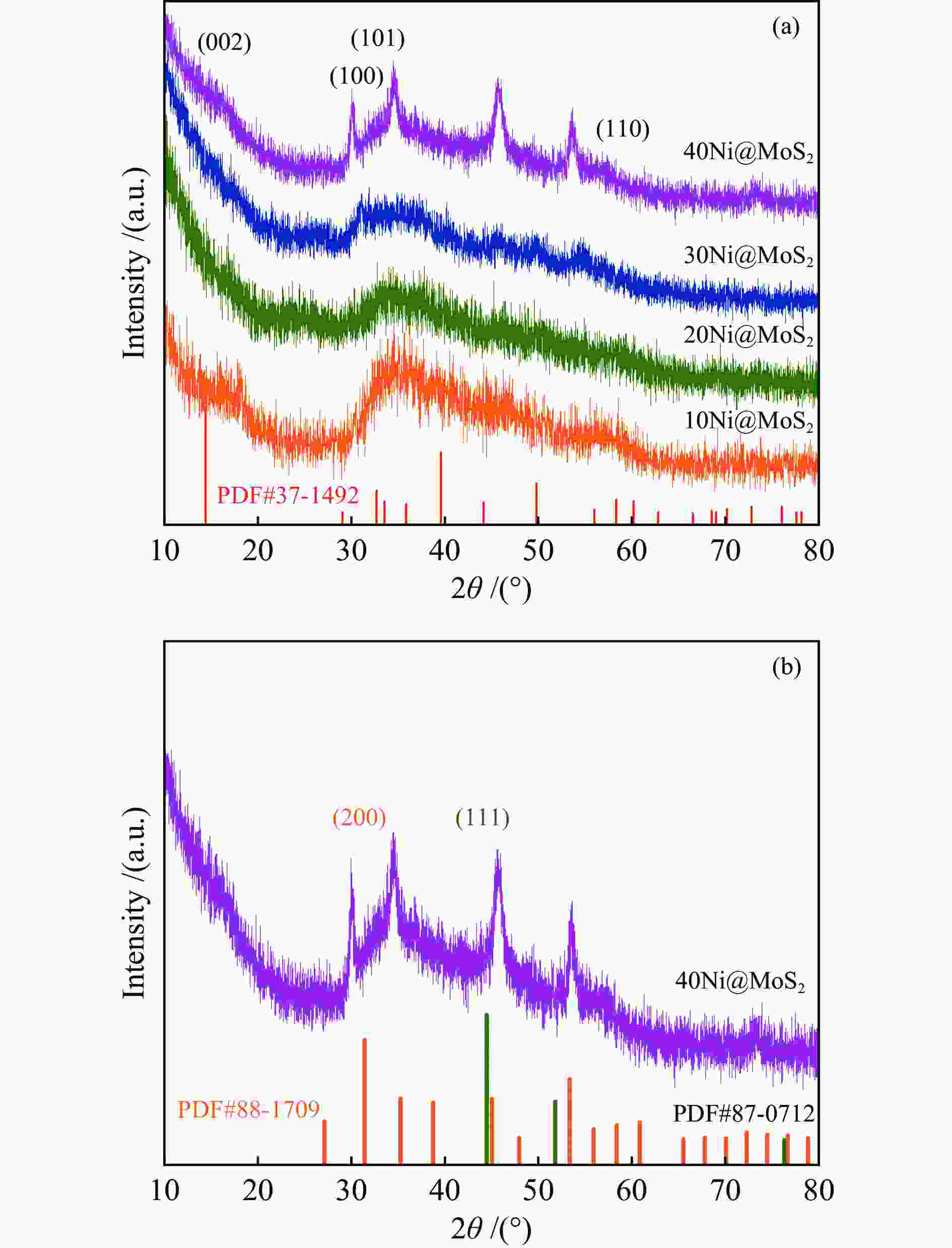
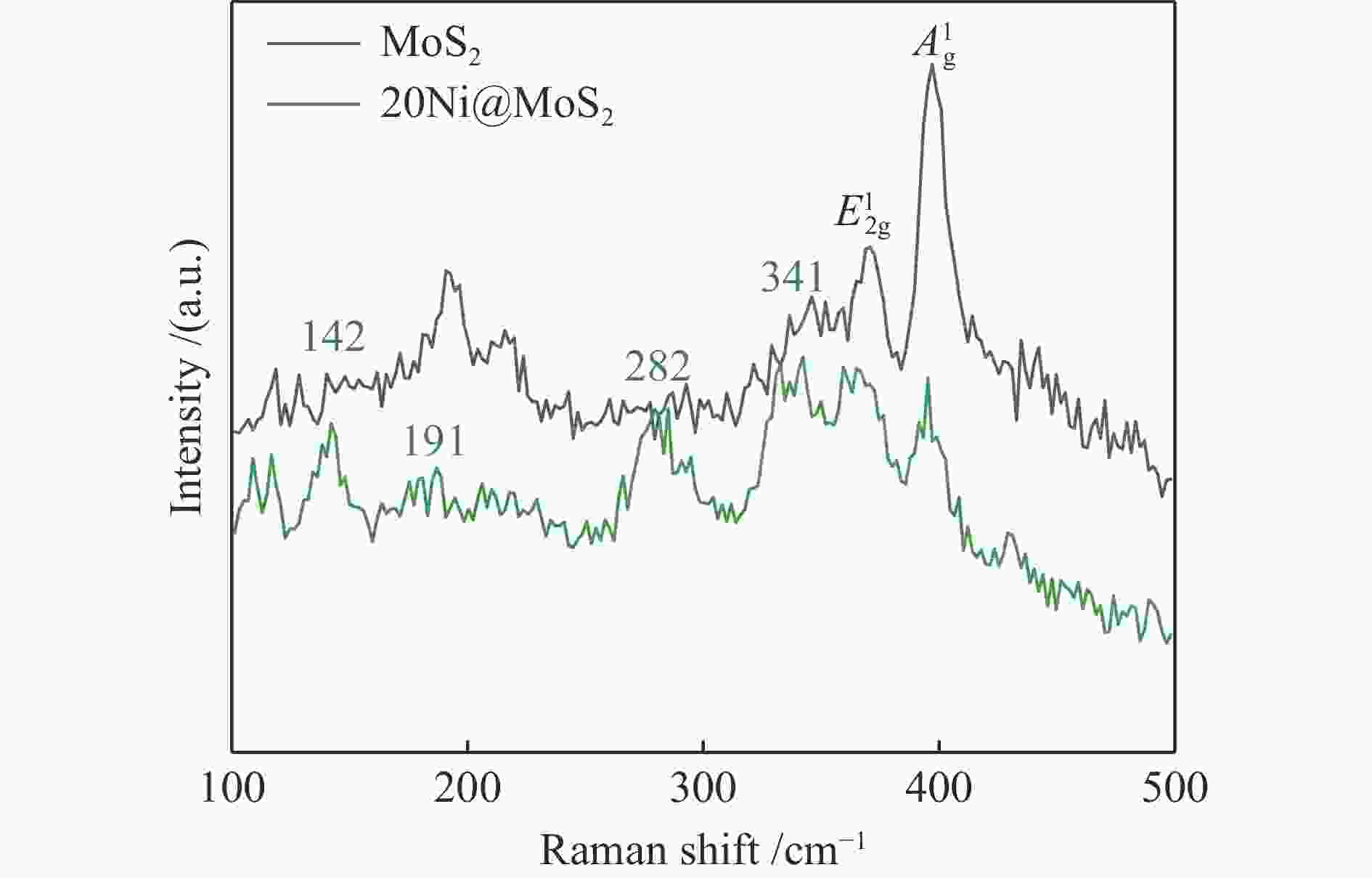
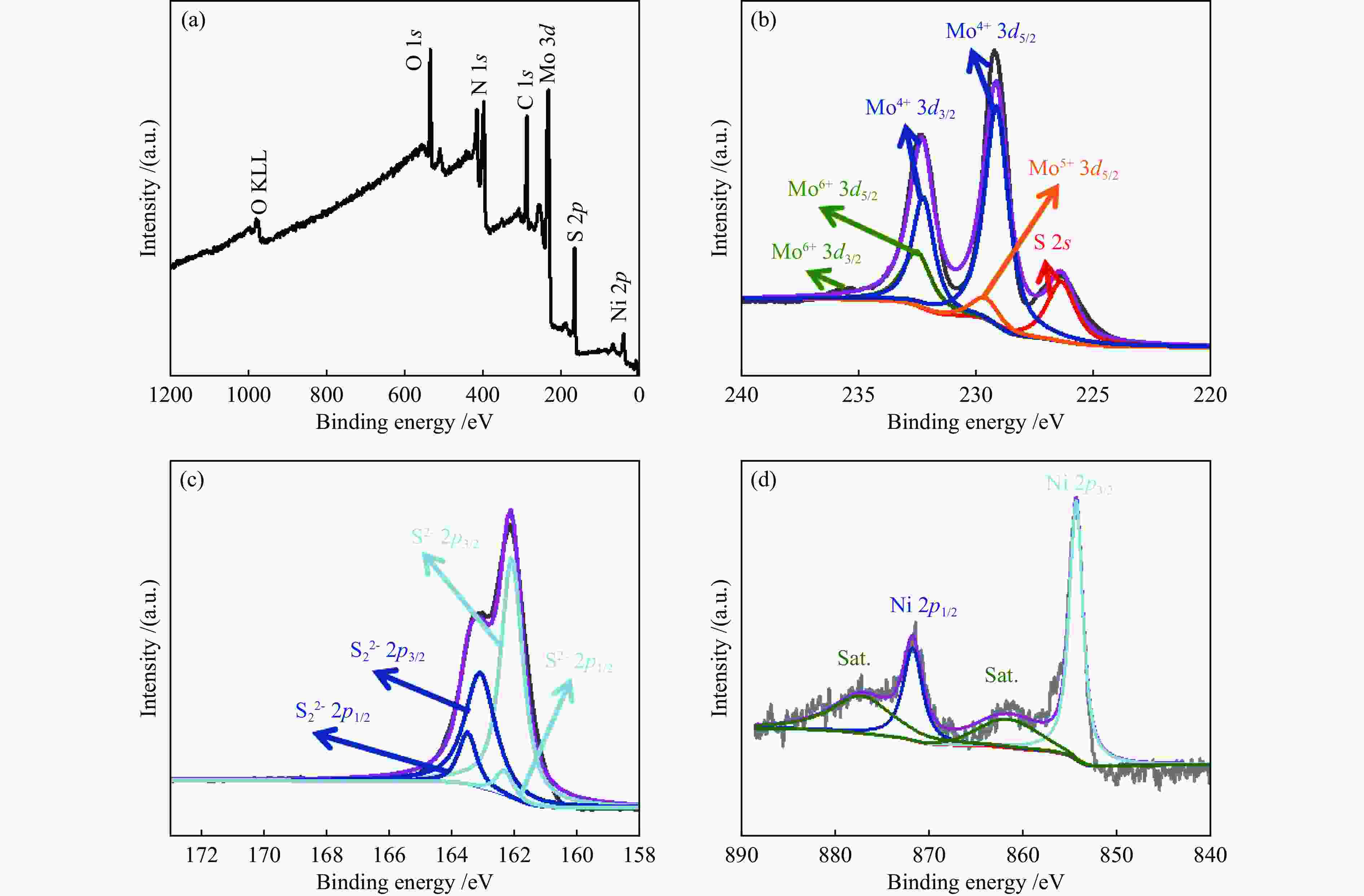
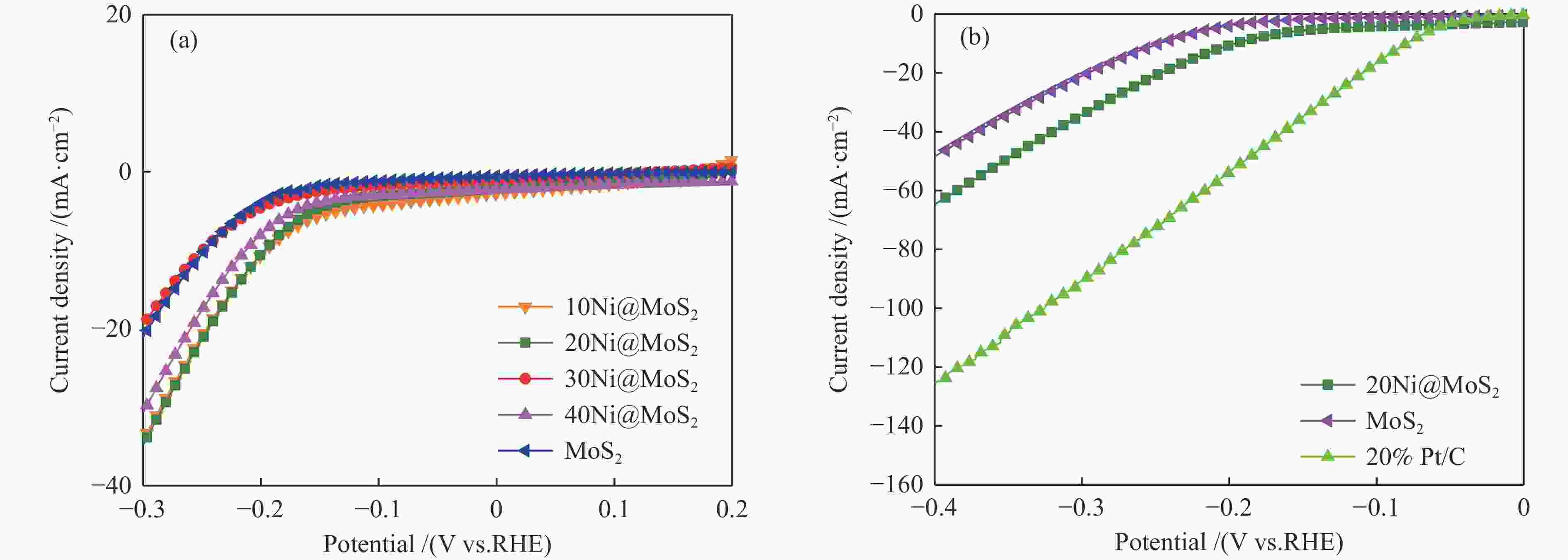
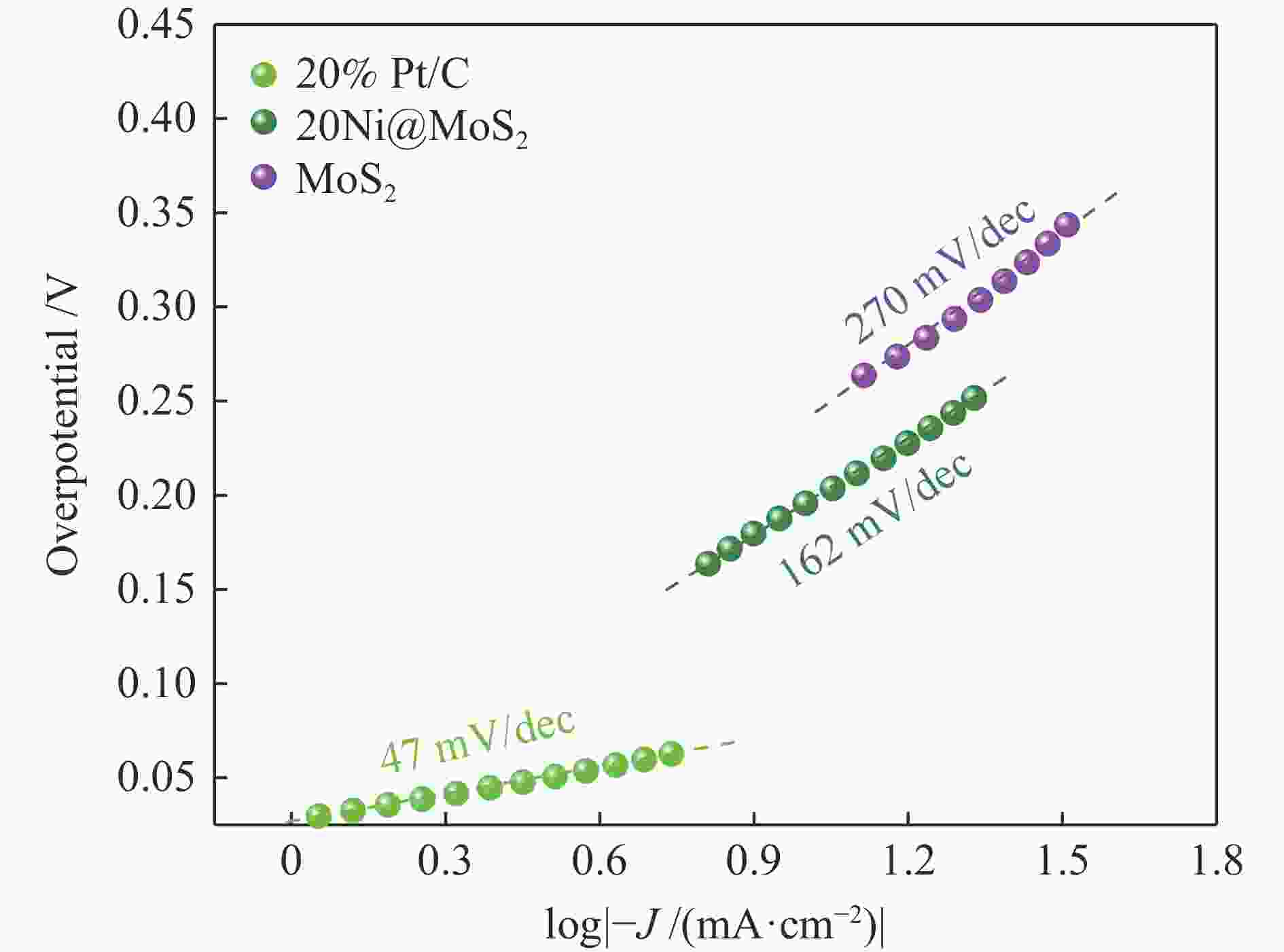
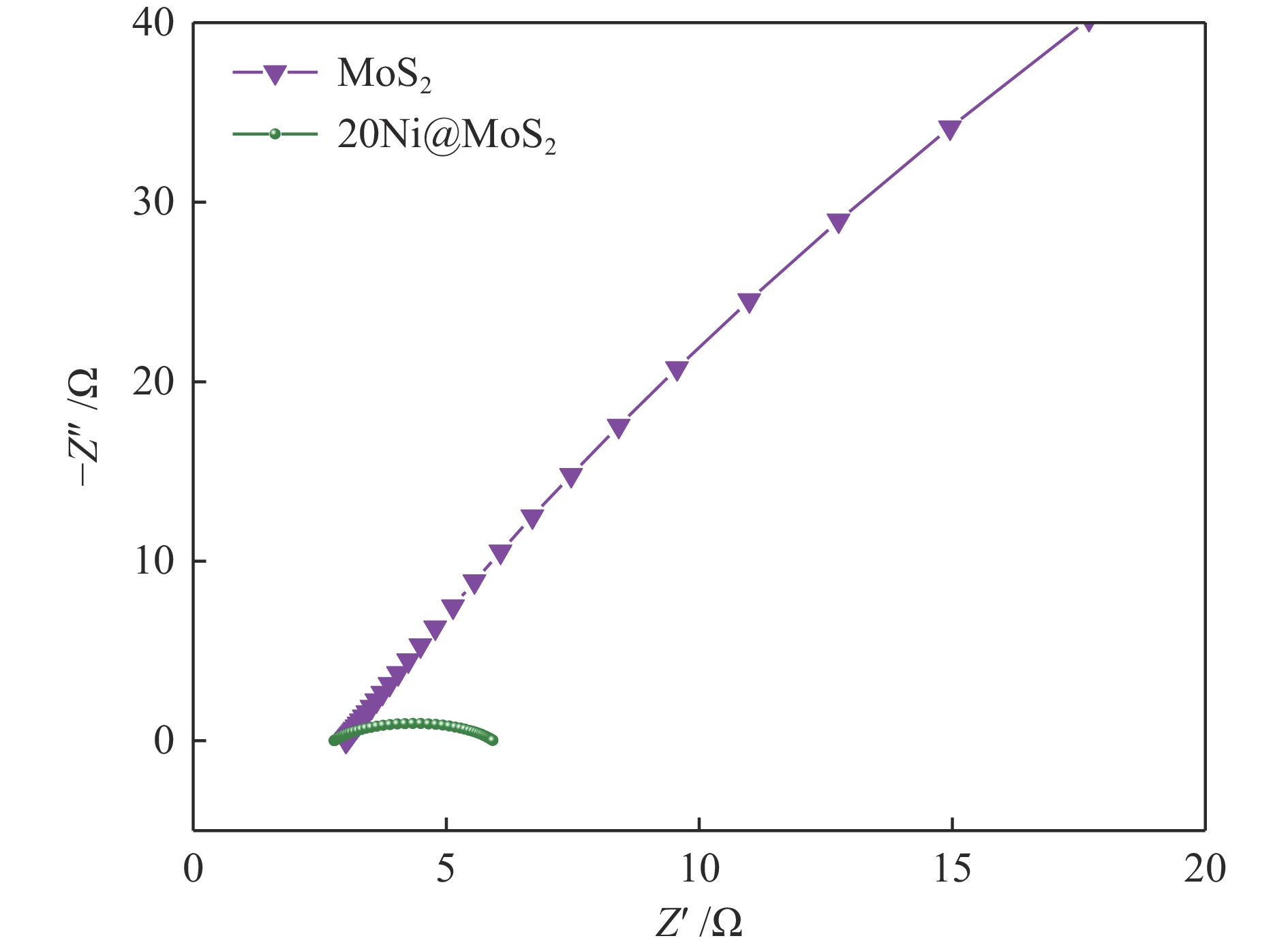
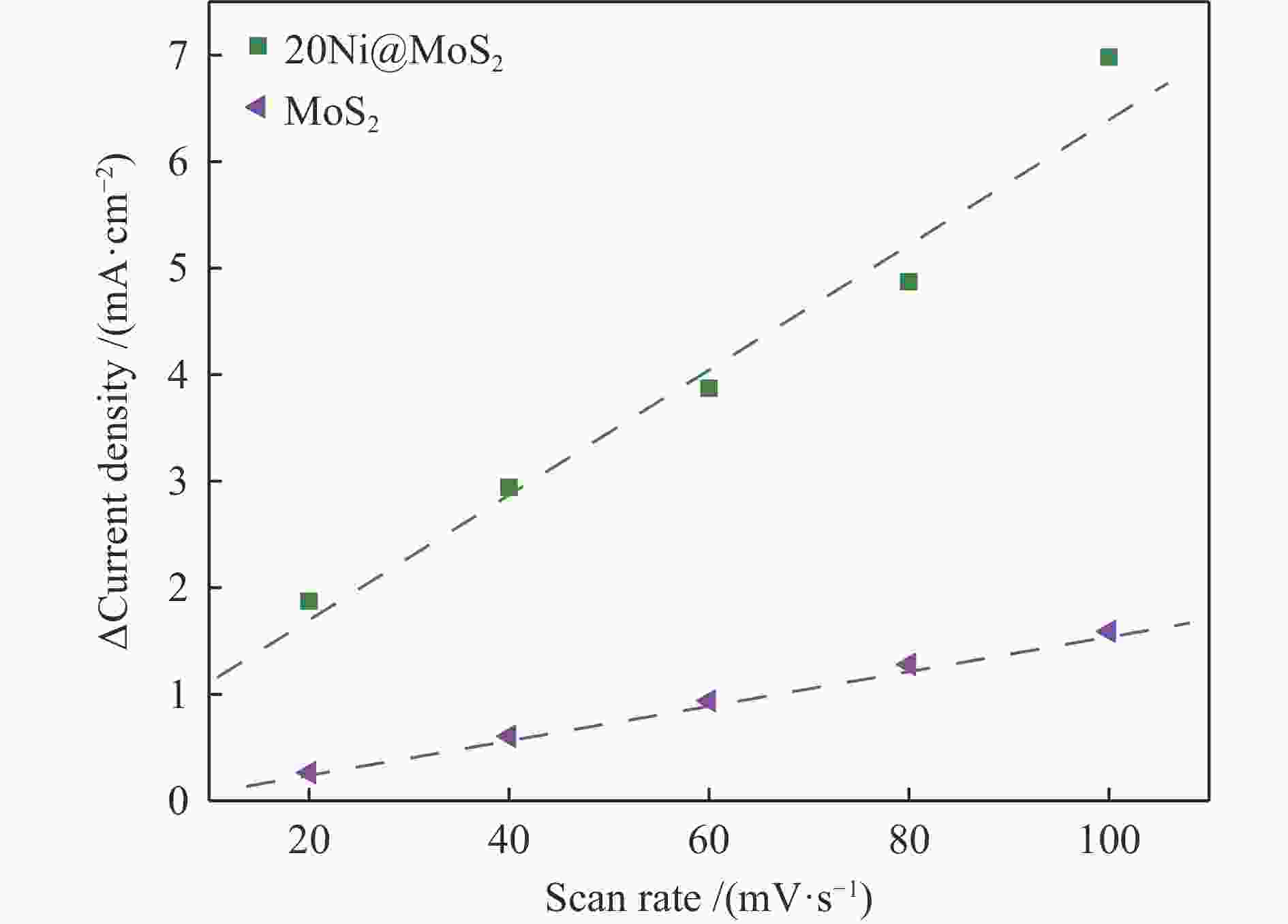
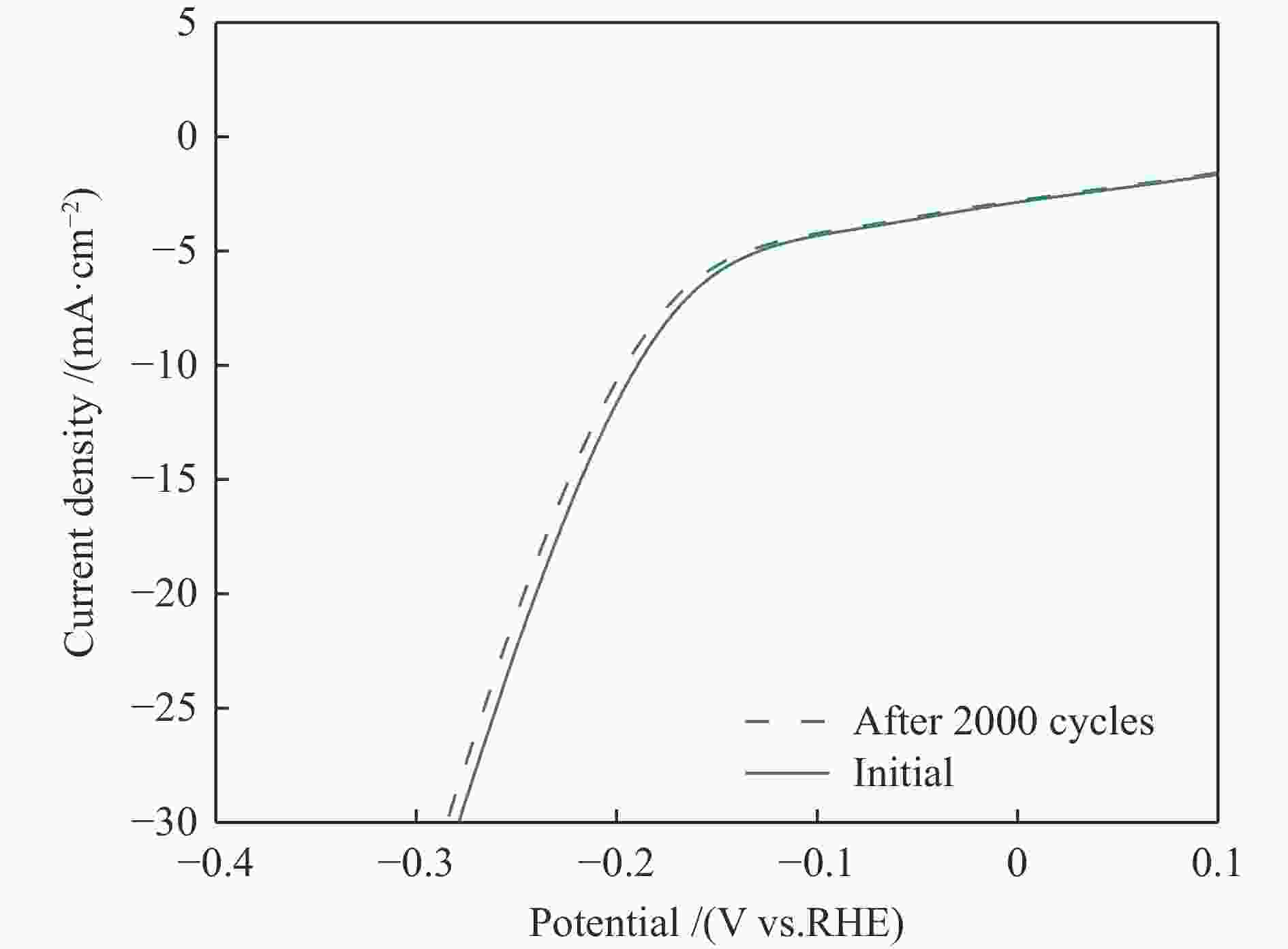
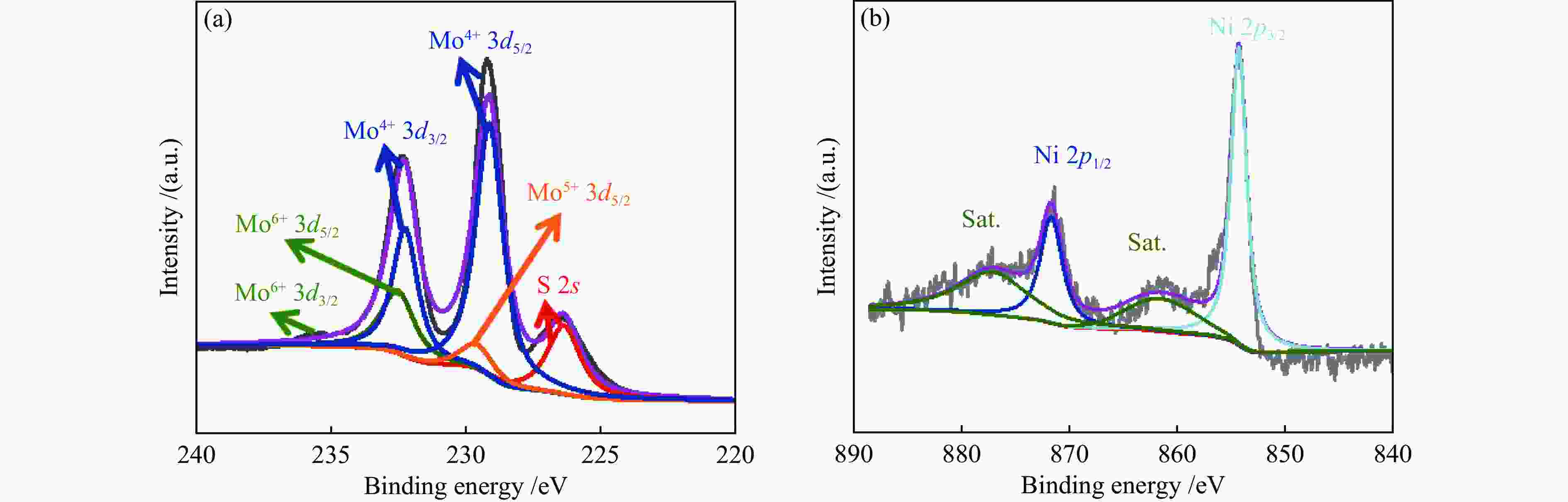
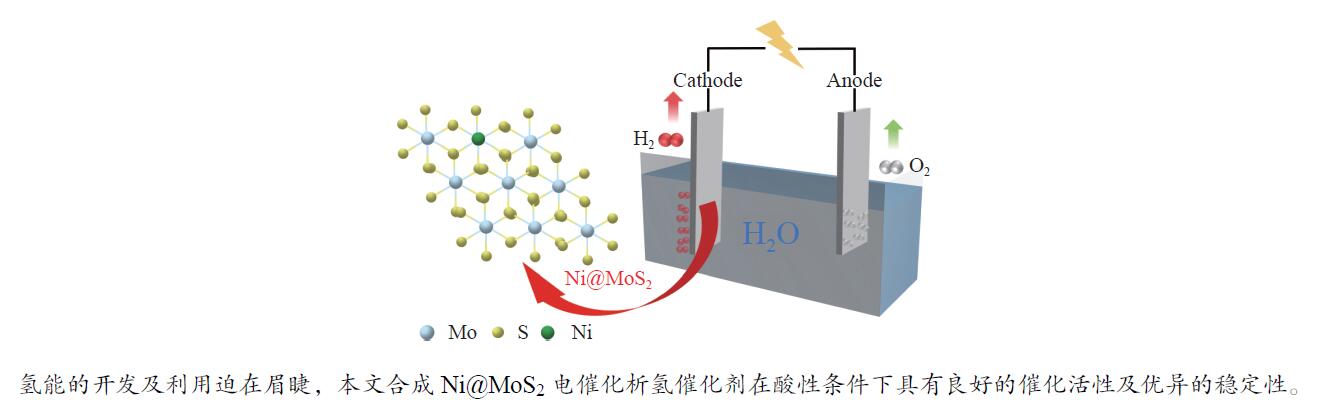
| [1] |
FU Q, HAN J C, WANG X J, XU P, YAO T, ZHONG J, ZHONG W W, LIU S W, GAO T L, ZHANG Z H, XU L L, SONG B. 2D transition metal dichalcogenides: Design, modulation, and challenges in electrocatalysis[J]. Adv Mater,2020,33(6):1907818.
|
| [2] |
李俊莉, 杨玉琴, 皇甫鑫强, 陈选欣. CoP/RGO复合材料的制备及结构性能研究[J]. 化工新型材料,2019,47(11):141−144.LI Jun-li, YANG Yu-qin, HUANGFU Xin-qiang, CHEN Xuan-xin. Study on the preparation and structure property of CoP/RGO[J]. New Chem Mater,2019,47(11):141−144.
|
| [3] |
王培灿, 雷青, 刘帅, 王保国. 电解水制氢MoS2催化剂研究与氢能技术展望[J]. 化工进展,2019,38(1):278−290.WANG Pei-can, LEI Qing, LIU Shuai, WANG Bao-guo. MoS2-based electrocatalysts for hydrogen evolution and the prospect of hydrogen energy technology[J]. Chem Eng Prog,2019,38(1):278−290.
|
| [4] |
马海霞, 王太和, 赵玉洁, 王旭, 李嘉辰. MoS2/Ru异质结构的制备及其电催化析氢反应性能[J]. 材料工程,2022,50(4):44−52. doi: 10.11868/j.issn.1001-4381.2020.000991MA Hai-xia, WANG Tai-he, ZHAO Yu-jie, WANG Xu, LI Jia-chen. Synthesis and electrochemical hydrogen evolution reaction properties of MoS2/Ru heterostructures[J]. J Mater Eng,2022,50(4):44−52. doi: 10.11868/j.issn.1001-4381.2020.000991
|
| [5] |
LIANG J S, MA F, HWANG S, WANG X, SU D. Atomic arrangement engineering of metallic nanocrystals for energy-conversion electrocatalysis[J]. Joule,2019,3(4):956−991. doi: 10.1016/j.joule.2019.03.014
|
| [6] |
米芳芳, 王庆涛. 碳化钼电解水析氢催化剂的性能调控研究进展[J]. 材料科学与工程学报,2022,40(3):520−527.MI Fang-fang, WANG Qing-tao. Research progress on performance regulation of molybdenum carbide hydrogen evolution catalyst[J]. J Mater Sci Eng,2022,40(3):520−527.
|
| [7] |
LI T F, LI S L, LIU Q Y, YIN J W, SUN D M, ZHANG M Y, XU L, TANG Y W, ZHANG Y W. Immobilization of Ni3Co nanoparticles into N-doped carbon nanotube/nanofiber integrated hierarchically branched architectures toward efficient overall water splitting[J]. Adv Sci,2020,7(1):1902371. doi: 10.1002/advs.201902371
|
| [8] |
DU H L, HODGETTS R Y, CHATTI M, NGUYEN C K, SIMONOV A N. Is molybdenum disulfide modified with molybdenum metal catalytically active for the nitrogen reduction reaction?[J]. J Electrochem Soc,2020,167(14):146507. doi: 10.1149/1945-7111/abc1a8
|
| [9] |
ZHANG H. Ultrathin two-dimensional nanomaterials. [J] ACS Nano, 2015, 9(10): 9451−9469.
|
| [10] |
乔劲松, 韩苗苗. 多孔二元过渡金属纳米片阵列电极制备及电催化析氢研究[J]. 分子催化,2021,35(5):449−455. doi: 10.16084/j.issn1001-3555.2021.05.006QIAO Jin-song, HAN Miao-miao. Preparation of porous binary transition metal nanosheets array electrode and its electrocatalytic hydrogen evolution[J]. J Mol Catal,2021,35(5):449−455. doi: 10.16084/j.issn1001-3555.2021.05.006
|
| [11] |
徐祥福, 陈佳, 赖国霞, 李天乐, 许诗圳, 陈星源, 朱伟玲. 单层MoS2在合金化及应力调控下光催化裂解水产氢的理论研究[J]. 燃料化学学报,2020,48(3):76−82.XU Xiang-fu, CHEN Jia, LAI Guo-xia, LI Tian-le, XU Shi-zhen, CHEN Xing-yuan, ZHU Wei-ling. Theoretical study on enhancing the monolayer MoS2 photocatalytic water splitting with alloying and stress[J]. J Fuel Chem Technol,2020,48(3):76−82.
|
| [12] |
LAURSEN A B, KEGNAES S, DAHL S, CHORKENDORFF I. Molybdenum sulfidesefficient and viable materials for electro and photoelectrocatalytic hydrogen evolution[J]. Energy Environ Sci,2012,5(2):5577−5591.
|
| [13] |
DU J F, ZHAO J H, REN J. Interface effect of C3N4-Ti4O7 -MoS2 composite toward enhanced electrocatalytic hydrogen evolution reaction[J]. J Fuel Chem Technol,2021,49(7):986−996. doi: 10.1016/S1872-5813(21)60109-3
|
| [14] |
RHEEM Y, PARK S H, YU S, LEE K H, MYUNG N V. Electrospun hybrid MoS2 nanofibers for high-efficiency electrocatalytic hydrogen evolution reaction[J]. J Electrochem Soc,2020,167(6):066522. doi: 10.1149/1945-7111/ab86c2
|
| [15] |
VOIRY D A, SALEHI M, SILVA R, FUJITA T, CHHOWALLA M. Conducting MoS2 nanosheets as catalysts for hydrogen evolution reaction[J]. Nano Lett,2013,13(12):6222−6227. doi: 10.1021/nl403661s
|
| [16] |
LIU GUO L, ROBERTSON A W, Li M M J, KUO W C H, DARBY M T, MUHIEDDINE M H, LIN Y C, SUENAGA K, STAMATAKIS M, WARNER J H, TSANG S C E. MoS2 monolayer catalyst doped with isolated Co atoms for the hydrodeoxygenation reaction[J]. Nat Chem,2017,9:810−816. doi: 10.1038/nchem.2740
|
| [17] |
WANG H T, TSAI C, KONG D S, CHAN K, ABILD-PEDERSEN F, NORSKOV J K, CUI Y. Transition-metal doped edge sites in vertically aligned MoS2 catalysts for enhanced hydrogen evolution[J]. Nano Res,2015,2:566−575.
|
| [18] |
YIN Y, HAN J C, ZHANG Y M, ZHANG X H, XU P, YUAN Q, LEITH S, WANG X J, WANG Y, ZHANG Z H, ZHANG P, CAO X Z, SONG B, JIN S. Contributions of phase, sulfur vacancies, and edges to the hydrogen evolution reaction catalytic activity of porous molybdenum disulfide nanosheets[J]. J Am Chem Soc,2016,138(25):7965−7972. doi: 10.1021/jacs.6b03714
|
| [19] |
张爽, 王子鸣, 卢雅宁, 唐梦, 王英财, 柳玉辉. 熔盐电解法制备NbS2@MoS2复合材料及其电催化析氢性能[J]. 复合材料学报,2022,39(8):3882−3890.ZHANG Shuang, WANG Zi-ming, LU Ya-ning, TANG Meng, WANG Ying-cai, LIU Yu-hui. Molten salt electrolysis synthesis of NbS2@MoS2 and its performance for water splitting into hydrogen[J]. Acta Mater Composit Sin,2022,39(8):3882−3890.
|
| [20] |
JI L, YAN P F, ZHU C H, MA C Y, WU W Z, WEI C, SHEN Y L, CHU S Q, WANG J OU, DU Y, CHEN J, YANG X A, XU Q. One-pot synthesis of porous 1T-phase MoS2 integrated with single-atom Cu doping for enhancing electrocatalytic hydrogen evolution reaction[J]. Appl Catal B: Environ,2019,251:87−93. doi: 10.1016/j.apcatb.2019.03.053
|
| [21] |
HU J, ZHANG C X, YANG P, XIAO J Y, DENG T, LIU Z Y, HUANG B L, MICHAEL K H L, YANG S H. Kinetic-oriented construction of MoS2 synergistic interface to boost pH-universal hydrogen evolution[J]. Adv Funct Mater,2020,30(6):1908520.1−1908520.9.
|
| [22] |
WANG D Z, ZHOU P, WU Z Z, WANG Z P, LIU Z H. Hydrothermal synthesis of MoS2 nanoflowers as highly efficient hydrogen evolution reaction catalysts[J]. J Power Sources,2014,264:229−234. doi: 10.1016/j.jpowsour.2014.04.066
|
| [23] |
LUO Z Y, OUYANG Y X, ZHANG H, XIAO M L, GE J J, JIANG Z, WANG J L, TANG D M, CAO X Z, LIU C P, XING W. Chemically activating MoS2 via spontaneous atomic palladium interfacial doping towards efficient hydrogen evolution[J]. Nat Commun,2018,9:2120. doi: 10.1038/s41467-018-04501-4
|
| [24] |
LI R C, YANG L J, XIONG T L, WU Y S, CAO L D, YUAN D S, ZHOU W J. Nitrogen doped MoS2 nanosheets synthesized via a low-temperature process as electrocatalysts with enhanced activity for hydrogen evolution reaction[J]. J Power Sources,2017,356:133−139.
|
| [25] |
JIA J, ZHOU W J, LI G X, YANG L J, WEI Z Q, CAO L D, WU Y S, ZHOU K, CHEN S W. Regulated synthesis of Mo sheets and their derivative MoX sheets (X: P, S, C) as efficient electrocatalysts for hydrogen evolution reaction[J]. ACS Appl Mater Interfaces,2017,9(9):8041. doi: 10.1021/acsami.6b12103
|
| [26] |
MOLINA S A, WIRTZ L. Phonons in single-Layer and few-Layer MoS2 and WS2[J]. Phys Rev B,2011,84(15):155413. doi: 10.1103/PhysRevB.84.155413
|
| [27] |
HUANG Y C, SUN Y H, ZHENG X L, AOKI T, PATTENGALE B, HUANG J E, HE X, BIAN W, YOUNAN S, WILLIAMS N, HU J, GE J X, PU N, YAN X X, PAN X Q, ZHANG L J, WEI Y G, GU J. Atomically engineering activation sites onto metallic 1T-MoS2 catalysts for enhanced electrochemical hydrogen evolution[J]. Nat Commun,2019,10(1):1−11. doi: 10.1038/s41467-018-07882-8
|
| [28] |
LI H, ZHANG Q, YAP C C R, TAY B K, EDWIN T H T, OLIVIER A, BAILLARGEAT D. From bulk to monolayer MoS2: Evolution of Raman Scattering[J]. Adv Funct Mater,2012,22(7):1385−1390. doi: 10.1002/adfm.201102111
|
| [29] |
GANTA D, SINHA S, Haasch R T. 2-D material molybdenum disulfide analyzed by XPS[J]. Surf Sci Spectra,2014,21(1):19−27. doi: 10.1116/11.20140401
|
| [30] |
XIANG J H, CAO H Q, Wu Q Z, ZHANG S C. L-cysteine-assisted synthesis and optical properties of Ag2S nanospheres[J]. J Phys Chem C,2008,112(10):3580−3584. doi: 10.1021/jp710597j
|
| [31] |
MCDONNELL S, ADDOU R, BUIE C, WALLACE R M, HINKLE C L. Defect-dominated doping and contact resistance in MoS2[J]. ACS Nano,2014,8(3):2880−2888. doi: 10.1021/nn500044q
|
| [32] |
YANG Y Q, ZHANG K, LIN H L, LI X, CHAN H C, YANG L C, GAO Q S. MoS2-Ni3S2 heteronanorods as efficient and stable bifunctional electrocatalysts for overall water splitting[J]. ACS Catal,2017,7(4):2357−2366. doi: 10.1021/acscatal.6b03192
|
| [33] |
SUN L, WANG T, ZHANG L, SUN Y J, XU K W, DAI Z F, MA F. Mace-like hierarchical MoS2/NiCo2S4 composites supported by carbon fiber paper: An efficient electrocatalyst for the hydrogen evolution reaction[J]. J Power Sources,2018,377(15):142−150.
|
| [34] |
YE J B, YU Z T, CHEN W X, CHEN Q N, MA L. Ionic-liquid mediated synthesis of molybdenum disulfide/graphene composites: An enhanced electrochemical hydrogen evolution catalyst[J]. Int J Hydrogen Energy,2016,41(28):12049−12061. doi: 10.1016/j.ijhydene.2016.05.186
|
| [35] |
WU L Q, XU X B, ZHAO Y Q, ZHANG K Y, SUN Y, WANG T T, WANG Y Q, ZHONG W, DU Y W. Mn doped MoS2/reduced graphene oxide hybrid for enhanced hydrogen evolution[J]. Appl Surf Sci,2017,425:470−477. doi: 10.1016/j.apsusc.2017.06.223
|
| [36] |
YANG L, HONG W W, ZHANG Y, TIAN Y, GAO X, ZHU Y R, ZOU G Q, HOU H S, JI X B. Hierarchical NiS2 Modified with Bifunctional Carbon for Enhanced Potassium‐Ion Storage[J]. Adv Funct Mater,2019,29(50):1903454. doi: 10.1002/adfm.201903454
|
| [37] |
ZENG L L, YANG L J, LU J, JIA J, YU J Y, DENG Y Q, SHAO M F, ZHOU W J. One-step synthesis of Fe-Ni hydroxide nanosheets derived from bimetallic foam for efficient electrocatalytic oxygen evolution and overall water splitting[J]. Chin Chem Lett,2018,29(12):1875−1878. doi: 10.1016/j.cclet.2018.10.026
|
| [38] |
ZHAO L L, YUAN H F, SUN D H, JIA J, YU J Y, ZHANG X L, LIU X Y, LIU H, ZHOU W J. Active facet regulation of highly aligned molybdenum carbide porous octahedrons via crystal engineering for hydrogen evolution reaction[J]. Nano Energy,2020,77:105056. doi: 10.1016/j.nanoen.2020.105056
|




 下载:
下载: