Study on co-pyrolysis and co-gasification of hydrothermal carbonized biomass and coal
-
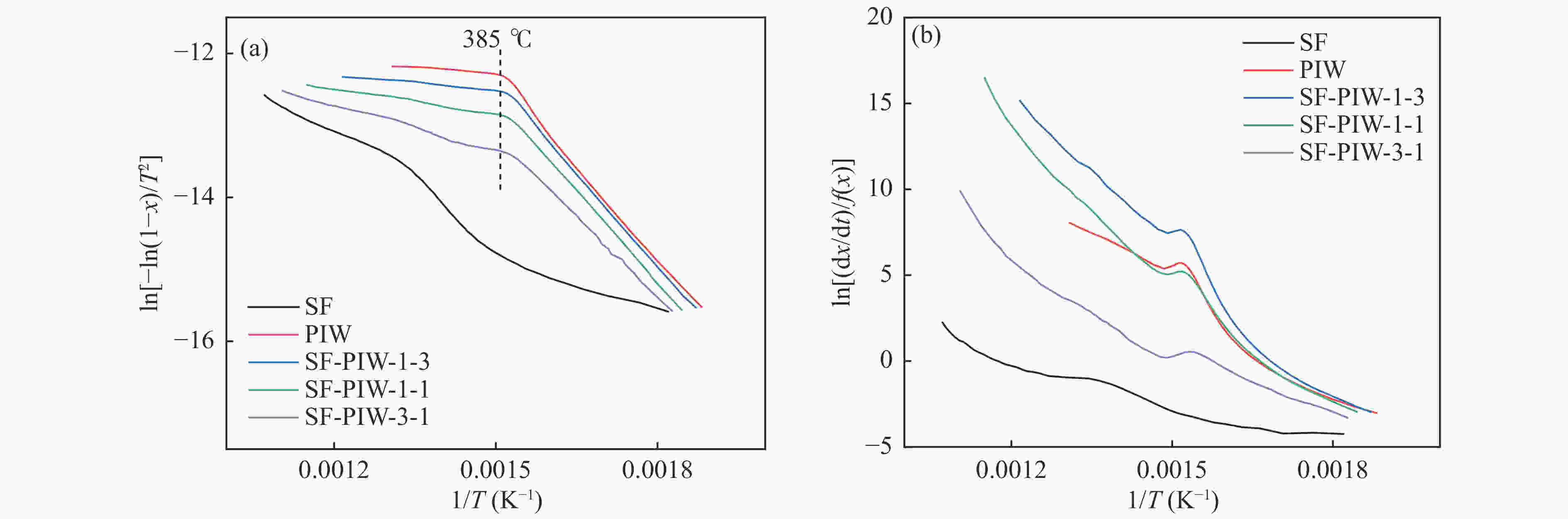
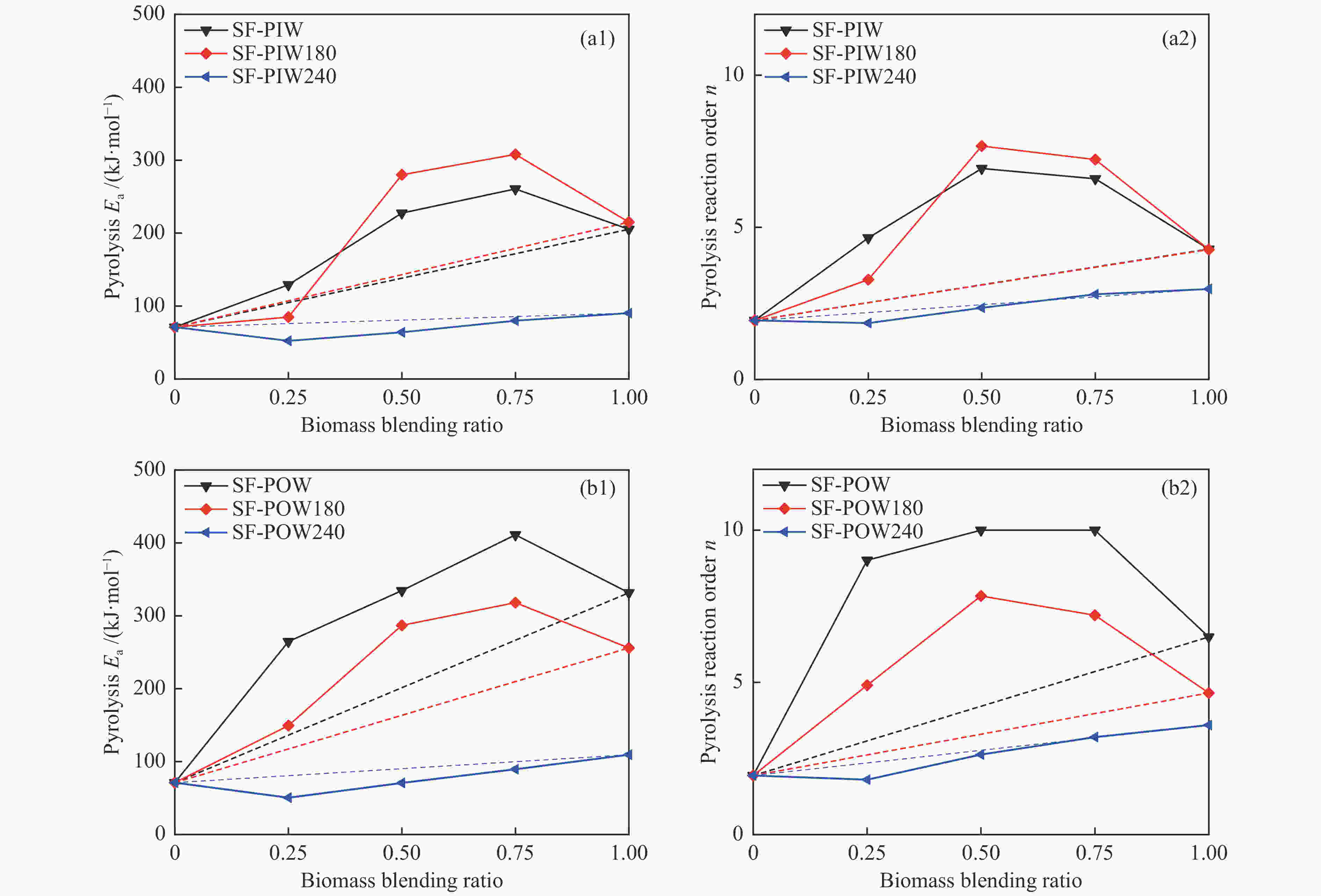
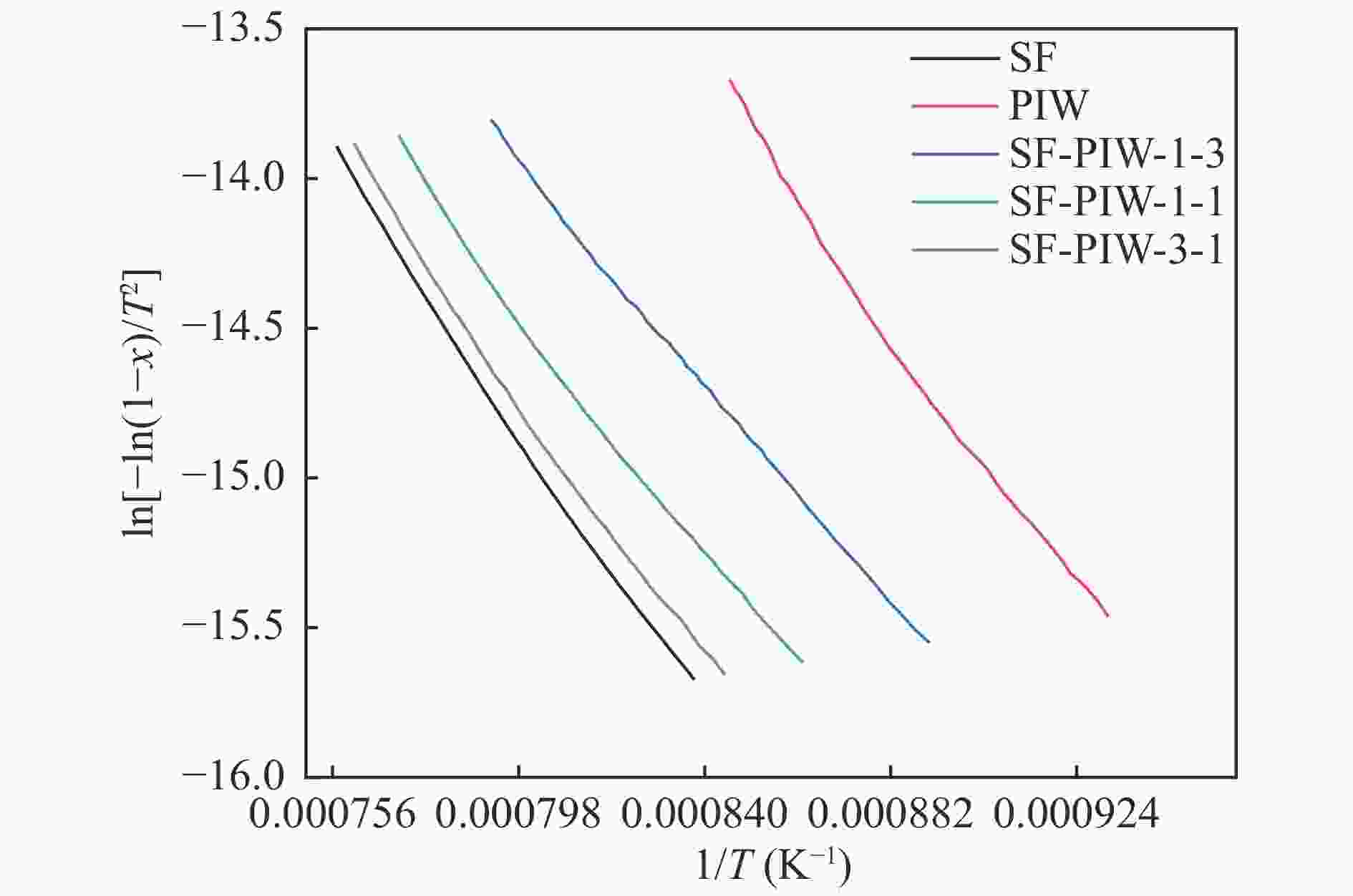
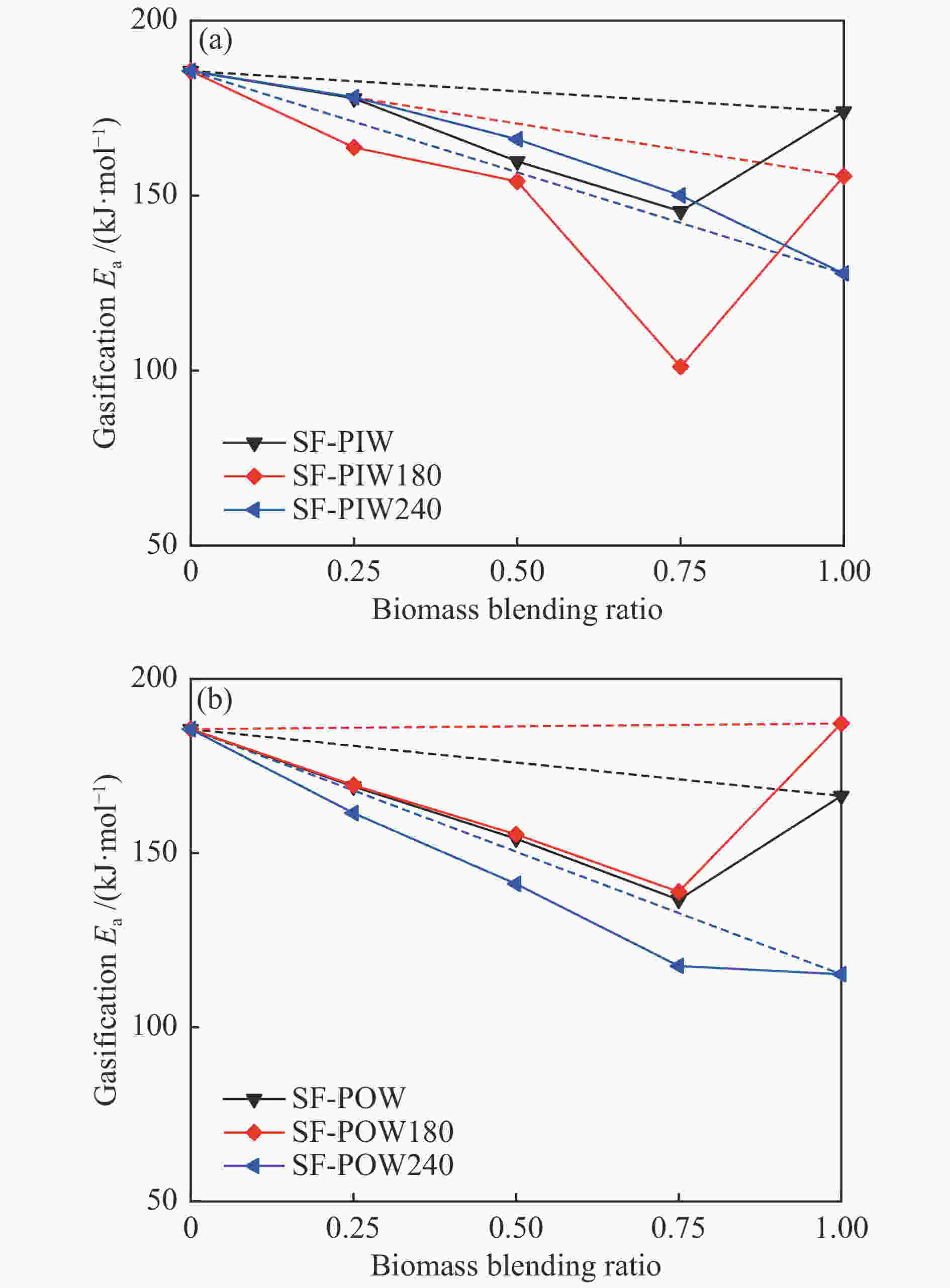
摘要: 煤和生物质共热化学转化有助于当前化石能源系统的低碳化发展。本研究以烟煤和木质生物质为原料,研究煤和生物质共热解和共气化特性,并考察了不同水热炭化温度和生物质掺混比的影响。利用热重分析仪和在线质谱分析共热解和共气化的协同作用和氢气释放特性。采用Model-fitting方法,单独分析热解和气化阶段的整体反应动力学。结果表明,煤和生物质共气化阶段的协同作用显著强于共热解阶段。生物质比例越高,共气化协同作用越明显,水热炭化会削弱共气化的协同作用。共热解过程,H2的产生受抑制。共气化过程可采用一级模型描述,而共热解过程需遵循n级反应模型。未处理的或轻度水热炭化的生物质与煤的混合物,共热解整体活化能和反应级数大于加权平均值,而其共气化的活化能变化趋势相反。重度水热炭化生物质与煤的混合物,共热解和共气化的活化能均接近加权平均值。Abstract: The co-thermochemical conversion of coal and biomass can contribute to the low carbonization of current fossil energy system. In this work, the bituminous and lignocellulosic biomass were selected to study the co-pyrolysis and co-gasification of coal and biomass, with the consideration of different hydrothermal carbonization (HTC) temperature and biomass blending ratio. The synergistic effect of co-pyrolysis and co-gasification was analyzed by using the thermogravimetric analyzer, and the H2 release property was investigated by the online mass spectrometer. The model-fitting method was adopted to analyze the overall kinetics during pyrolysis and gasification stage, respectively. The results showed that the synergistic effect of coal and biomass in co-gasification stage was much stronger than that in co-pyrolysis stage. The gasification synergy was enhanced with the biomass blending ratio, while the HTC pretreatment could weaken the synergy. The H2 production was inhibited during co-pyrolysis. The first-order reaction model could well describe the co-gasification process, while the n-order reaction model was suitable for the co-pyrolysis process. For the blends of raw or the slight HTC biomass and coal, the overall pyrolysis activation energy (Ea) was greater than that calculated by the weighted average, whereas the overall gasification Ea showed the opposite trend. For the blends of the severe HTC biomass and coal, the Ea of co-pyrolysis and co-gasification were both close to the weighted average value.
-
Key words:
- co-pyrolysis /
- co-gasification /
- H2 generation /
- kinetics /
- synergistic effect
-
表 1 样品的工业分析和元素分析
Table 1 Proximate and ultimate analyses of samples
Sample Proximate analysis wd/% Ultimate analysis wdaf/% A V FC C H O* N S PIW 2.84 81.20 15.96 43.31 5.71 50.59 0.19 0.19 PIW180 1.71 83.79 14.50 46.01 6.14 47.42 0.28 0.15 PIW240 1.69 65.34 32.97 60.14 5.58 33.81 0.32 0.15 POW 9.79 74.33 15.88 40.57 5.49 53.34 0.45 0.15 POW180 7.51 76.20 16.29 43.34 5.69 50.40 0.43 0.14 POW240 11.07 57.00 31.93 50.44 4.81 44.16 0.44 0.15 SF 6.65 30.82 62.53 80.90 4.37 13.06 1.11 0.56 V: volatile; A: ash; FC: fixed carbon; ad: air dry basis; daf: dry ash-free basis; *: by difference 表 2 样品的灰分组成
Table 2 Ash composition of samples
Sample Content w/% SiO2 CaO Al2O3 Fe2O3 K2O MgO Na2O P2O5 PIW 35.76 31.45 8.97 6.43 6.05 4.00 3.07 0.73 PIW180 48.69 17.58 15.77 6.91 3.59 3.07 1.61 0.49 PIW240 48.96 13.42 19.20 6.25 4.17 3.04 1.54 0.70 POW 50.57 15.59 14.02 6.32 4.55 4.21 2.19 1.01 POW180 55.00 13.26 14.63 6.95 3.24 3.21 1.35 0.94 POW240 59.23 8.68 17.08 5.82 3.04 2.97 1.31 0.66 SF 29.03 19.20 17.33 6.73 0.78 6.63 1.23 0.07 表 3 热解气化反应主要参数
Table 3 Main parameters for pyrolysis and gasification
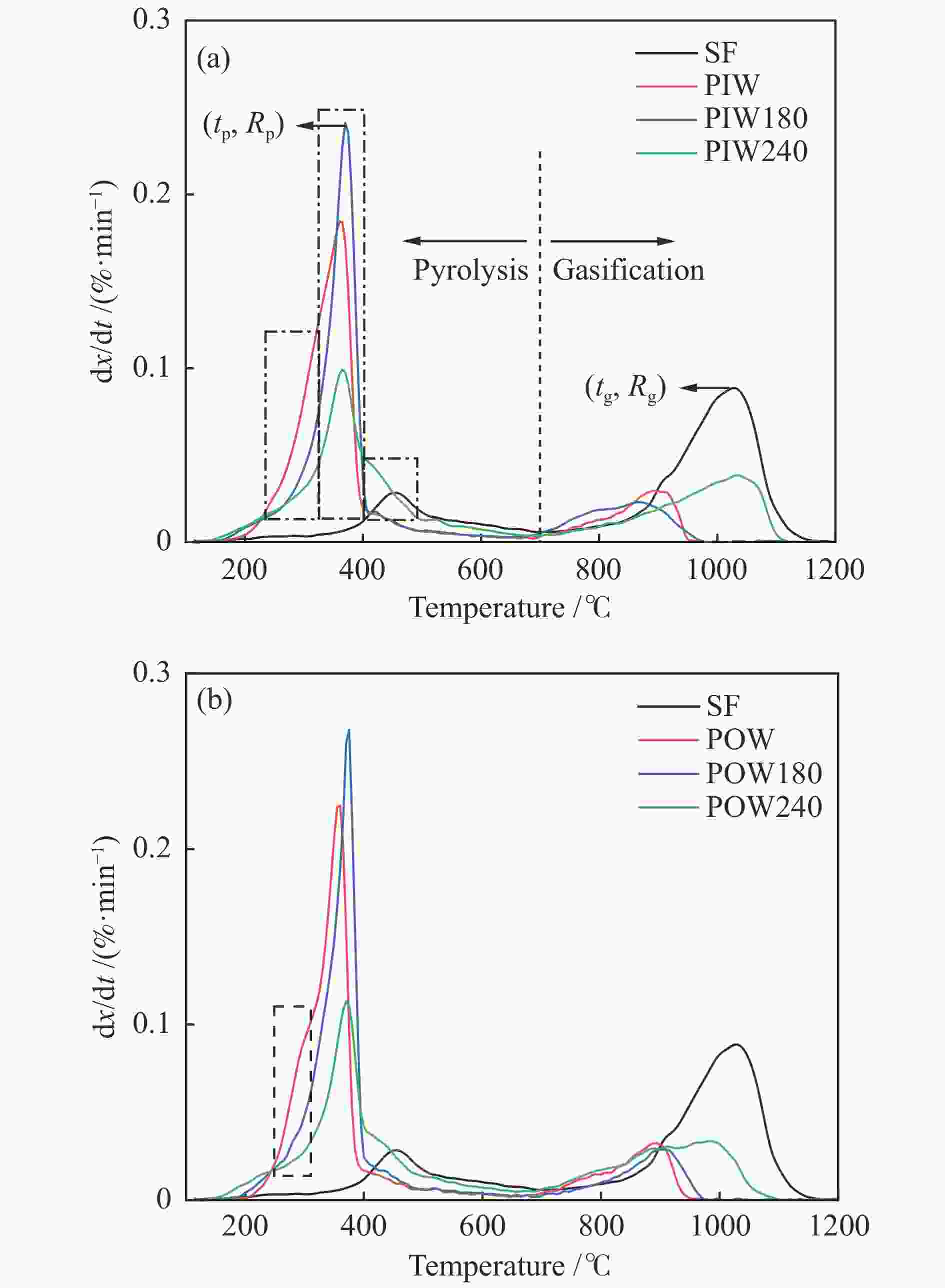
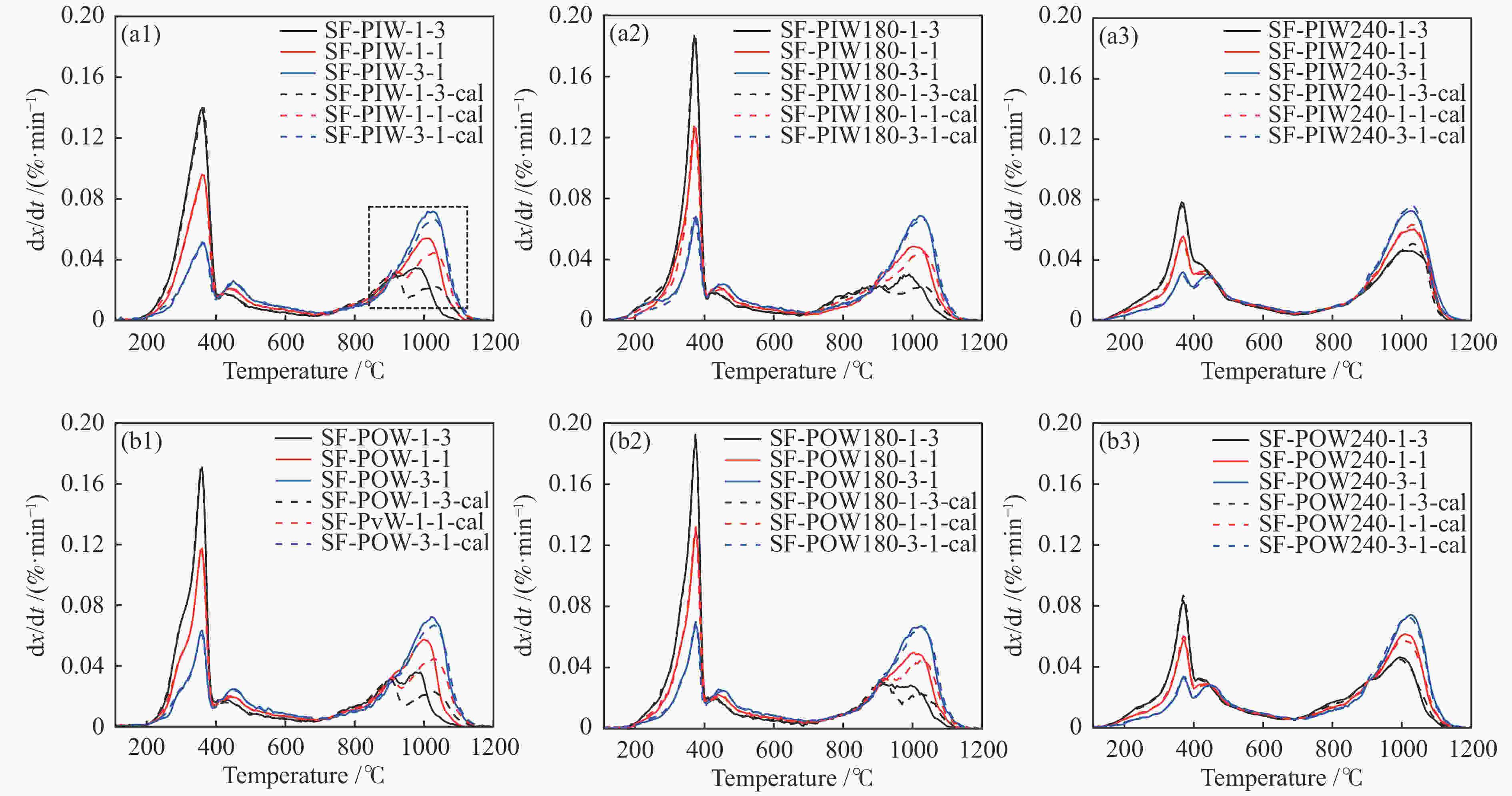
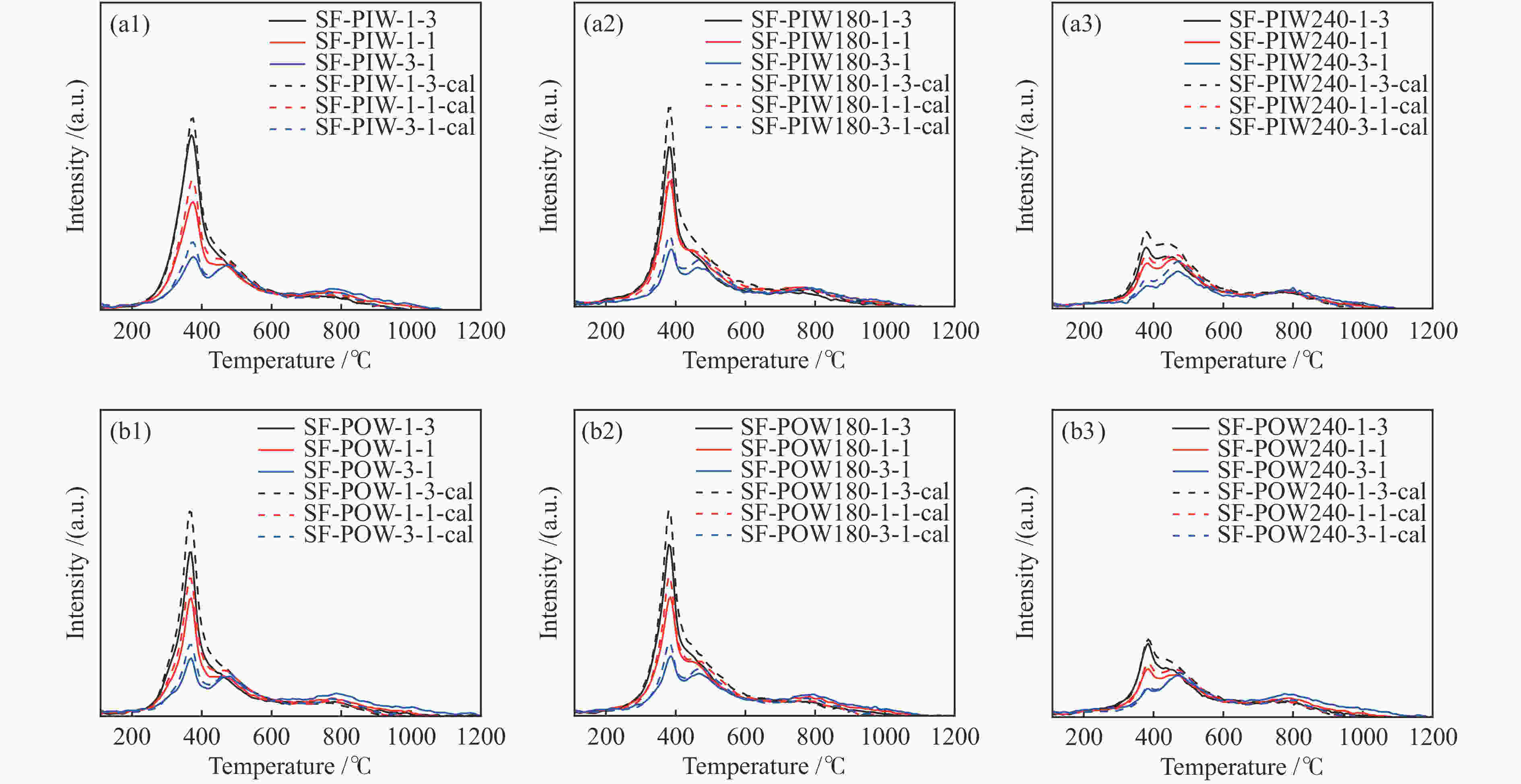
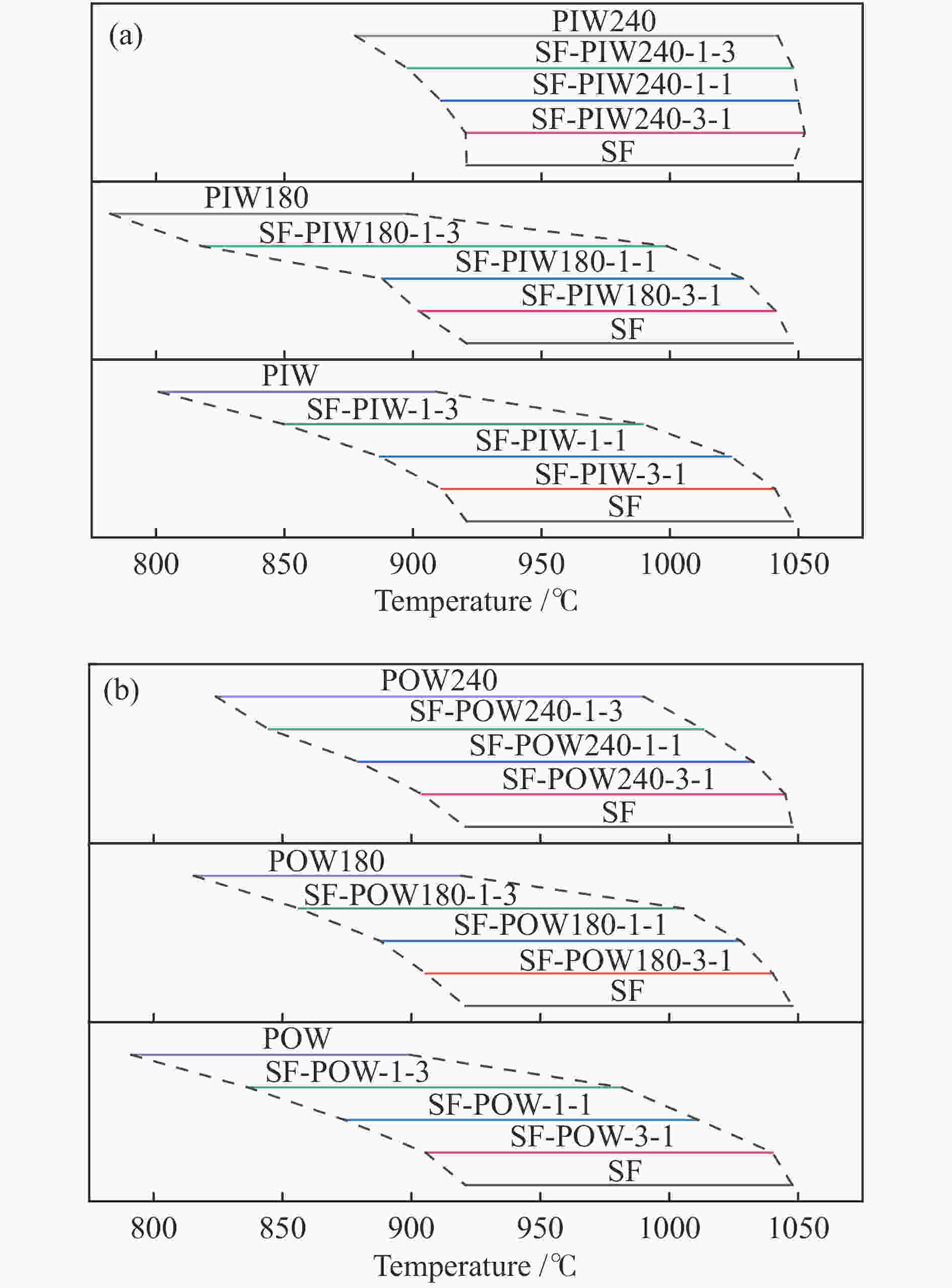
Sample Pyrolysis Gasification H2 release mass fraction* tp /℃ Rp /(%·min−1) mass fraction tg /℃ Rg /(%·min−1) tH /℃ PIW 80.6 363 0.184 19.4 894 0.03 372 PIW180 80.6 368 0.241 19.4 867 0.023 381 PIW240 61.9 368 0.099 38.1 1034 0.038 379 POW 79.5 358 0.225 20.5 891 0.032 366 POW180 81.6 371 0.268 18.4 901 0.029 380 POW240 60.8 371 0.114 39.2 979 0.034 384 SF 26.2 456 0.028 73.8 1028 0.088 479 *: ash-free base -
[1] GOUWS S M, CARRIER M, BUNT J R, NEOMAGUS H W J P. Co-pyrolysis of coal and raw/torrefied biomass: A review on chemistry, kinetics and implementation[J]. Renewable Sustainable Energy Rev,2021,135:110189. [2] HE Q, GUO Q, UMEKI K, DING L, WANG F, YU G. Soot formation during biomass gasification: A critical review[J]. Renewable Sustainable Energy Rev,2021,139:110710. doi: 10.1016/j.rser.2021.110710 [3] PATEL M, ZHANG X, KUMAR A. Techno-economic and life cycle assessment on lignocellulosic biomass thermochemical conversion technologies: A review[J]. Renewable Sustainable Energy Rev,2016,53:1486−1499. doi: 10.1016/j.rser.2015.09.070 [4] HE Q, DING L, RAHEEM A, GUO Q, GONG Y, YU G. Kinetics comparison and insight into structure-performance correlation for leached biochar gasification[J]. Chem Eng J,2021,417:129331. doi: 10.1016/j.cej.2021.129331 [5] ARRIOLA E, CHEN W-H, CHIH Y-K, DE LUNA M D, SHOW P L. Impact of post-torrefaction process on biochar formation from wood pellets and self-heating phenomena for production safety[J]. Energy,2020,207:118324. [6] HE Q, DING L, GONG Y, LI W, WEI J, YU G. Effect of torrefaction on pinewood pyrolysis kinetics and thermal behavior using thermogravimetric analysis[J]. Bioresour Technol,2019,280:104−111. doi: 10.1016/j.biortech.2019.01.138 [7] ZHANG S, CHEN T, XIONG Y, DONG Q. Effects of wet torrefaction on the physicochemical properties and pyrolysis product properties of rice husk[J]. Energy Convers Manage,2017,141:403−409. doi: 10.1016/j.enconman.2016.10.002 [8] ZHUANG X, SONG Y, ZHAN H, YIN X, WU C. Gasification performance of biowaste-derived hydrochar: The properties of products and the conversion processes[J]. Fuel,2020,260:116320. [9] OLSZEWSKI M P, NICOLAE S A, ARAUZO P J, TITIRICI M-M, KRUSE A. Wet and dry? Influence of hydrothermal carbonization on the pyrolysis of spent grains[J]. J Clean Prod,2020,260:121101. [10] HE Y, CHANG C, LI P, HAN X, LI H, FANG S, CHEN J, MA X. Thermal decomposition and kinetics of coal and fermented cornstalk using thermogravimetric analysis[J]. Bioresour Technol,2018,259:294−303. doi: 10.1016/j.biortech.2018.03.043 [11] CHEN C, MA X, HE Y. Co-pyrolysis characteristics of microalgae Chlorella vulgaris and coal through TGA[J]. Bioresour Technol,2012,117:264−273. doi: 10.1016/j.biortech.2012.04.077 [12] HU Q, TANG Z, YAO D, YANG H, SHAO J, CHEN H. Thermal behavior, kinetics and gas evolution characteristics for the co-pyrolysis of real-world plastic and tyre wastes[J]. J Clean Prod,2020,260:121102. [13] WANG C, BI H, LIN Q, JIANG X, JIANG C. Co-pyrolysis of sewage sludge and rice husk by TG-FTIR-MS: Pyrolysis behavior, kinetics, and condensable/non-condensable gases characteristics[J]. Renewable Energy,2020,160:1048−1066. doi: 10.1016/j.renene.2020.07.046 [14] LIU X, BURRA K G, WANG Z, LI J, CHE D, GUPTA A K. On deconvolution for understanding synergistic effects in co-pyrolysis of pinewood and polypropylene[J]. Appl Energy,2020,279:115811. [15] TONG S, SUN Y, LI X, HU Z, WORASUWANNARAK N, LIU H, HU H, LUO G, YAO H. Gas-pressurized torrefaction of biomass wastes: Co-gasification of gas-pressurized torrefied biomass with coal[J]. Bioresour Technol,2021,321:124505. [16] DING G, HE B, YAO H, KUANG Y, SONG J, SU L. Synergistic effect, kinetic and thermodynamics parameters analyses of co-gasification of municipal solid waste and bituminous coal with CO2[J]. Waste Manage,2021,119:342−355. doi: 10.1016/j.wasman.2020.10.028 [17] MERDUN H, LAOUGÉ Z B. Kinetic and thermodynamic analyses during co-pyrolysis of greenhouse wastes and coal by TGA[J]. Renewable Energy,2021,163:453−464. doi: 10.1016/j.renene.2020.08.120 [18] WU Z, ZHANG J, ZHANG B, GUO W, YANG G, YANG B. Synergistic effects from co-pyrolysis of lignocellulosic biomass main component with low-rank coal: Online and offline analysis on products distribution and kinetic characteristics[J]. Appl Energy,2020,276:115461. [19] CHEN X, LIU L, ZHANG L, ZHAO Y, QIU P. Gasification reactivity of co-pyrolysis char from coal blended with corn stalks[J]. Bioresour Technol,2019,279:243−251. doi: 10.1016/j.biortech.2019.01.108 [20] HE Q, RAHEEM A, DING L, XU J, CHENG C, YU G. Combining wet torrefaction and pyrolysis for woody biochar upgradation and structural modification[J]. Energy Convers Manage,2021,243:114383. doi: 10.1016/j.enconman.2021.114383 [21] VYAZOVKIN S, BURNHAM A K, CRIADO J M, PéREZ-MAQUEDA L A, POPESCU C, SBIRRAZZUOLI N. ICTAC Kinetics Committee recommendations for performing kinetic computations on thermal analysis data[J]. Thermochim Acta,2011,520(1/2):1−19. doi: 10.1016/j.tca.2011.03.034 [22] ALMAZROUEI M, JANAJREH I. Model-fitting approach to kinetic analysis of non-isothermal pyrolysis of pure and crude glycerol[J]. Renewable Energy,2020,145:1693−1708. doi: 10.1016/j.renene.2019.07.095 [23] ŠESTÁK J, BERGGREN G. Study of the kinetics of the mechanism of solid-state reactions at increasing temperatures[J]. Thermochim Acta,1971,3(1):1−12. doi: 10.1016/0040-6031(71)85051-7 [24] TIAN X, DAI L, WANG Y, ZENG Z, ZHANG S, JIANG L, YANG X, YUE L, LIU Y, RUAN R. Influence of torrefaction pretreatment on corncobs: A study on fundamental characteristics, thermal behavior, and kinetic[J]. Bioresour Technol,2020,297:122490. doi: 10.1016/j.biortech.2019.122490 [25] MISHRA R K, MOHANTY K. Pyrolysis kinetics and thermal behavior of waste sawdust biomass using thermogravimetric analysis[J]. Bioresour Technol,2018,251:63−74. doi: 10.1016/j.biortech.2017.12.029 [26] ANCA-COUCE A, OBERNBERGER I. Application of a detailed biomass pyrolysis kinetic scheme to hardwood and softwood torrefaction[J]. Fuel,2016,167:158−167. doi: 10.1016/j.fuel.2015.11.062 [27] ZHAO H, LI Y, SONG Q, LIU S, YAN J, WANG X, MA Q, SHU X. Investigation on the physicochemical structure and gasification reactivity of nascent pyrolysis and gasification char prepared in the entrained flow reactor[J]. Fuel,2019,240:126−137. doi: 10.1016/j.fuel.2018.11.145 [28] WANG G, ZHANG J, SHAO J, LIU Z, WANG H, LI X, ZHANG P, GENG W, ZHANG G. Experimental and modeling studies on CO2 gasification of biomass chars[J]. Energy,2016,114:143−154. doi: 10.1016/j.energy.2016.08.002 [29] HE Q, YU J, SONG X, DING L, WEI J, YU G. Utilization of biomass ash for upgrading petroleum coke gasification: Effect of soluble and insoluble components[J]. Energy,2020,192:116642. doi: 10.1016/j.energy.2019.116642 [30] LIU J, JIANG X, SHEN J, ZHANG H. Pyrolysis of superfine pulverized coal. Part 1. Mechanisms of methane formation[J]. Energy Convers Manage,2014,87:1027−1038. doi: 10.1016/j.enconman.2014.07.053 [31] CHEN T, DAI R, YELLEZUOME D, ZHANG K, ZHAO R, LIU G, WU J. Effect of density on physicochemical and thermal conversion characteristic of Naomaohu coal[J]. Fuel,2021,284:119045. [32] YANG P, ZHAO S, ZHANG Q, HU J, LIU R, HUANG Z, GAO Y. Synergistic effect of the cotton stalk and high-ash coal on gas production during co-pyrolysis/gasification[J]. Bioresour Technol,2021,336:125336. doi: 10.1016/j.biortech.2021.125336 [33] NZIHOU A, STANMORE B, SHARROCK P. A review of catalysts for the gasification of biomass char, with some reference to coal[J]. Energy,2013,58:305−317. doi: 10.1016/j.energy.2013.05.057 [34] HU S, JESS A, XU M. Kinetic study of Chinese biomass slow pyrolysis: Comparison of different kinetic models[J]. Fuel,2007,86(17):2778−2788. [35] SONG H, LIU G, ZHANG J, WU J. Pyrolysis characteristics and kinetics of low rank coals by TG-FTIR method[J]. Fuel Process Technol,2017,156:454−460. doi: 10.1016/j.fuproc.2016.10.008 [36] REN S, LEI H, WANG L, BU Q, CHEN S, WU J. Thermal behaviour and kinetic study for woody biomass torrefaction and torrefied biomass pyrolysis by TGA[J]. Biosystems Eng,2013,116(4):420−426. doi: 10.1016/j.biosystemseng.2013.10.003 [37] MA Z, CHEN D, GU J, BAO B, ZHANG Q. Determination of pyrolysis characteristics and kinetics of palm kernel shell using TGA-FTIR and model-free integral methods[J]. Energy Convers Manage,2015,89:251−259. doi: 10.1016/j.enconman.2014.09.074 [38] GÓMEZ-BAREA A, OLLERO P, FERNÁNDEZ-BACO C. Diffusional effects in CO2 gasification experiments with single biomass char particles. 1. Experimental investigation[J]. Energy Fuels,2006,20(5):2202−2210. doi: 10.1021/ef050365a [39] OLLERO P, SERRERA A, ARJONA R, ALCANTARILLA S. Diffusional effects in TGA gasification experiments for kinetic determination[J]. Fuel,2002,81(15):1989−2000. doi: 10.1016/S0016-2361(02)00126-6 -





 下载:
下载: