Study on CO/CO2 formation mechanism of Zigzag model coke with high oxygen coverage based on DFT theory
-
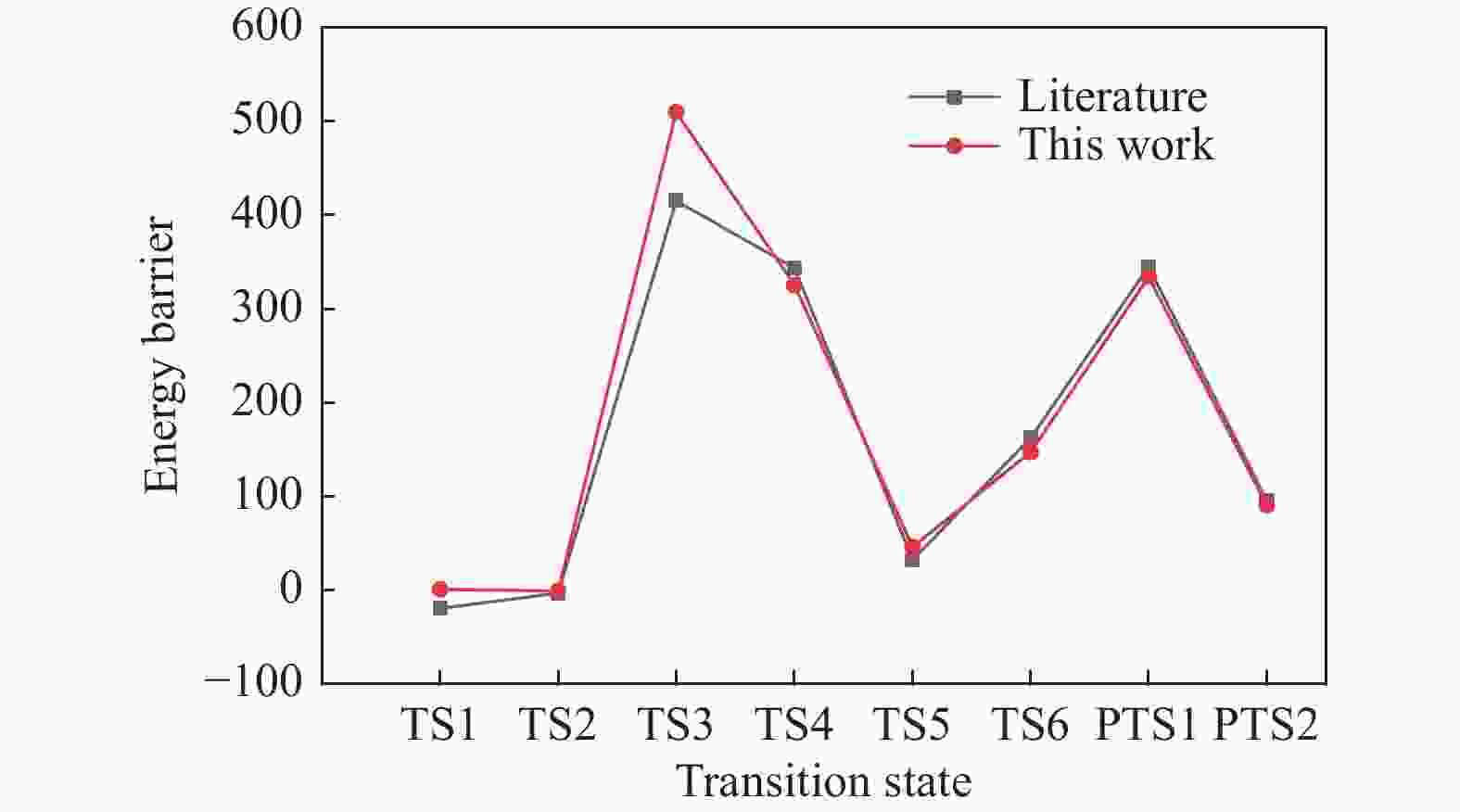
摘要: 碳资源在能源、材料及化工等领域的清洁高效利用日益重要,而焦炭氧化特别是脱附产生CO2/CO的机理研究并不充分。其中较高焦炭表面氧覆盖率相应于较低温度或较高压力的反应条件,对此,本研究基于第一性原理研究讨论了该情况下焦炭Zigzag结构碳环簇氧化脱附过程的反应路径。计算表明,表面吸附氧热解生成CO2过程需要经过重排形成含O−C−O团簇的结构,最终至CO2完成脱附需多个中间反应步,与对比文献中形成碳氧六元环再依次断掉两个C−O 键而脱附 CO2 不同,本研究得到了相关的两种路径,分别为形成 CO2−C−官能团再断掉 C−C 而脱附CO2以及基于碳氧六元环结构直接断裂两个C−O 键而脱附CO2的可能反应路径。另外,研究了CO脱附过程的不同反应路径。模型计算结果与相关文献理论和实验结果具有良好的符合。Abstract: The clean and efficient utilization of carbon resources is becoming more and more important, in energy, material, and chemical engineering field, but the mechanism of coke oxidation, especially that of the CO2/CO desorption is not fully studied yet. In this paper, density functional theory was used to study the oxidation mechanism of Zigzag char structure with high coverage of O2, which is related to an oxidation under lower temperature or high pressure. Based on the corresponding quantum chemistry calculation, it is shown that there are several possible pathways for the CO2 desorption process, which may need rearrange to form the structure containing O−C−O clusters. And successively, multiple intermediate reaction steps are required to complete the desorption of CO2. Other than in the literature that the COO–O–C functional group formed first, with then the C–O bond broken and CO2 desorbed respectively, a novel pathway with two C–O bonds broken simultaneously to generate CO2 was found. It results from a functional group of COO–char formed, and certain alternative pathways via C–C bonds breaking were also dealt with, as well as related CO desorptions. The reaction model built was validated by theoretical and experimental results from literature satisfactorily.
-
Key words:
- char /
- oxidation /
- desorption /
- reaction path /
- DFT theory
-
表 1 边缘活性位CO/CO2的生成与脱附过程的能垒、焓变及过渡态虚频
Table 1 Energy barrier, enthalpy change and imaginary frequency of each step in the CO/CO2 formation and desorption process
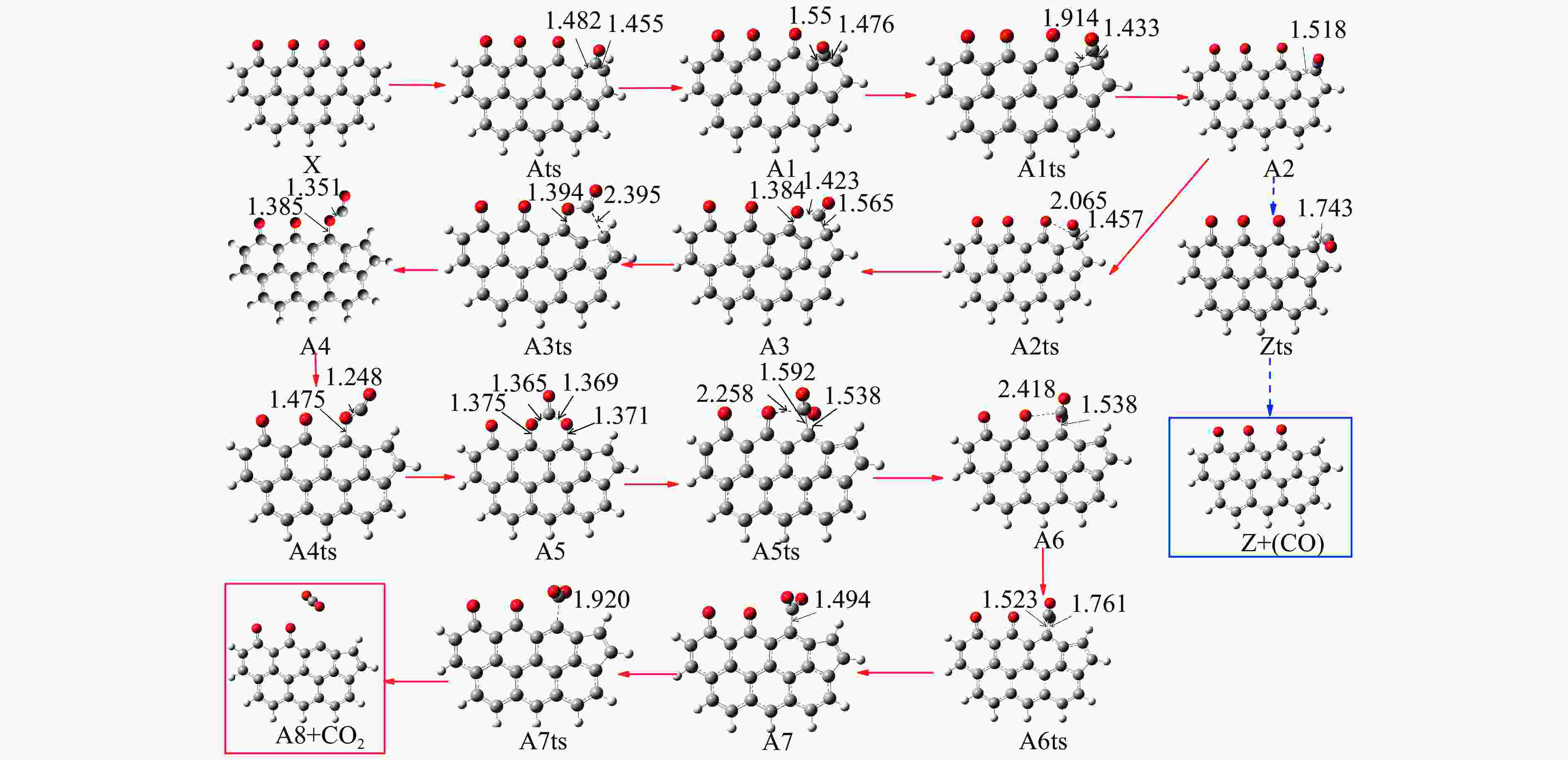
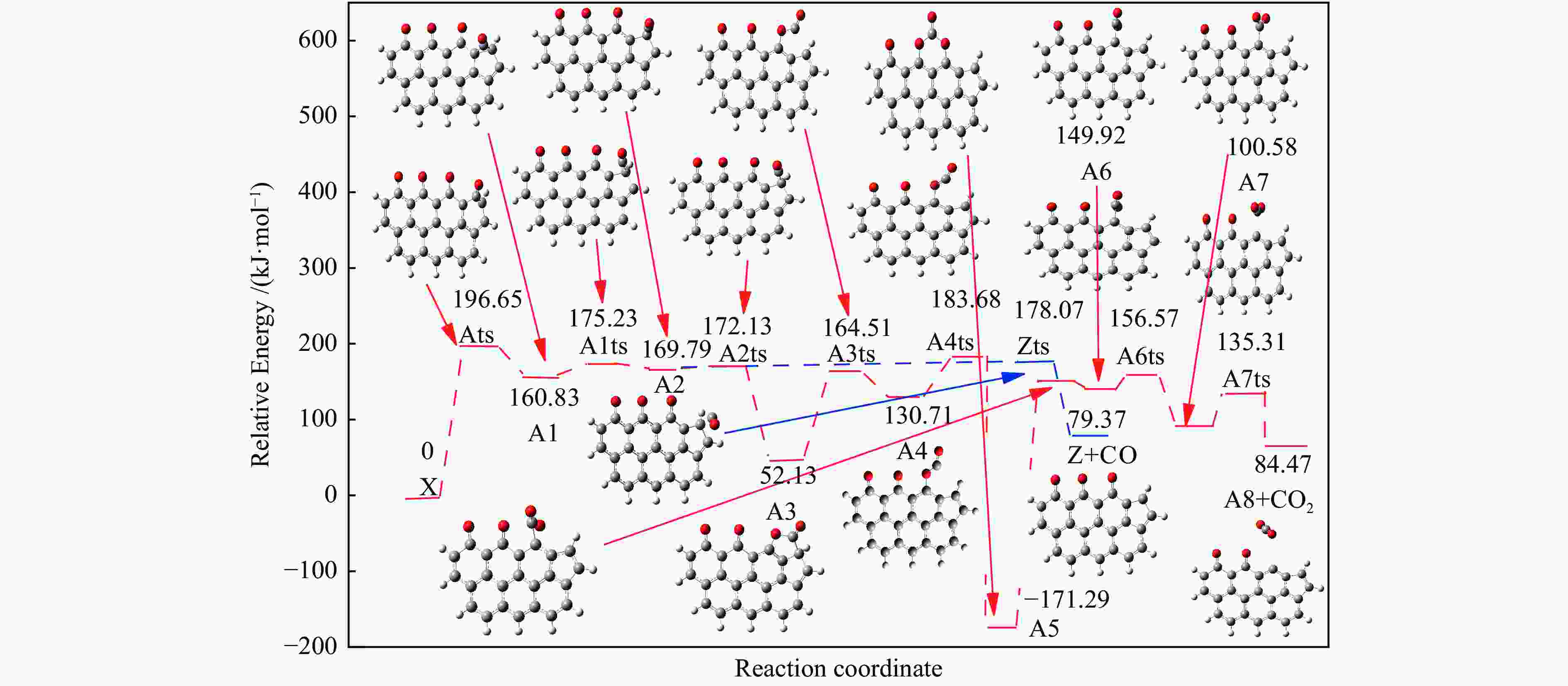
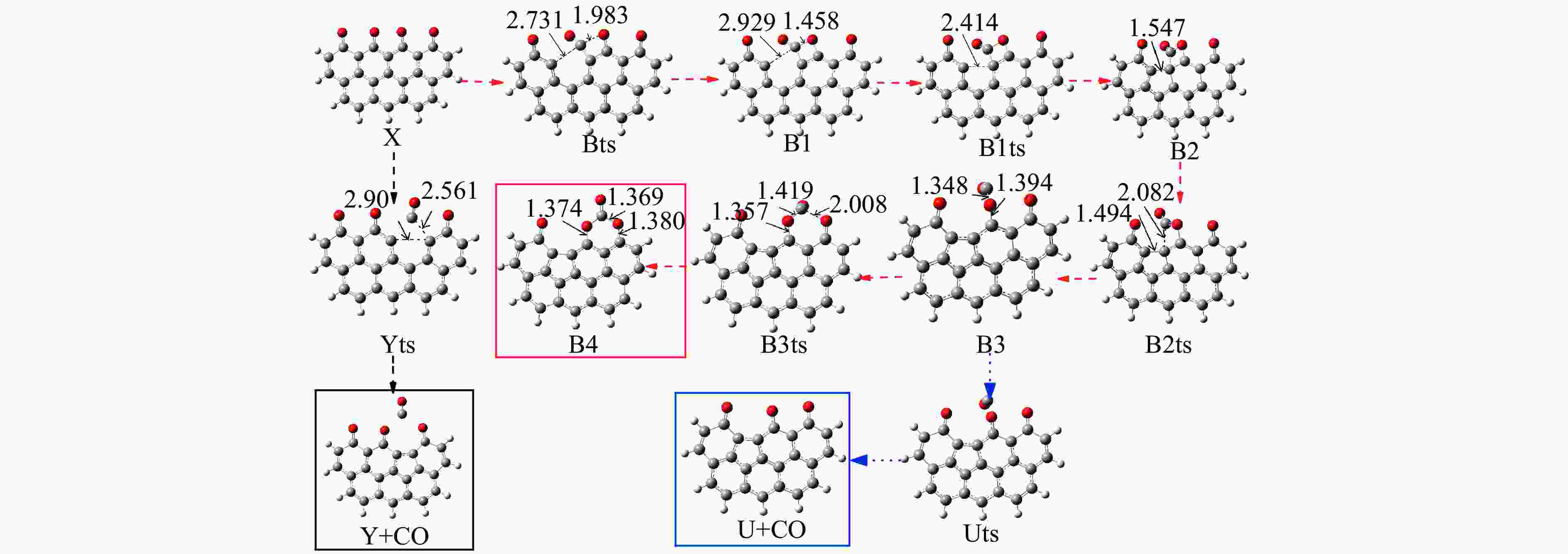
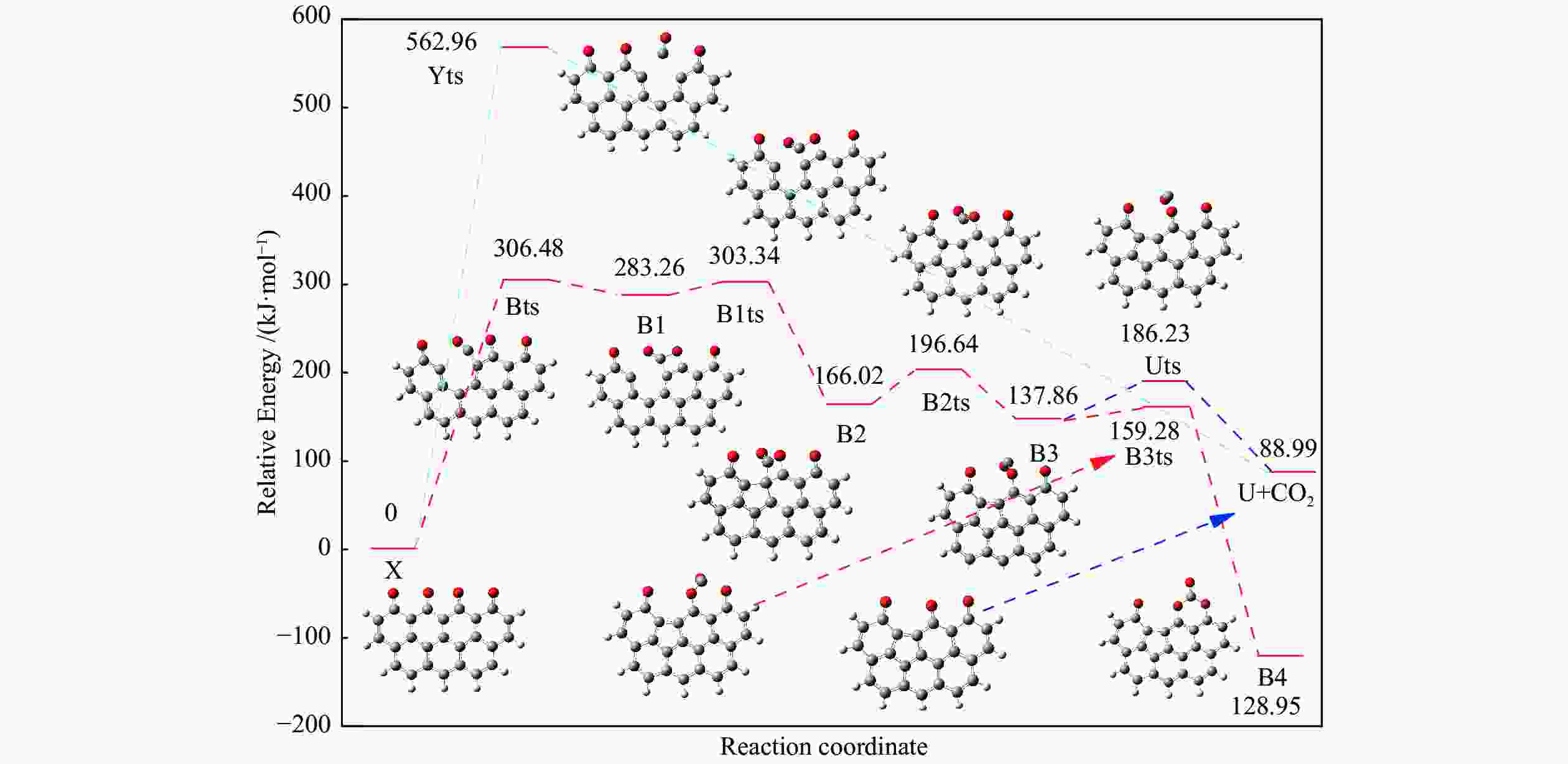
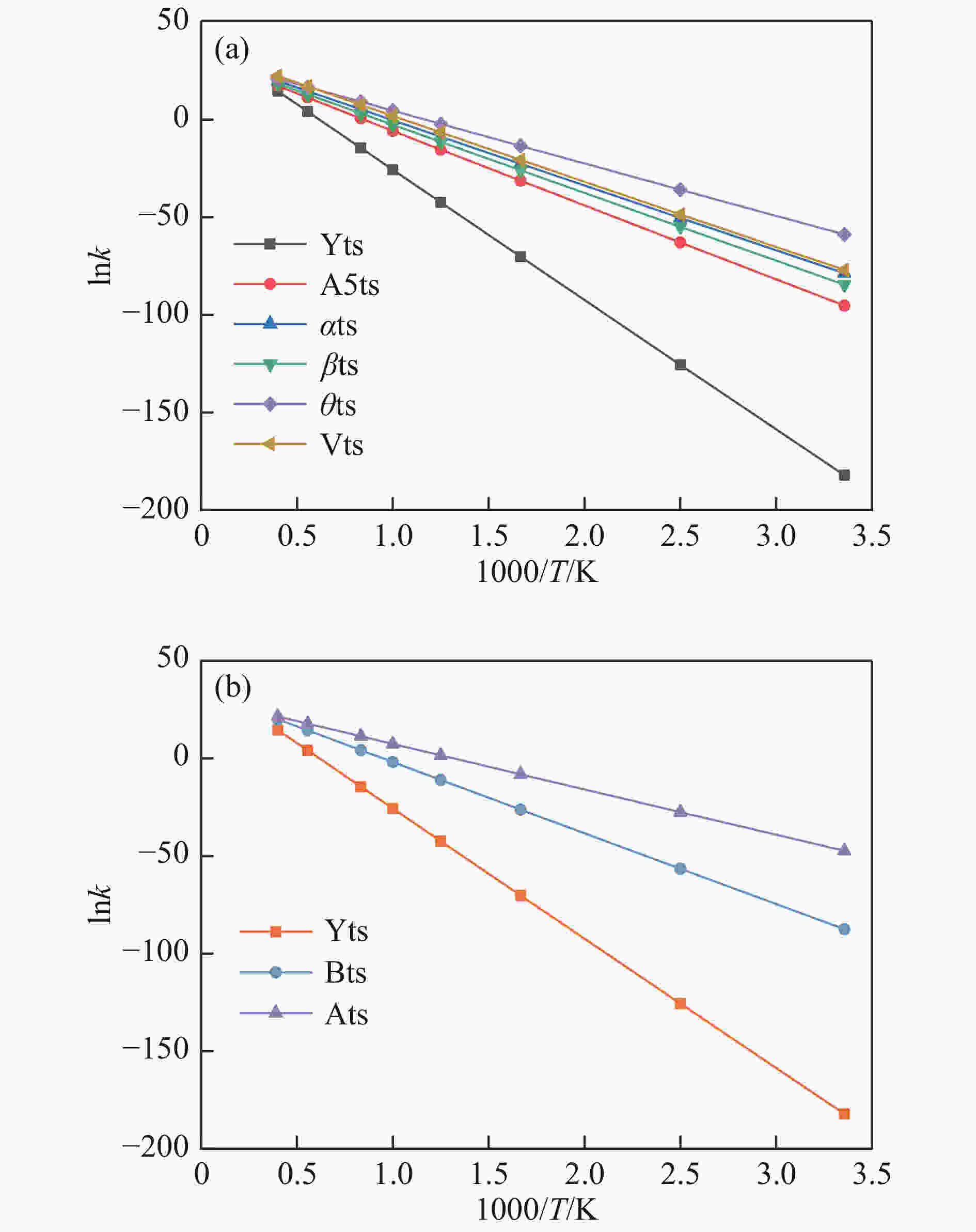
Reaction ΔE/(kJ·mol−1) ΔH/(kJ·mol−1) Imaginary frequency/cm−1 X → Yts → Y+CO 562.96 20.56 91.42 i X→ Ats → A1 196.65 159.41 411.10 i A1→ A1ts → A2 14.40 2.04 271.17 i A2→ A2ts → A3 2.34 −27.06 155.45 i A2 → Zts → Z+CO 8.28 −21.45 308.18 i A3→ A3ts → A4 112.38 17.58 377.67 i A4→ A4ts →A5 52.97 −293.34 336.70 i A5→ A5ts →A6 323.71 309.16 275.81 i A6→ A6ts →A7 6.65 −57.24 395.01 i A7→ A7ts →A8+CO2 34.73 −35.15 351.6 i 表 2 中间位点重排及脱附过程中各基元步的能垒、焓变以及虚频
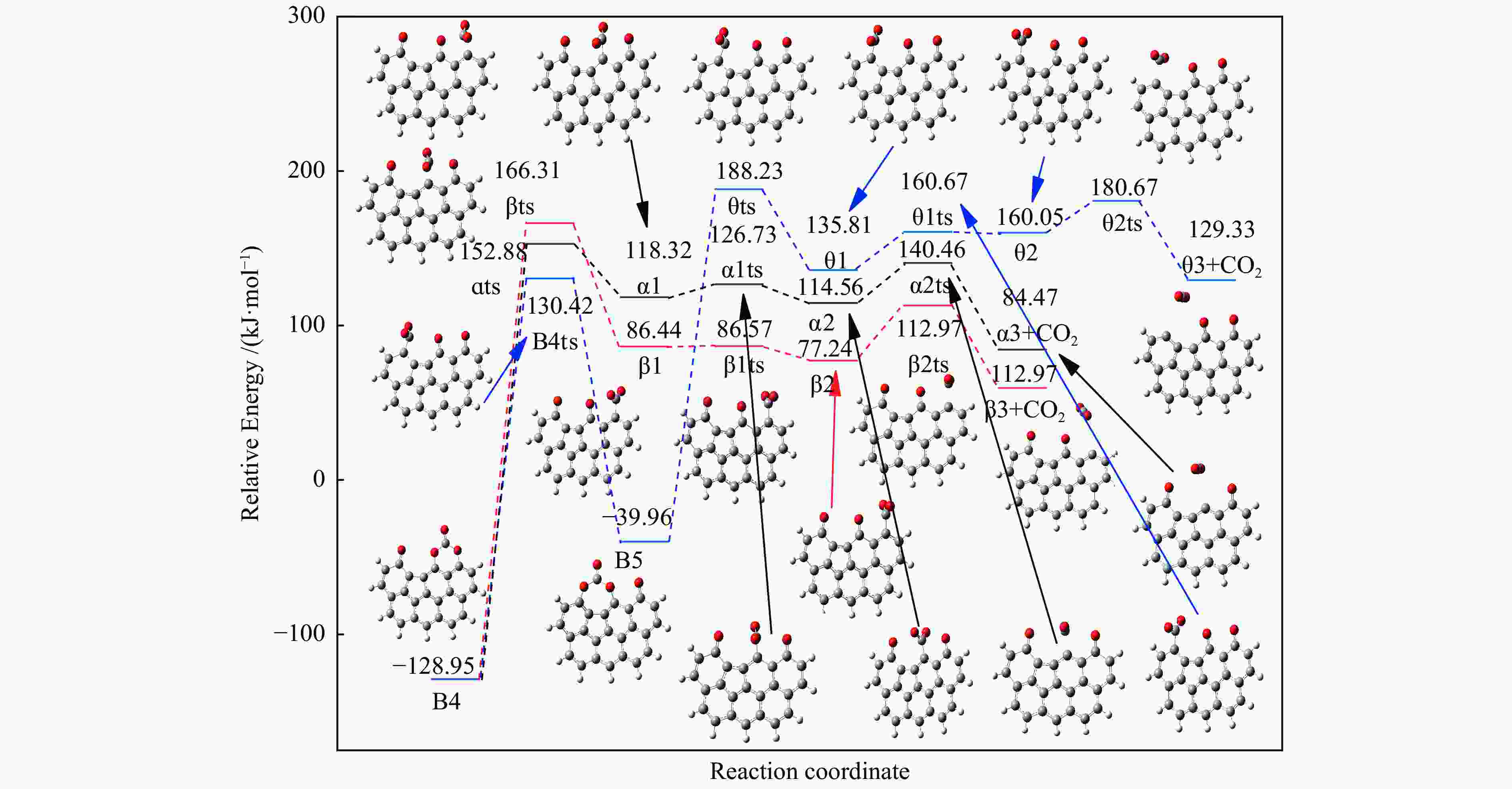
Table 2 Energy Barrier, enthalpy change and imaginary frequency of each step in the processes of rearrangement and desorption
Reaction ΔE
/(kJ·mol−1)ΔH
/(kJ·mol−1)Imaginary frequency
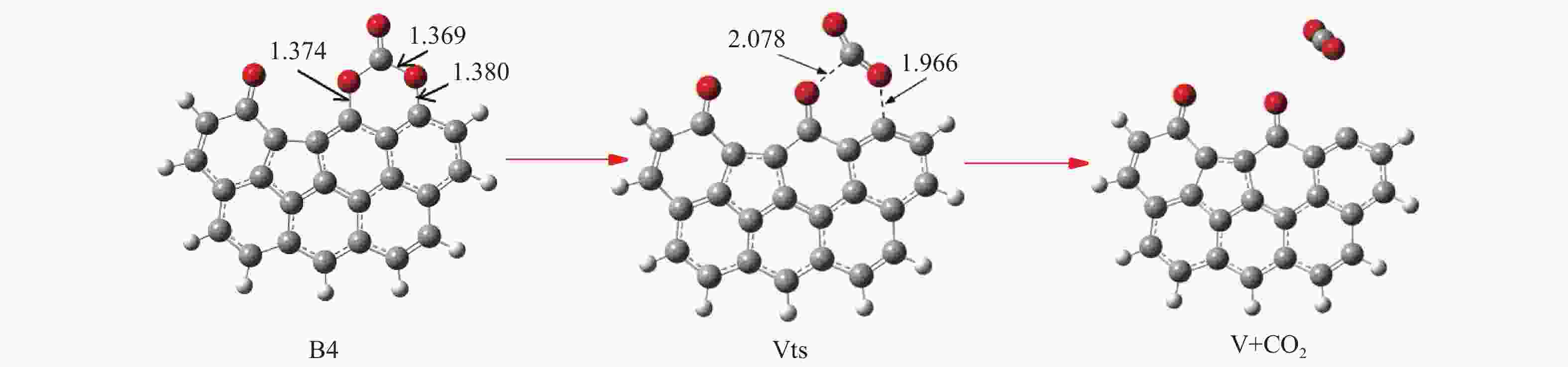
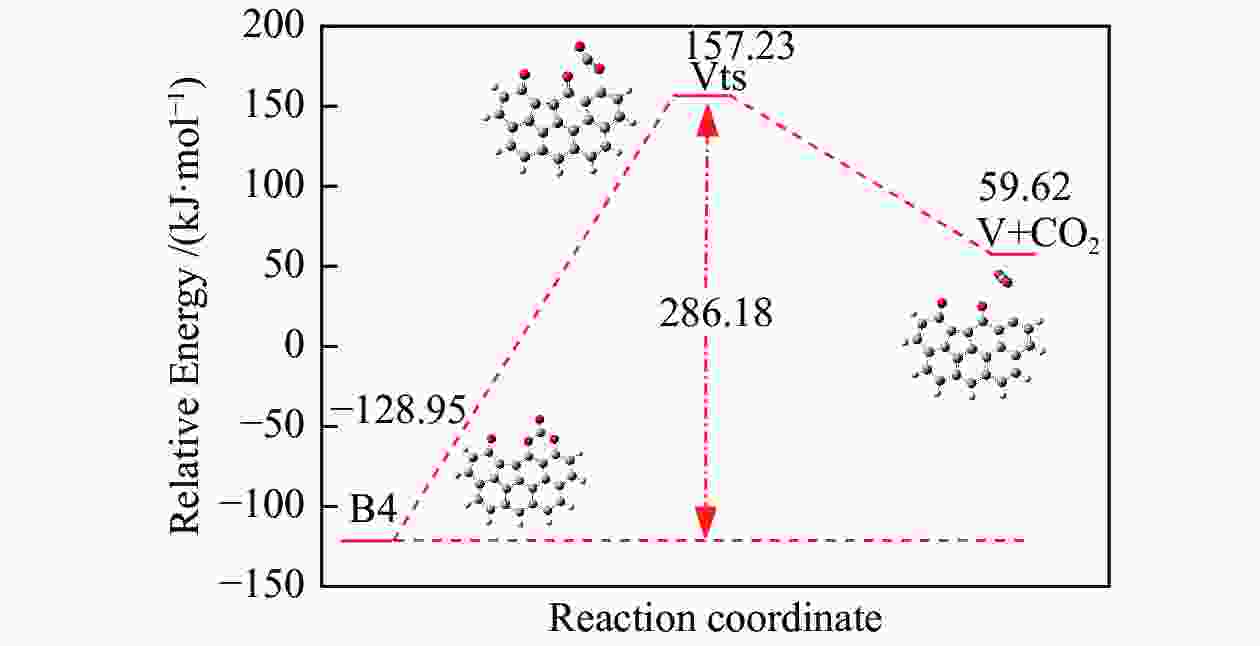
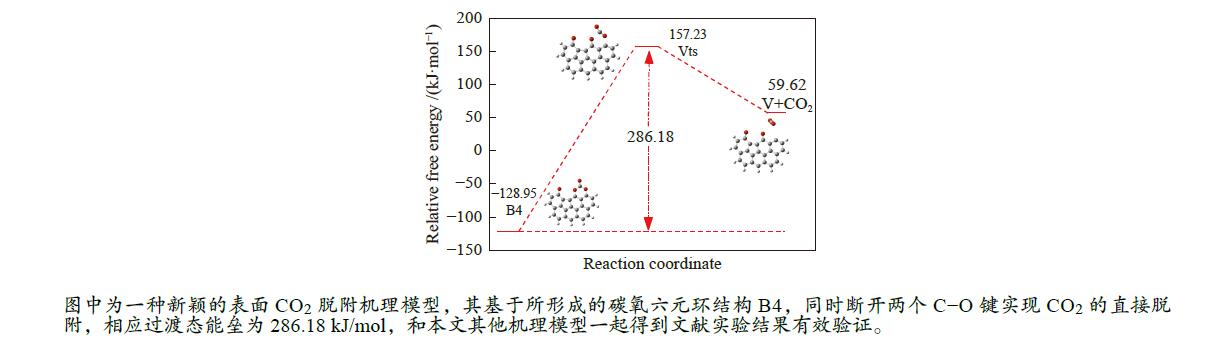
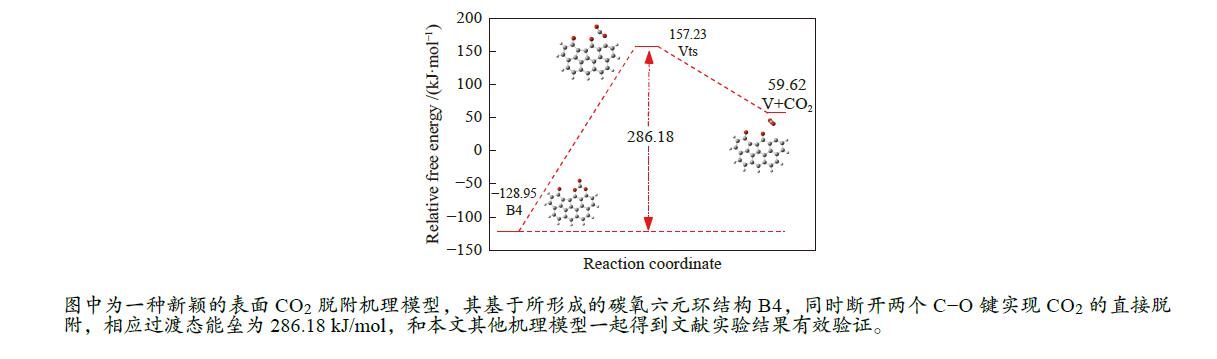
/(kJ·mol−1)X → Yts → Y+CO 562.96 20.56 91.42 i X → Bts → B1 306.48 271.29 368.64 i B1→ B1ts →B2 20.08 −103.93 208.84 i B2→ B2ts → B3 30.62 −38.95 217.17 i B3→ Uts → U+CO 48.37 −71.72 727.24 i B3 → B3ts → B4 21.42 −247.52 712.15 i B4→ B4ts → B5 259.37 86.15 116.25 i B4 → αts → ɑ1 281.83 234.81 547.88 i α1 → α1ts → α2 8.41 −10.29 351.53 i α2 → α2ts → α2+CO2 25.90 −46.19 366.38 i B4 → βts → β1 295.26 198.44 582.07 i β1 → β1ts → β2 0.13 −12.26 207.88 i β2 → β2ts → β3+CO2 35.73 −22.64 352.48 i B5 → θts → θ1 228.19 162.46 551.45 i θ1 → θ1ts → θ2 24.86 13.89 325.85 i θ2 → θ2ts → θ3+CO2 20.62 −46.15 383.07 i B4→ Vts→ V+CO2 286.18 163.54 822.10 i -
[1] 潘登. 新环保形势下焦炭市场发展趋势分析[J]. 煤炭经济研究,2019,39(8):15−19.PAN Deng. Analysis on the development trend of coke market under the new environmental protection situation[J]. Coal Econ Res,2019,39(8):15−19. [2] 陈勇, 张俊晓, 杜仲军, 夏勇, 周研, 马凯. 延迟焦化装置焦炭自燃的原因及对策[J]. 山东化工,2019,48(21):123−124+126. doi: 10.3969/j.issn.1008-021X.2019.21.049CHEN Yong, ZHANG Jun-xiao, DU Zhong-jun, XIA Yong, ZHOU Yan, MA Kai. Causes and countermeasures of coke spontaneous combustion in delayed coking units[J]. Shandong Chem Ind,2019,48(21):123−124+126. doi: 10.3969/j.issn.1008-021X.2019.21.049 [3] 李金虎. 基于活性位点产生和氧化的热侵煤体煤自燃特性及抑制途径研究[D]. 徐州: 中国矿业大学, 2020.LI Jin-hu. Study on spontaneous combustion characteristics and inhibition pathway of thermal invaded coal based on the generation and oxidation of active sites[D]. Xuzhou: China University of Mining and Technology, Bei Jing, 2020. [4] ZHU Z H, LU G Q(Max), FINNERTY J, YANG R T. Electronic structure methods applied to gas-carbon reactions[J]. Carbon,2003,41(4):635−658. doi: 10.1016/S0008-6223(02)00380-9 [5] MONTOYA A, MONDRAGON F, TRUONG T N. Formation of CO precursors during char gasification with O2, CO2 and H2O[J]. Fuel Process Technol,2002,77−78:125−130. doi: 10.1016/S0378-3820(02)00013-9 [6] MONTOYA A, MONDRAGON F, TRUONG T N. CO2 adsorption on carbonaceous surfaces: a combined experimental and theoretical study[J]. Carbon,2003,41(1):29−39. doi: 10.1016/S0008-6223(02)00249-X [7] ESPINAL J F, MONTOYA A, MONDRAGON F, TRUONG T N. A DFT study of interaction of carbon monoxide with carbonaceous materials[J]. J Phys Chem B,2004,108(3):1003−1008. doi: 10.1021/jp0308211 [8] BACKREEDY R, JONES J M, POURKASHANIAN M, WILLLAMS A. A study of the reaction of oxygen with graphite: Model chemistry[J]. Faraday Discuss,2002,119:385−394. [9] ZHUANG Q L, KYOTANI T, TOMITA A. Dynamics of surface oxygen complexes during carbon gasification with oxygen[J]. Energy Fuels,1995,9(4):630−634. doi: 10.1021/ef00052a009 [10] SENDT K, HAYNES B S. Density functional study of the reaction of O2 with a single site on the zigzag edge of graphene[J]. Proc Combust Inst,2011,33(2):1851−1858. doi: 10.1016/j.proci.2010.06.021 [11] SENDT K, HAYNES B S. Density functional study of the chemisorption of O2 on the zigzag surface of graphite[J]. Combust Flame,2005,143:629−643. doi: 10.1016/j.combustflame.2005.08.026 [12] RADOVIC L R. The mechanism of CO2 chemisorption on Zigzag carbon active sites: A computational chemistry study[J]. Carbon,2005,43(5):907−915. doi: 10.1016/j.carbon.2004.11.011 [13] 钟俊, 高正阳, 丁艺, 余岳溪, 杨维结. Zigzag煤焦表面异相还原N2O反应[J]. 煤炭学报,2017,42(11):3028−3034.ZHONG Jun, GAO Zheng-Yang, DING Y, YU Y X, YANG W J. Heterogeneous reduction reaction of N2O by char based on Zigzag carbonaceous model[J]. J China Coal Soc,2017,42(11):3028−3034. [14] CHEN Y F, SU S, ZHANG C X, WANG Z H, XIE Y X, ZHANG H, QING M X, WANG Y, HU S, ZHANG Z X, XIANG J. Experimental and DFT research on role of sodium in NO reduction on char surface under H2O/Ar atmosphere[J]. Fuel,2021,302(10):121105. [15] YANG H P, DONG Z G, LIU B, CHEN Y Q, GONG M, LI S J, CHEN H P. A new insight of lignin pyrolysis mechanism based on functional group evolutions of solid char[J]. Fuel,2020,288(11):119719. [16] FRANKCOMBE T J, SMITH S C. OH-initiated oxidation of toluene. 1. Quantum chemistry investigation of the reaction path[J]. J Phys Chem A,2007,111(19):3686−3690. doi: 10.1021/jp067112i [17] SIMONS J, NICHOLS J. Quantum mechanics in chemistry [M]. New York: Oxford University Press, 1997. [18] ZHANG H, LIU J X, WANG X Y, LUO L, JIANG X M. DFT study on the C(N)-NO reaction with isolated and contiguous active sites[J]. Fuel,2017,203:715−724. doi: 10.1016/j.fuel.2017.05.023 [19] 周赛, 刘虎, 于鹏飞, 车得福. 基于密度泛函理论的CO2对NO异相还原影响的机理研究[J]. 燃料化学学报,2021,49(9):1234−1238.ZHOU Sai, LIU Hu, YU Peng-fei, CHE De-fu. Study on the mechanism of oxidation of nitrogen–containing char by CO2 based on density functional theory[J]. J Fuel Chem Technol,2021,49(9):1234−1238. [20] ORREGO J F, ZAPATA F, TRUONG T N, MONDRAGON F. Heterogeneous CO2 evolution from oxidation of aromatic carbon-based materials[J]. J Phys Chem A,2009,113(29):8415−8420. doi: 10.1021/jp903362g [21] SÁNCHEZ A, MONDRAGON F. Role of the epoxy group in the heterogeneous CO2 evolution in carbon oxidation reactions[J]. J Phys Chem C,2007,111(2):612−617. doi: 10.1021/jp065701i [22] RAADOVIC L R. Active sites in graphene and the mechanism of CO2 formation in carbon oxidation[J]. J Am Chem Soc,2009,131(47):17166−17175. doi: 10.1021/ja904731q [23] HURT R H, CALO J M. Semi-global intrinsic kinetics for char combustion modeling[J]. Combust Flame,2001,125:1138−1149. doi: 10.1016/S0010-2180(01)00234-6 [24] 田向红. 焦炭氧化的密度泛函理论研究[D]. 郑州: 郑州大学, 2019.TIAN Xiang-hong. Density functional theory study of coke oxidation[D]. Zhengzhou: Zhengzhou University, 2019. [25] CREHUT R, BOFILL J M. The reaction path intrinsic reaction coordinate method and the Hamilton-Jacobi theory[J]. J Chem Phys,2005,122(23):234105. doi: 10.1063/1.1927521 [26] CHUANG Y Y, CORCHADO J C, TRUHLAR D G. Mapped interpolation scheme for single-point energy corrections in reaction rate calculations and a critical evaluation of Dual-Level reaction path dynamics methods[J]. J Phys Chem A,1999,103(8):1140−1149. doi: 10.1021/jp9842493 [27] 刘国杰, 黑恩成. 化学反应活化能的定义及其与势垒的关系[J]. 大学化学,2013,28(5):73−76.LIU Guo-jie, HEI En-cheng. The definition of activation energy of chemical reaction and its relation with potential barrier[J]. Univ Chem,2013,28(5):73−76. [28] 傅献彩, 沈文霞, 姚天扬. 物理化学(第四版)(下册)[M]. 北京: 高等教育出版社, 1990: 64−65FU Xian-cai, SHEN Wen-xia, YAO Tian-yang. Physical Chemistry [M]. 4th Ed. Beijing: Higher Education Press, 1990: 64−65. [29] ROBERTS M J, EVERSON R C, DOMAZETIS G, NEOMAGUS H W J P, JONES J M, VAN SITTERTE C G C E. OKOLO G N, NIEKERKF D V N, MATHEWS J P, The DFT molecular modeling and experimental particle kinetics studies of CO2-char gasification[J]. Carbon,2015,93:295−314. doi: 10.1016/j.carbon.2015.05.053 [30] MA M C, BROWN T C, HAYNES B S. Evaluation of thermal desorption spectra for heterogeneous surfaces: Application to carbon surface oxides[J]. Surf Sci,1993,297(3):312−326. doi: 10.1016/0039-6028(93)90220-E -




 下载:
下载: