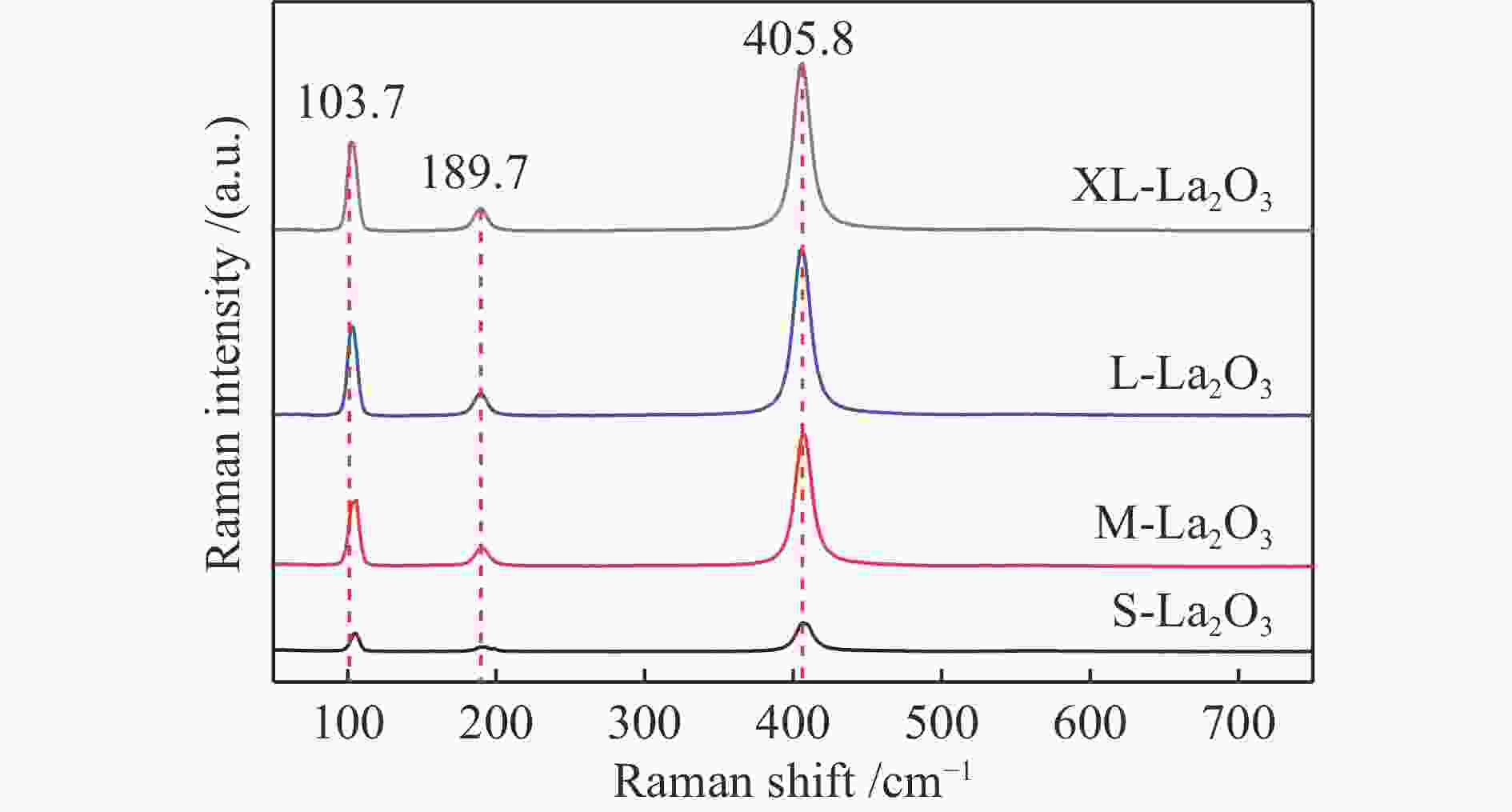
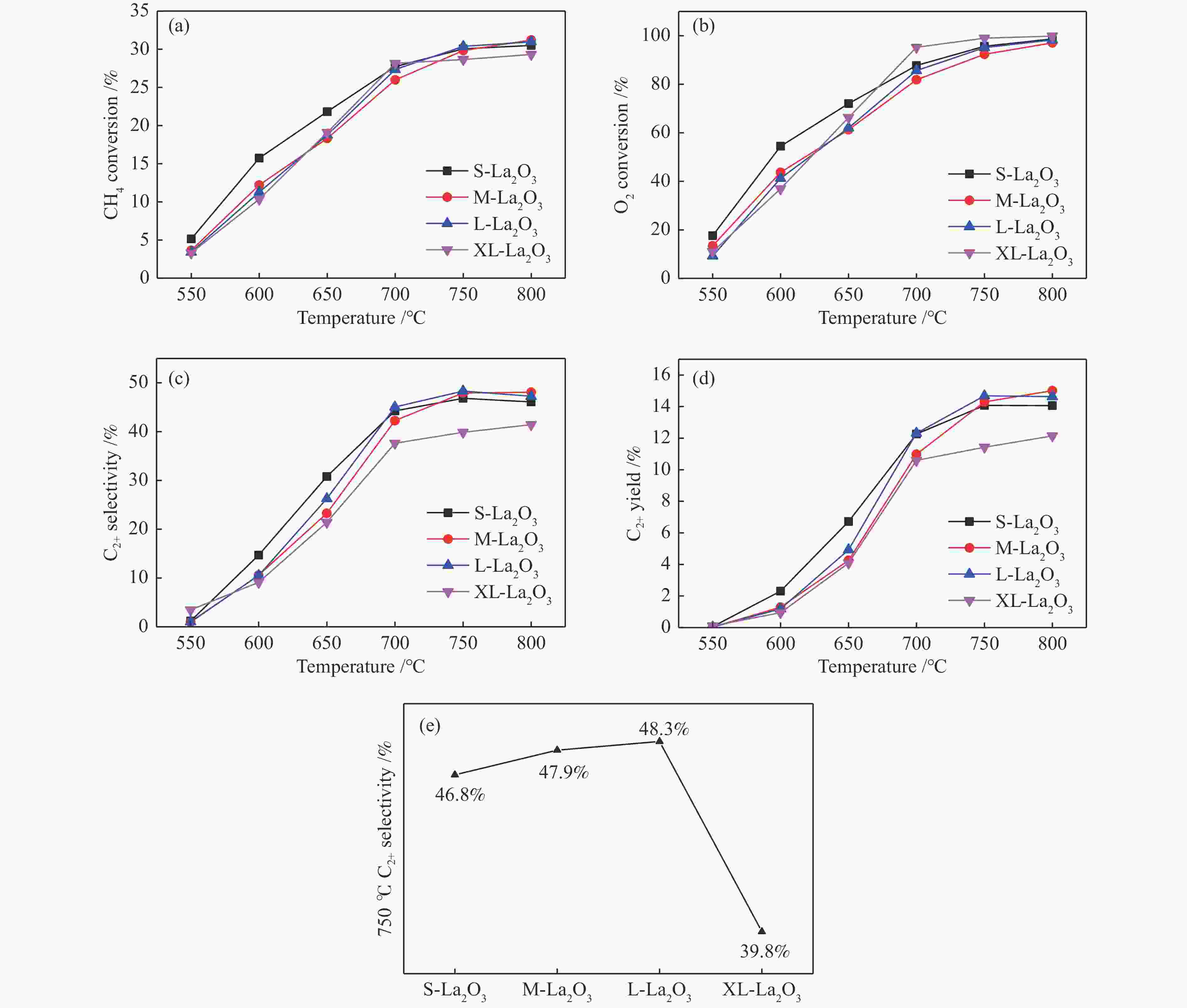
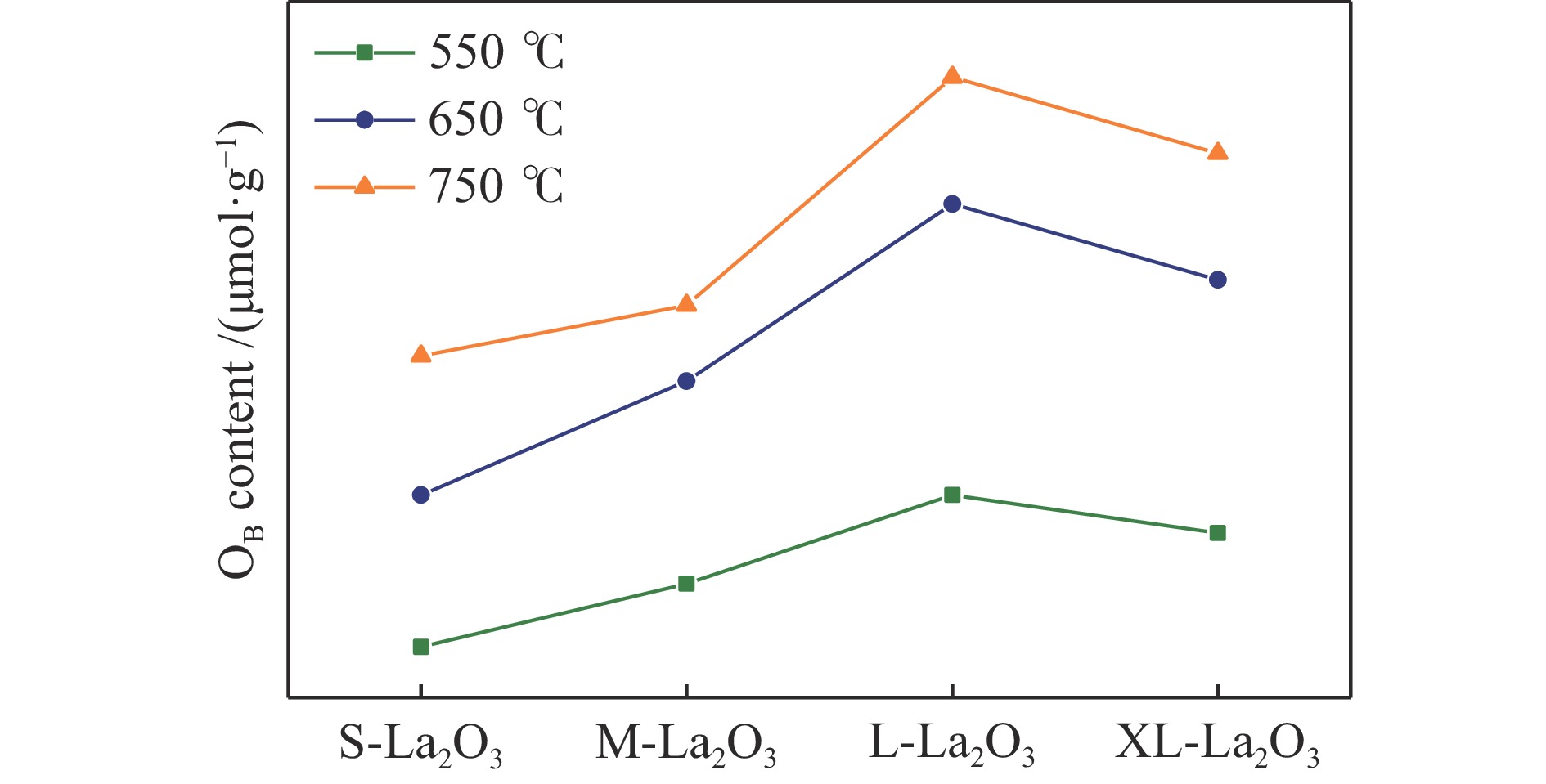
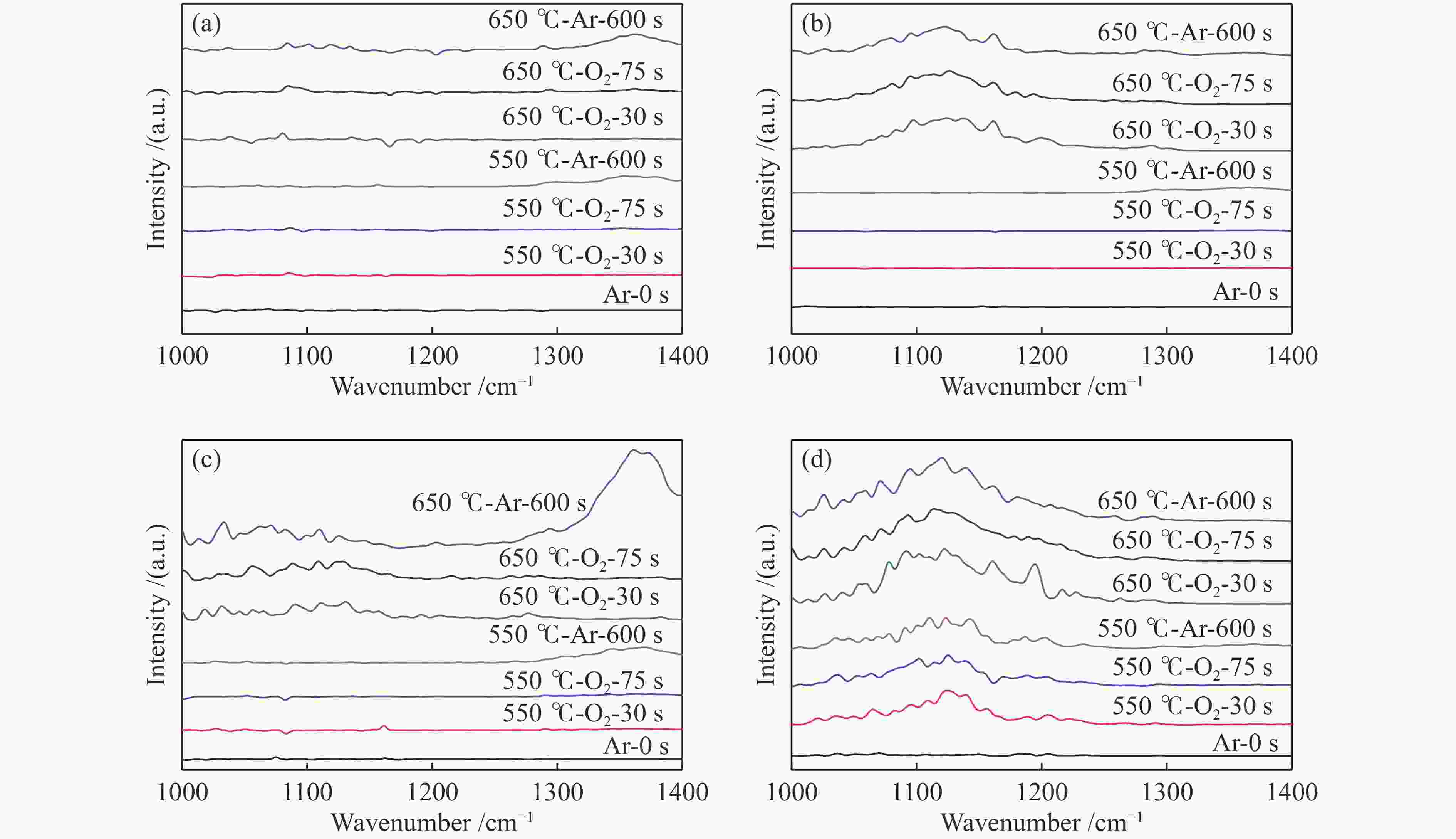
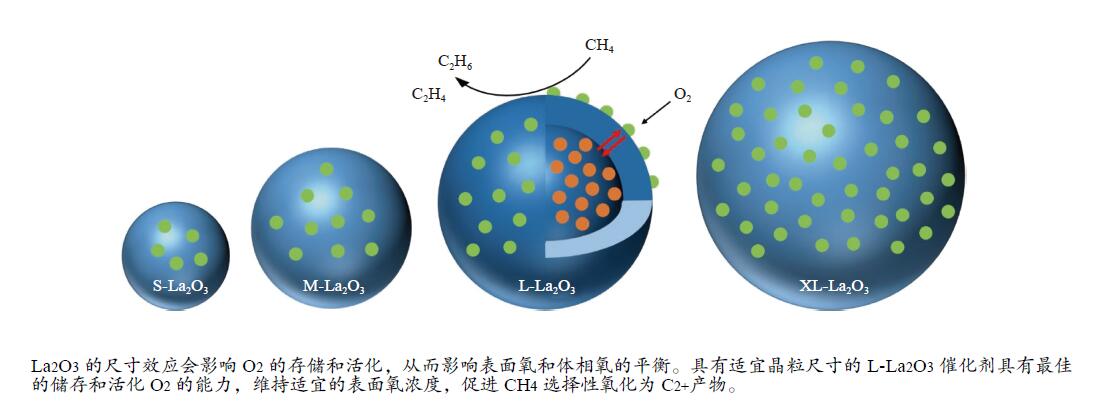
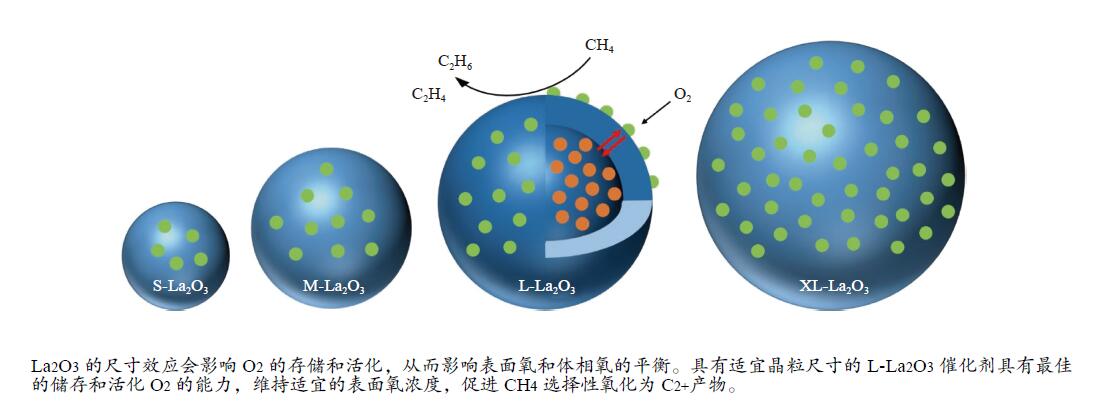
摘要: 采用水热合成法,制备了不同晶粒尺寸的La2O3催化剂。运用XRD、原位拉曼光谱、原位红外漫反射光谱、H2-TPR和O2-TPD等表征手段研究了不同晶粒尺寸La2O3催化剂的OCM反应性能和催化剂之间的构效关系。结果表明,La2O3催化剂的La−O键会随着温度的升高出现明显的伸长,从而影响La2O3对O2的吸附和动态储存。当晶粒尺寸增大至57.4 nm时,La2O3催化剂的储氧能力开始下降,同时伴随着表面氧物种,特别是超氧物种在催化剂表面富集,导致CH4和产物的过度氧化,降低OCM反应的选择性。晶粒尺寸为52.3 nm的L-La2O3催化剂在750 ℃时,表面氧物种含量适宜,储氧能力强。在CH4/O2为3,空速为1.6 × 105 mL/(g·h)的条件下表现出最佳的C2 + 烃选择性。Abstract: La2O3 catalysts with different grain sizes were prepared under hydrothermal condition. The structure activity relationship of La2O3 catalysts with different grain sizes were investigated by using in-situ XRD, Raman, FT-IR and H2-TPR, O2-TPD. The results showed that the La−O bond of the La2O3 catalyst showed a significant elongation with increasing temperature, which affected its adsorption and dynamic storage of O2. When increasing the grain size up to 57.4 nm, the oxygen storage capacity of the La2O3 catalyst started to decrease, accompanied by the enrichment of surface oxygen species, especially superoxide species, on the catalyst surface, which led to the over-oxidation of CH4 and products and reduced the C2 + hydrocarbons selectivity. The L-La2O3 catalyst with a grain size of 52.3 nm had a suitable content of surface oxygen species and a high oxygen storage capacity at 750 ℃. It exhibited the best C2 + hydrocarbon selectivity up to a CH4/O2 of 3 and a vacancy rate of 1.6 × 105 mL/(g·h).
-
Key words:
- methane oxidation coupling /
- La2O3 /
- grain size /
- oxygen storage capacity
-
表 1 催化剂的织构性质
Table 1 Texture properties of catalyst
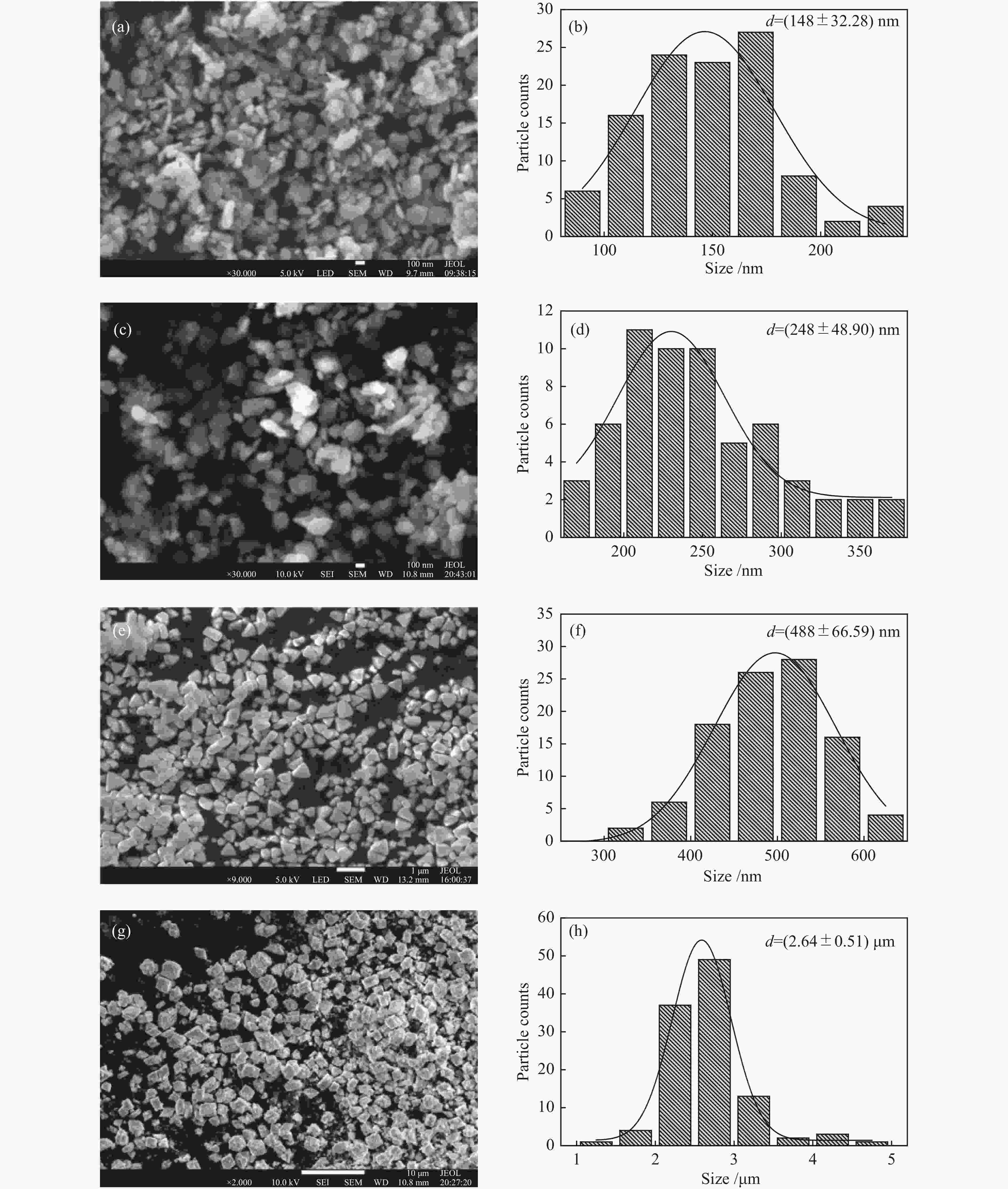
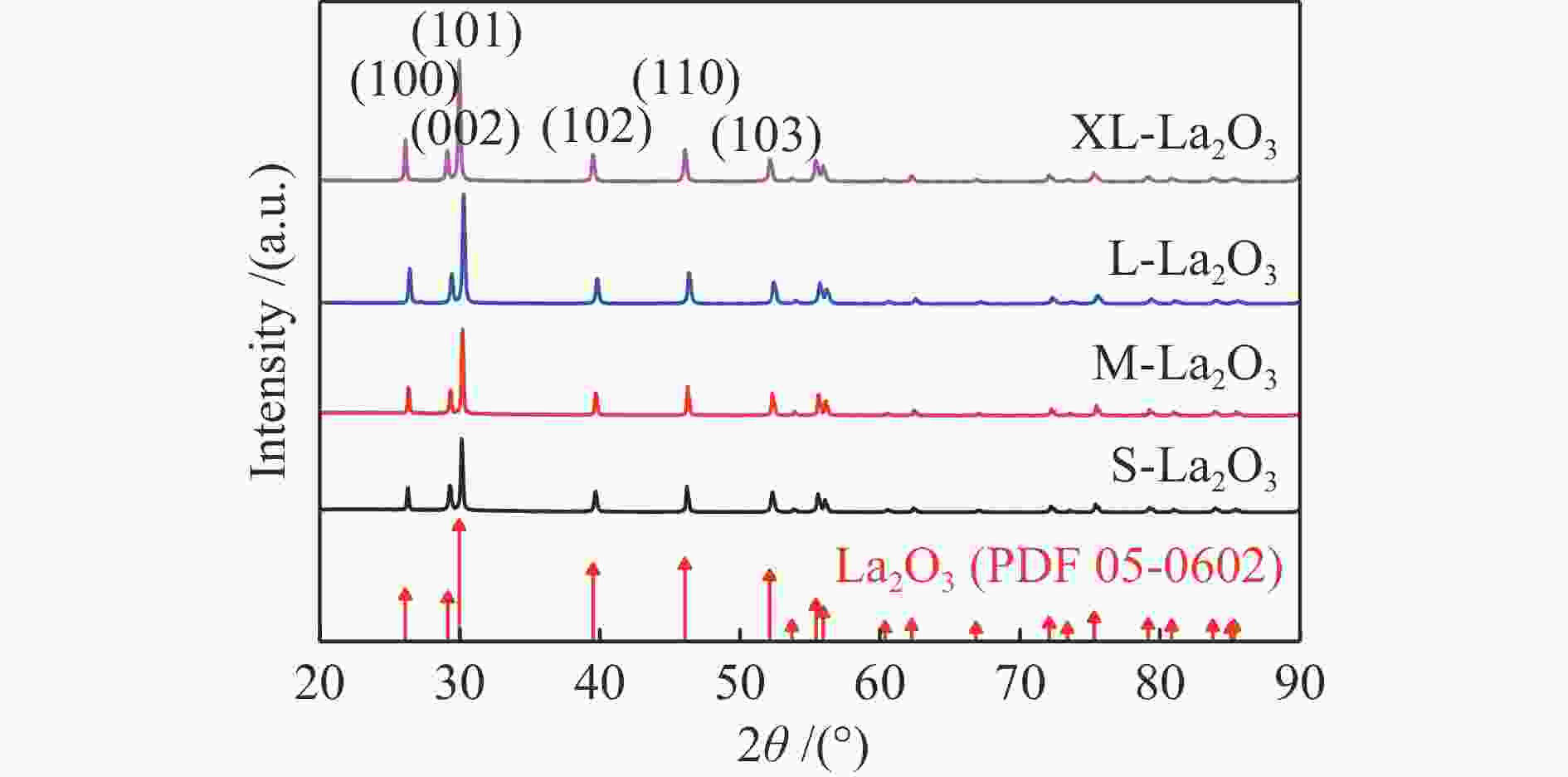
Catalyst ABET/(m2·g−1)a vp/(cm3·g−1)b dp/nmc Crystallite size/nmd S-La2O3 9 0.07 29 42.5 M-La2O3 5 0.03 38 47.3 L-La2O3 3 0.02 41 52.3 XL-La2O3 4 0.02 40 57.4 a: BET surface area. b: BJH desorption cumulative volum of pores. c: BJH desorption average pore diameter (4V/A).
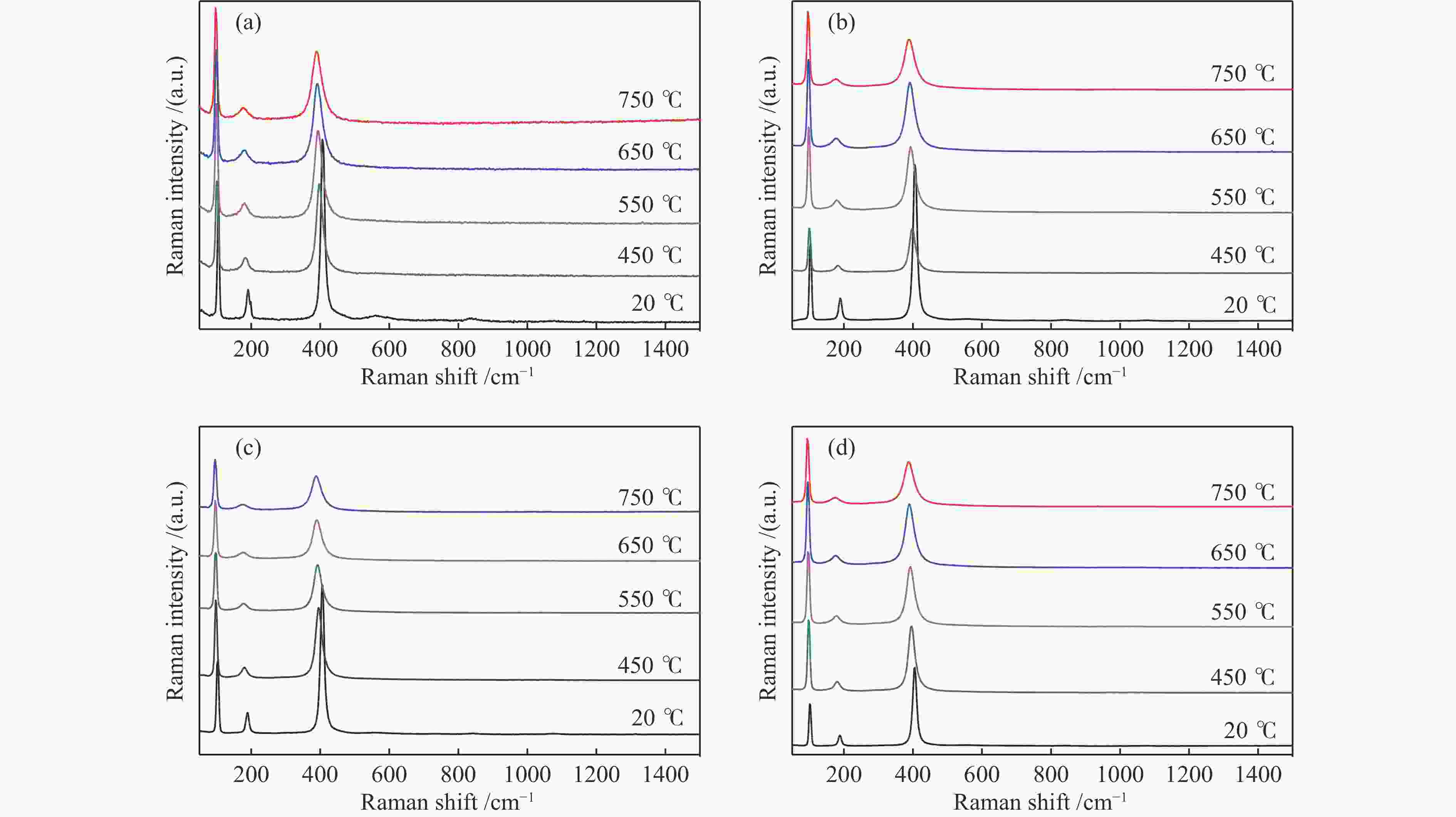
d: crystallite size was calculated by the Scherer formula based on the strongest diffraction (2θ=30°)表 2 不同温度下不同晶粒尺寸La2O3的拉曼峰偏移率
Table 2 Raman peak shift rates of La2O3 with different grain sizes at different temperatures
Catalyst Raman shift/cm−1 Drift rate/%a 20 ℃ 400 ℃ 550 ℃ 650 ℃ 750 ℃ 400 ℃ 550 ℃ 650 ℃ 750 ℃ S-La2O3 407 397 392 391 388 2.5 3.7 3.9 4.7 M-La2O3 407 396 392 391 388 2.7 3.7 3.9 4.7 L-La2O3 407 398 394 392 389 2.2 3.1 3.7 4.4 XL-La2O3 406 396 393 390 387 2.5 3.2 3.9 4.7 a: 400, 550, 650, 750 ℃ drift rate(%) = (20 ℃ Raman shift – 400, 550, 650, 750 ℃ Raman shift)100/20 ℃ Raman shift 表 3 不同晶粒尺寸La2O3在不同O2气预处理温度下吸附体相中近表层氧物种相对含量
Table 3 Relative content of oxygen species near surface oxygen species in bulk phase of La2O3 with different grain sizes at different O2 gas pretreatment temperatures
Catalyst Relative content of oxygen species in
adsorbate phase/(μmol·g−1)550 ℃ 650 ℃ 750 ℃ S-La2O3 24 36 47 M-La2O3 29 45 51 L-La2O3 36 59 69 XL-La2O3 33 53 63 -
[1] ALVAREZ-GALVAN M C, MOTA N, OJEDA M, ROJAS S, NAVARRO R. M. FIERRO J. L. G. Direct methane conversion routes to chemicals and fuels[J]. Catal Today,2011,171(1):15−23. doi: 10.1016/j.cattod.2011.02.028 [2] LUNSFORD J H. Catalytic conversion of methane to more useful chemicals and fuels: A challenge for the 21st century[J]. Catal Today,2000,63(2/4):165−174. doi: 10.1016/S0920-5861(00)00456-9 [3] HORN R, SCHLOGL R. Methane activation by heterogeneous catalysis[J]. Catal Lett,2015,145(1):23−39. doi: 10.1007/s10562-014-1417-z [4] KELLER G E, BHASIN M M. Synthesis of ethylene via oxidative coupling of methane: I. Determination of active catalysts[J]. Chem Inform,1982,73(1):9−19. [5] QIAN K, YOU R, GUAN Y, WEN W, TIAN Y C, PAN Y, HUANG W X. Single-site catalysis of Li-MgO catalysts for oxidative coupling of methane reaction[J]. ACS Catal,2020,10(24):15142−15148. doi: 10.1021/acscatal.0c03896 [6] YAMASHITA H, MACHIDA Y, TOMITA A. Oxidative coupling of methane with peroxide ions over barium lanthanum oxygen mixed-oxide[J]. Appl Catal A: Gen,1991,79(2):203−214. doi: 10.1016/0926-860X(91)80006-K [7] ITO T, LUNSFORD J H. Synthesis of ethylene and ethane by partial oxidation of methane over lithium-doped magnesium oxide[J]. Nature,1985,314(6013):721−722. [8] SOURAV S, WANG Y X, KIANI D, BALTRUSAITIES J, FUSHIMI R R, WACHS I E. Resolving the types and origin of active oxygen species present in supported Mn-Na2WO4/SiO2 catalysts for oxidative coupling of methane[J]. ACS Catal,2021,11(16):10288−10293. doi: 10.1021/acscatal.1c02315 [9] SOURAV S, WANG Y X, KIANI D, BALTRUSAITIES J, FUSHIMI R R, WACHS I E. New mechanistic and reaction pathway insights for oxidative coupling of methane (OCM) over supported Na2WO4/SiO2 Catalysts[J]. Angew Chem Int Ed,2021,60(39):21502−21511. doi: 10.1002/anie.202108201 [10] KIANI D, SOURAV S, BALTRUSAITIS J, WACHS I E. Elucidating the effects of Mn promotion on SiO2-supported Na-promoted tungsten oxide catalysts for oxidative coupling of methane (OCM)[J]. ACS Catal,2021,11(16):10131−10137. doi: 10.1021/acscatal.1c01392 [11] XU J, ZHANG Y, XU X, FANG , XI R, LIU Y, ZHENG R, WANG X. Constructing La2B2O7 (B = Ti, Zr, Ce) compounds with three typical crystalline phases for the oxidative coupling of methane: The effect of phase structures, superoxide anions, and alkalinity on the reactivity[J]. ACS Catal,2019,9(5):4030−4045. doi: 10.1021/acscatal.9b00022 [12] WANG Z, ZHOU G, JIANG D, WANG S. Recent development of A2B2O7 system transparent ceramics[J]. J Adv Ceram,2018,7(4):289−306. doi: 10.1007/s40145-018-0287-z [13] PETIT C, REHSPRINGER J L, KADDOURI A, LIBS S, POIX P, KIENNEMANN A. Oxidative coupling of methane by pyrochlore oxide A2B2O7 (A = rare earth, B = Ti, Zr, Sn). Relation between C2 selectivity and B-O bond energy[J]. Catal Today,1992,13(2/3):409−416. doi: 10.1016/0920-5861(92)80166-K [14] ITO T, WANG J, LIN C H, LUNSFORD J H. Oxidative dimerization of methane over a lithium-promoted magnesium oxide catalyst[J]. ChemInform,1985,16(51):255−265. [15] ARNDT S, SIMON U, HEITZ S, BERTHOLD A, BECK B, GORKE O, EPPING J D, OTREMBA T, AKSU Y, IRRAN E, LAUGEL G, DRIESS M, SCHUBERT H, SCHOMACKER R. Li-doped MgO from different preparative routes for the oxidative coupling of methane[J]. Top Catal,2011,54(16):1266. [16] 方学平, 李树本, 林景治, 褚衍来. 甲烷在W-Mn体系催化剂上氧化偶联制乙烯[J]. 分子催化,1992,(6):427−433. doi: 10.16084/j.cnki.issn1001-3555.1992.06.004FANG Xue-ping, LI Shu-ben, LIN Jing-zhi, CHU Yan-lai. Oxidative coupling of methane to ethylene over W-Mn system catalyst[J]. J Mol Catal,1992,(6):427−433. doi: 10.16084/j.cnki.issn1001-3555.1992.06.004 [17] ORTIZ-BRAVO CARLOS A, FIGUEROA SANTIAGO J A, PORTELA RAQUEL, BANARES MIGUEL A, TONIOLO FABIO SOUZA. Elucidating the structure of the W and Mn sites on the Mn-Na2WO4/SiO2 catalyst for the oxidative coupling of methane (OCM) at real reaction temperatures[J]. J Catal,2021,408:423−435. [18] WANG P, ZHAO G, WANG Y, LU Y. MnTiO3-driven low-temperature oxidative coupling of methane over TiO2-doped Mn2O3-Na2WO4/SiO2 catalyst[J]. Sci Adv,2017,3(6):e1603180. doi: 10.1126/sciadv.1603180 [19] TEYMOURI M, BAGHERZADEH E, PETIT C, REHSPRINGER J L, LIBS S, KIENNEMANN A. Reactivity of perovskites on oxidative coupling of methane[J]. J Mater Sci,1995,30(11):3005−3009. doi: 10.1007/BF00349675 [20] ZHANG Y, XU J, XU X, XI R, LIU Y, FANG X, WANG X. Tailoring La2Ce2O7 catalysts for low temperature oxidative coupling of methane by optimizing the preparation methods[J]. Catal Today,2020,355:518−528. doi: 10.1016/j.cattod.2019.06.060 [21] FENG R, NIU P, WANG Q, HOU B, JIA L, LIN M, LI D. In-depth understanding of the crystal-facet effect of La2O2CO3 for low-temperature oxidative coupling of methane[J]. Fuel,2022,308:121848. doi: 10.1016/j.fuel.2021.121848 [22] DANIEL N, BAHMAN Z, SELIM S. Oxidative coupling of methane with La2O3-CeO2 nanofiber fabrics: A reaction engineering study[J]. J Nat Gas Sci Eng,2014,18:406−411. doi: 10.1016/j.jngse.2014.04.004 [23] SUNG J S, CHOO K Y, KIM T H, GREISH A, GLUKHOV L, FINASHINA E, KUSTOV L. Peculiarities of oxidative coupling of methane in redox cyclic mode over Ag-La2O3/SiO2 catalysts[J]. Appl Catal A: Gen,2010,380(1):28−32. [24] WANG S, LI S, DIXON D A. Mechanism of selective and complete oxidation in La2O3-catalyzed oxidative coupling of methane[J]. Catal Sci Technol,2020,10(8):2602−2614. [25] PALMER M S, NEUROCK M, OLKEN M M. Periodic density functional theory study of methane activation over La2O3: Activity of O2−, O−, O22−, oxygen point defect, and Sr2 + -doped surface sites[J]. J Am Chem Soc,2002,124(28):8452−8461. doi: 10.1021/ja0121235 [26] GUO Y, LIANG J, LIU Y, LIU Y, XU X, FANG X, ZHONG W, WANG X. Identifying surface active sites of SnO2: Roles of surface O2–, O22– anions and acidic species played for toluene deep oxidation[J]. Ind Eng Chem Res,2019,58(40):18569−18581. [27] SONG J, SUN Y, BA R, HUANG S, ZHAO Y, ZHANG J, SUN Y, ZHU Y. Monodisperse Sr-La2O3 hybrid nanofibers for oxidative coupling of methane to synthesize C2 hydrocarbons[J]. Nanoscale,2015,7(6):2260−2264. doi: 10.1039/C4NR06660J [28] HUANG P, ZHAO Y, ZHANG J, ZHU Y, SUN Y. Exploiting shape effects of La2O3 nanocatalysts for oxidative coupling of methane reaction[J]. Nanoscale,2013,5(22):10844−10848. doi: 10.1039/c3nr03617k [29] LUNSFORD J H. The catalytic oxidative coupling of methane[J]. Angew Chem Int Ed,1995,34(9):970−980. doi: 10.1002/anie.199509701 [30] LACOMBE S, GEANTET C, MIRODATOS C. Oxidative coupling of methane over lanthana catalysts[J]. J Catal,1995,151(2):439−452. doi: 10.1006/jcat.1995.1046 [31] LIN C H, CAMPBELL K D, WANG J X, LUNSFORD J H. Oxidative dimerization of methane over lanthanum oxide[J]. J Phys Chem,1986,90(4):534−537. doi: 10.1021/j100276a004 [32] AU C T, HE H, LAI S Y, NG C F. The oxidative coupling of methane over BaCO3/LaOBr—Catalysts of high ethylene yield[J]. J Catal,1996,163(2):399−408. doi: 10.1006/jcat.1996.0341 [33] 黎营涛, 牛鹏宇, 王强, 贾丽涛, 林明桂, 李德宝. Zn-Al共掺杂La2O3催化剂在甲烷氧化偶联中的性能研究[J]. 燃料化学学报,2021,49(10):1−10.LI Ying-tao, NIU Peng-yu, WANG Qiang, JIA Li-tao, LIN Ming-gui, LI De-bao. Study on the performance of Zn-Al co-doped La2O3 catalysts in oxidative coupling of methane[J]. J Fuel Chem Technol,2021,49(10):1−10. [34] LACOMBE S, ZANTHOFF H, MIRODATOS C. Oxidative coupling of methane over lanthana catalysts: II. A mechanistic study using isotope transient kinetics[J]. J Catal,1995,155(1):106−116. doi: 10.1006/jcat.1995.1192 [35] MIHAI O, CHEN D, HOLMEN A. Chemical looping methane partial oxidation: The effect of the crystal size and O content of LaFeO3[J]. J Catal,2012,293:175−185. doi: 10.1016/j.jcat.2012.06.022 [36] ORERA A, LARRAZ G, SANJUAN M L. Spectroscopic study of the competition between dehydration and carbonation effects in La2O3-based materials[J]. J Eur Ceram Soc,2013,33(11):2103−2110. doi: 10.1016/j.jeurceramsoc.2013.03.010 [37] ZHANG H, YANG Q, ZHANG B, LU S. Raman spectroscopic investigation of lanthana-doped neodymium-yttria transparent ceramics[J]. J Raman Spect,2011,42(6):1384−1387. doi: 10.1002/jrs.2840 [38] WEI Y J, YAN L Y, WANG C Z, XU X G, WU F, CHEN G. Effects of Ni doping on [MnO6] octahedron in LiMn2O4[J]. J Phys Chem B,2004,108(48):18547−18551. doi: 10.1021/jp0479522 [39] RONG S, ZHANG P, LIU F, YANG Y. Engineering crystal facet of α-MnO2 nanowire for highly efficient catalytic oxidation of carcinogenic airborne formaldehyde[J]. ACS Catal,2018,8(4):3435−3446. doi: 10.1021/acscatal.8b00456 [40] 冯茹. 镧基甲烷氧化偶联催化剂的合成及反应机理的研究[D]. 太原: 中国科学院山西煤炭化学研究所, 2021.FENG Ru. Studies on synthesis of lanthanum based catalysts and its reaction mechanism for oxidative coupling of methane[D]. Taiyuan: Shanxi Institute of Coal Chemistry, Chinese Academy of Sciences, 2021. [41] GAMBO Y, JALIL A A, TRIWAHYONO S, ABDULRASHEED A A. Recent advances and future prospect in catalysts for oxidative coupling of methane to ethylene: A review[J]. J Ind Eng Chem,2018,59:218−229. doi: 10.1016/j.jiec.2017.10.027 [42] BORCHERT H, BAERNS M. The effect of oxygen-anion conductivity of metal-oxide doped lanthanum oxide catalysts on hydrocarbon selectivity in the oxidative coupling of methane[J]. J Catal,1997,168(2):315−320. doi: 10.1006/jcat.1997.1662 [43] FENG R, NIU P, HOU B, WANG Q, JIA L, LIN M, LI D. Synthesis and characterization of the flower-like LaxCe1−xO1.5 + δ catalyst for low-temperature oxidative coupling of methane[J]. J Energy Chem,2022,67:342−353. doi: 10.1016/j.jechem.2021.10.018 [44] HOU Y, HAN W, XIA W, WANG H. Structure sensitivity of La2O2CO3 catalysts in the oxidative coupling of methane[J]. ACS Catal,2015,5(3):1663−1674. doi: 10.1021/cs501733r [45] SPINICCI R, TOFANARI A. Characterization of catalysts for methane-coupling by means of temperature programmed desorption[J]. Catal Today,1990,6(4):473−479. doi: 10.1016/0920-5861(90)85041-L [46] WU J, DACQUIN J P, DJELAL N, CORDIER C, DUJARDIN C, GRANGER P. Calcium and copper substitution in stoichiometric and La-deficient LaFeO3 compositions: A starting point in next generation of Three-Way-Catalysts for gasoline engines[J]. Appl Catal B: Environ,2020,119621. [47] SUTTHIUMPORN K, KAWI S. Promotional effect of alkaline earth over Ni-La2O3 catalyst for CO2 reforming of CH4: Role of surface oxygen species on H2 production and carbon suppression[J]. Int J Hydrogen Energy,2011,36(22):14435−14446. doi: 10.1016/j.ijhydene.2011.08.022 [48] DING W, CHEN Y, FU X. Oxidative coupling of methane over Ce4 + -doped Ba3WO6 catalysts: investigation on oxygen species responsible for catalytic performance[J]. Catal Lett,1994,23(1-2):69−78. doi: 10.1007/BF00812132 [49] WANG L, YI X, WENG W, WAN H. In situ IR and pulse reaction studies on the active oxygen species over SrF2/Nd2O3 catalyst for oxidative coupling of methane[J]. Catal Today,2008,131(1):135−139. [50] WENG W, CHEN M, WAN H, LIAO Y. High-temperature in situ FTIR spectroscopy study of LaOF and BaF2/LaOF catalysts for methane oxidative coupling[J]. Catal Lett,1998,53(1):43−50. [51] WAN H, CHAO Z, WENG W, ZHOU X, CAI J, TSAI K. Constituent selection and performance characterization of catalysts for oxidative coupling of methane and oxidative dehydrogenation of ethane[J]. Catal Today,1996,30(1):67−76. [52] ZHANG Z, VERYKIOS X E, BAERNS M. Effect of electronic properties of catalysts for the oxidative coupling of methane on their selectivity and activity[J]. Catal Rev,1994,36(3):507−556. doi: 10.1080/01614949408009470 -




 下载:
下载: