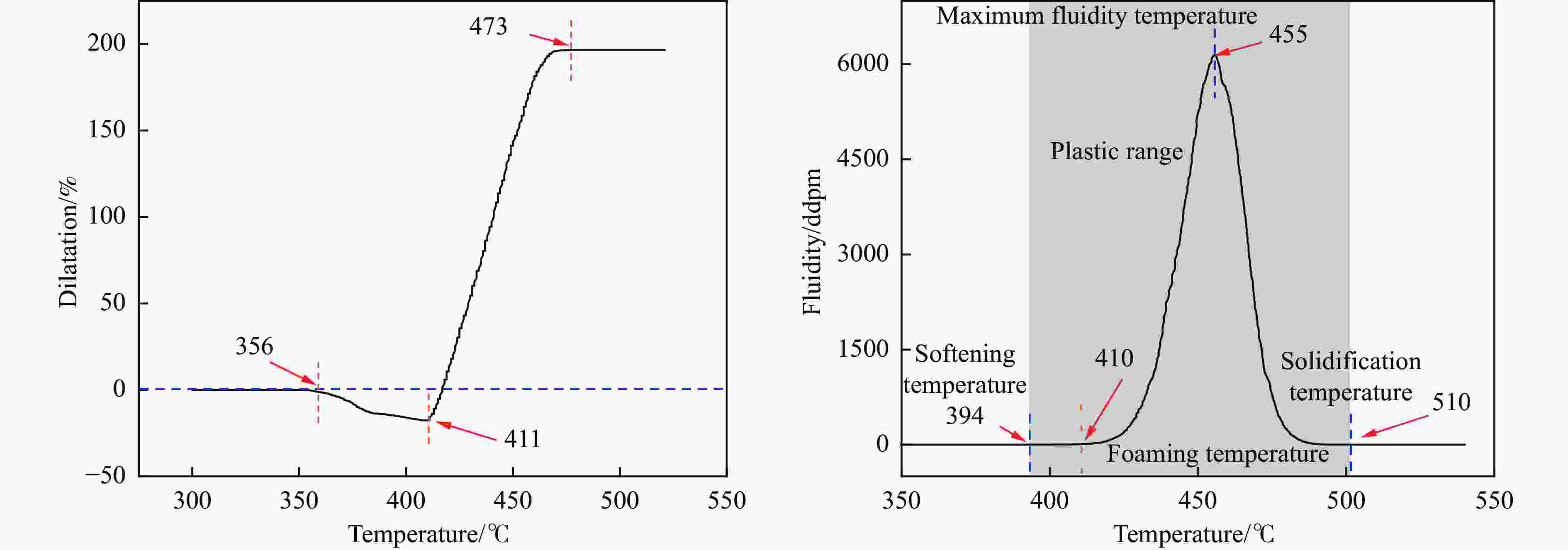
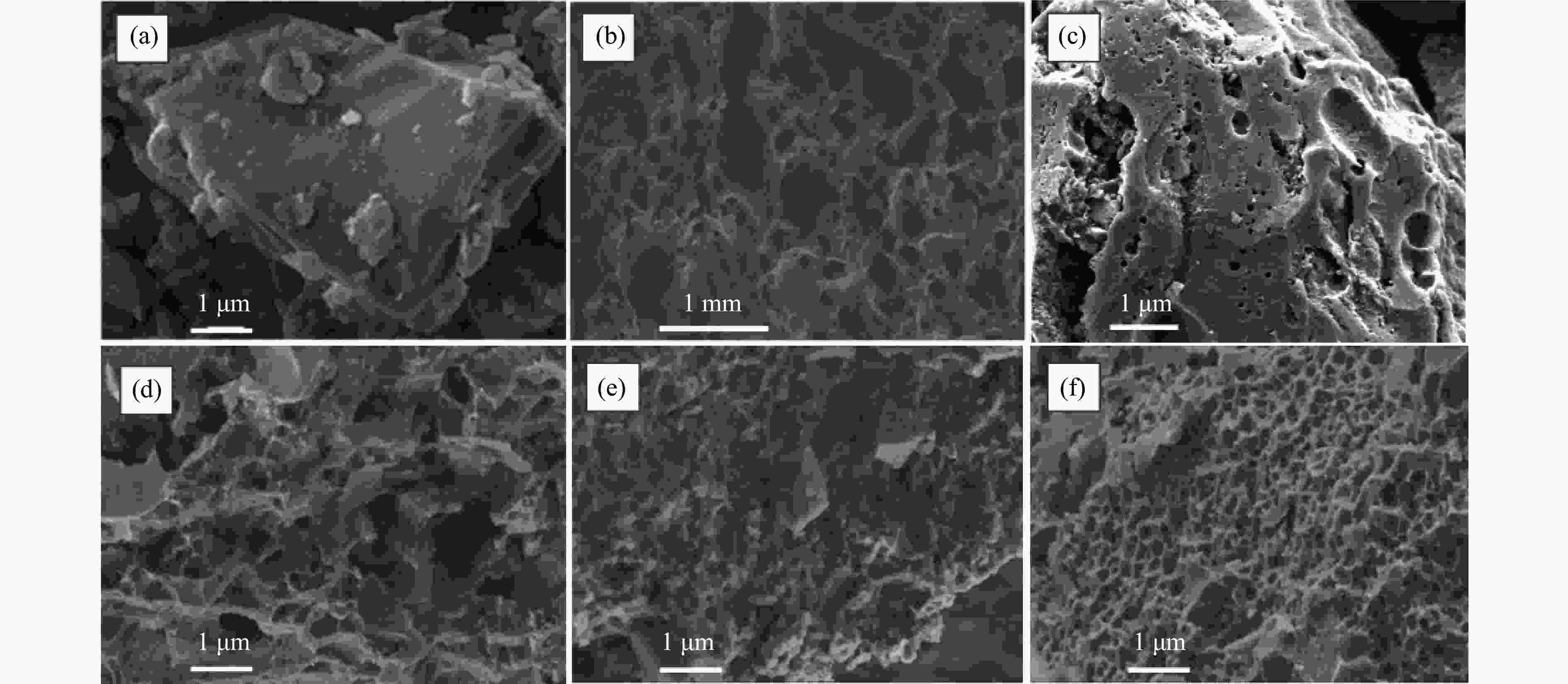
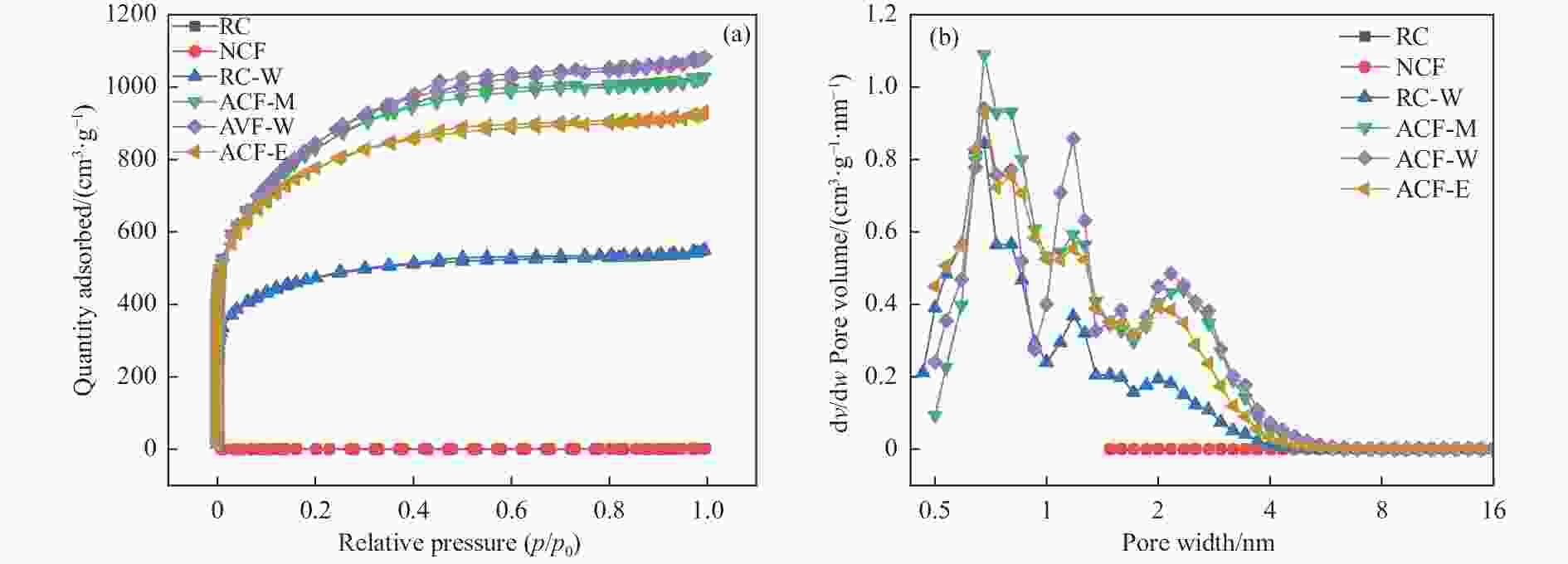
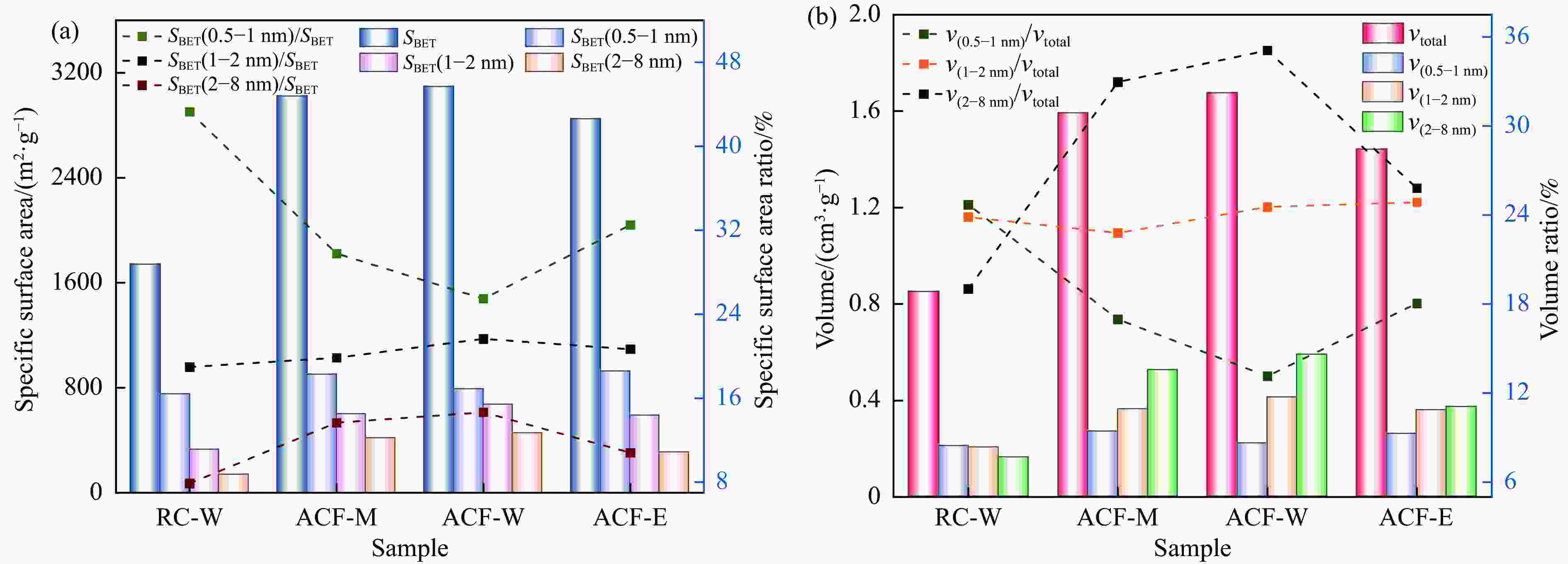
Effect of KOH addition on electrochemical properties of coal-based active carbon foams
-
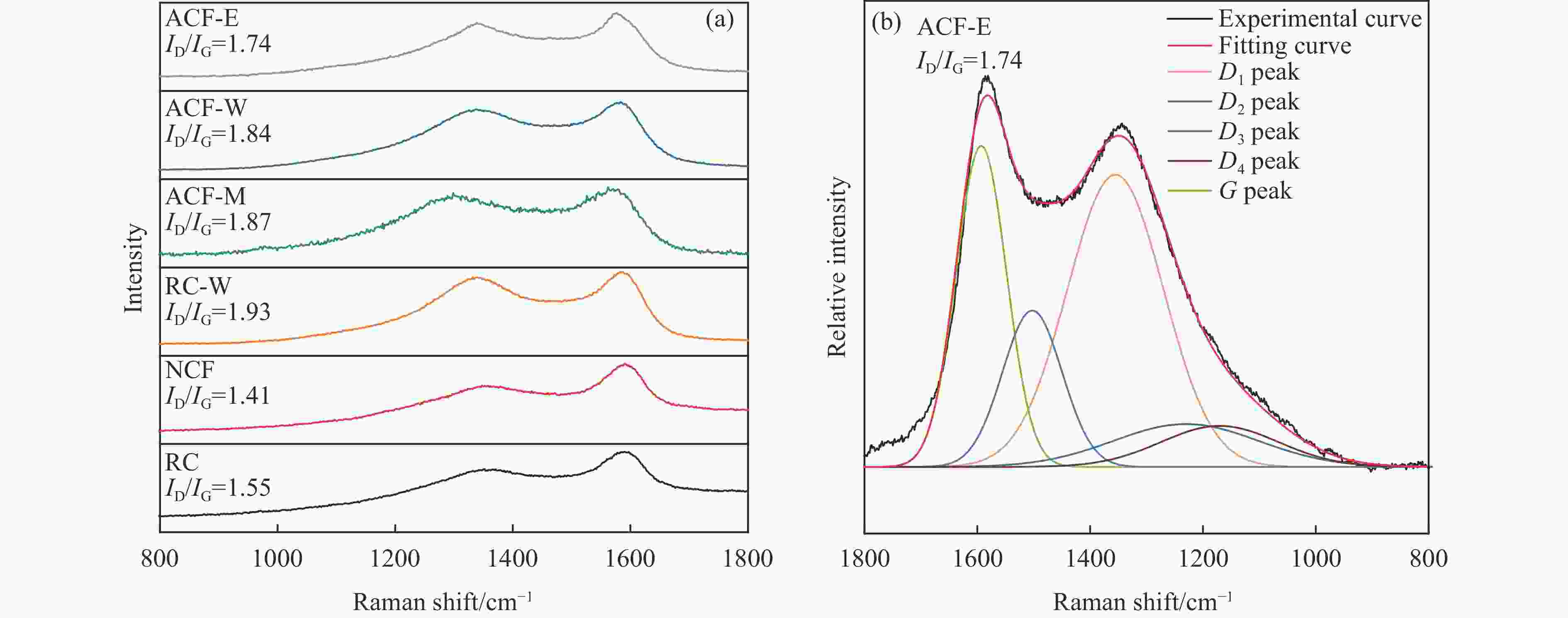
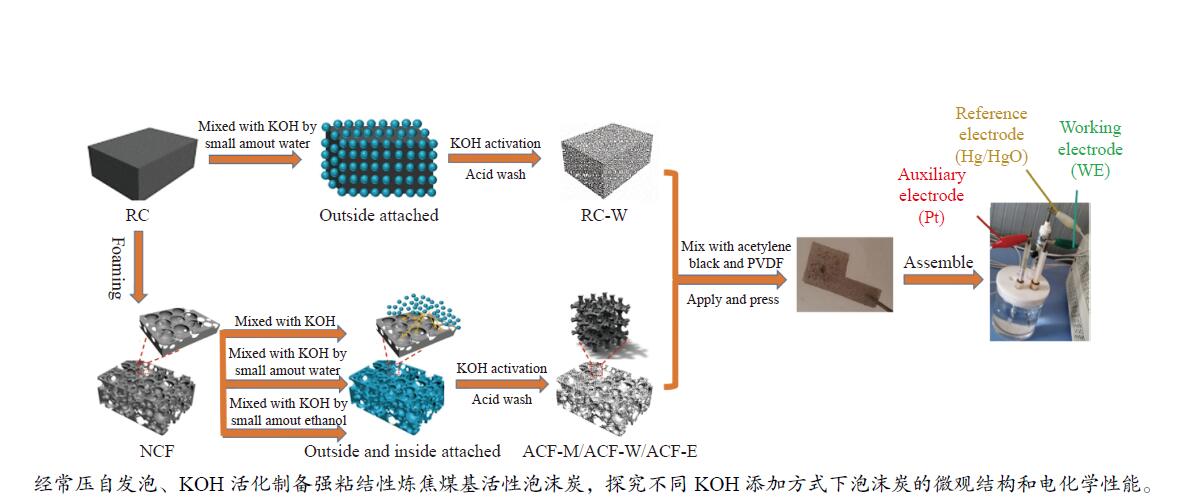
摘要: 以强黏性炼焦煤为原料,经常压自发泡法制得的煤基泡沫炭(NCF)为碳基底,KOH为活化剂,采用机械混合、水溶液浸渍、乙醇浸渍三种不同的方式制备煤基活性泡沫炭(HPCs),并将其用作双电层电容器的电极材料,研究了KOH添加方式对其微观结构和电化学性能的影响。结果表明,KOH分散度和附着性对煤基活性泡沫炭孔隙结构的生成、晶体结构、表面化学性质以及电化学性能有显著影响。煤基泡沫炭本身具有三维连通泡孔结构,有利于活化剂(KOH)深入材料的泡孔内部并为其提供大量附着位点,增大活化剂与碳基体的接触面积进而发生高效的活化。KOH水溶液的流动性较好,可以使K+更有效地穿插在NCF的泡孔结构中,在活化过程中作用于缺陷部位,在碳基体内部基质上产生更多的微孔以及介孔结构,有效地放大了活化效果。KOH水溶液浸渍泡沫炭材料制得的ACF-W样品拥有最高的比表面积(3098.35 m2/g)、总孔体积(1.68 cm3/g)、介孔体积比(59.13%),将其用作电极材料表现出优异的比电容(310 F/g)以及循环稳定性。Abstract: Using strong-caking coking coal as raw material, coal-based carbon foam (NCF) was prepared by constant pressing and self-foaming method and used as carbon base to produce coal-based active carbon foamed (HPCs) together with KOH activator, which was used as electrode material for double-layer capacitor. The effects of KOH added by mechanical mixing, aqueous solution impregnation and ethanol solution impregnation methods on microstructure and electrochemical properties of the prepared materials were studied. The results show that formation of pore structure, crystal structure, surface chemistry and electrochemical performance of HPCs are significantly affected by KOH dispersion and adhesion. The NCF itself has a three-dimensional connected bubble pore structure, which is conducive to the activator (KOH) penetrating into the bubble pore and providing a large number of attachment sites, thus increasing the contact area between the activator and the carbon matrix and resulting in efficient activation. The good fluidity of KOH solution can make K+ more effectively interspersed in the bubble structure of NCF, act on the defect site during activation, and generate more micropores and mesoporous structures on the internal matrix of carbon matrix, effectively amplifying the activation effect. ACF-W obtained by KOH aqueous impregnation has the highest specific surface area (3098.35 m2/g), total pore volume (1.68 cm3/g), mesoporous volume ratio (59.13%). It shows excellent specific capacitance (310 F/g) and cycle stability when used as electrode material.
-
Key words:
- carbon foam /
- KOH addition method /
- double layer capacitor /
- electrochemical performance
-
图 8 (a)样品的XPS全谱;(b)ACF-M、ACF-W、ACF-E的C 1s XPS分峰拟合谱图;(c)RC-W的C 1s XPS分峰拟合谱图;(d)ACF-M、ACF-W、ACF-E的O 1s XPS分峰拟合谱图;(e)RC-W的O 1s XPS分峰拟合谱图;(f)FT-IR谱图
Figure 8 (a) XPS survey spectra of samples; (b) Deconvolution C 1s XPS spectra of ACF-M, ACF-W, ACF-E; (c) Deconvolution C 1s XPS spectra of RC-W; (d) Deconvolution O 1s XPS spectra of ACF-M, ACF-W, ACF-E; (e) Deconvolution O 1s XPS spectra of RC-W; (f) FT-IR spectra
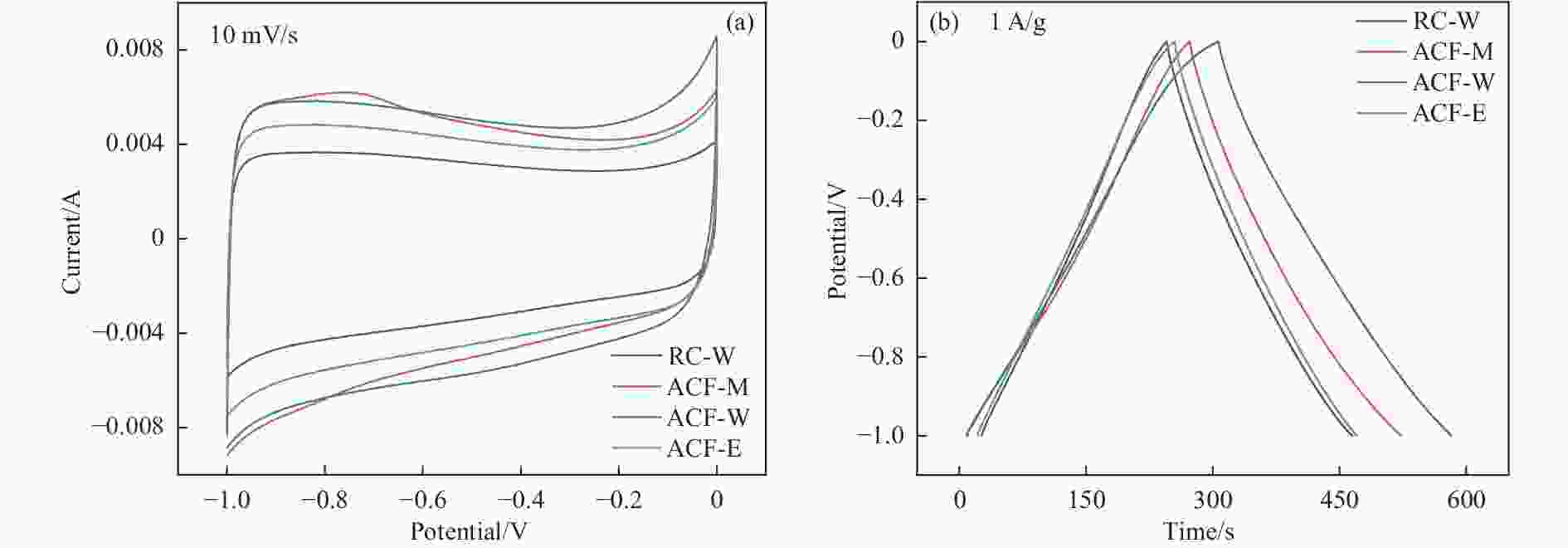
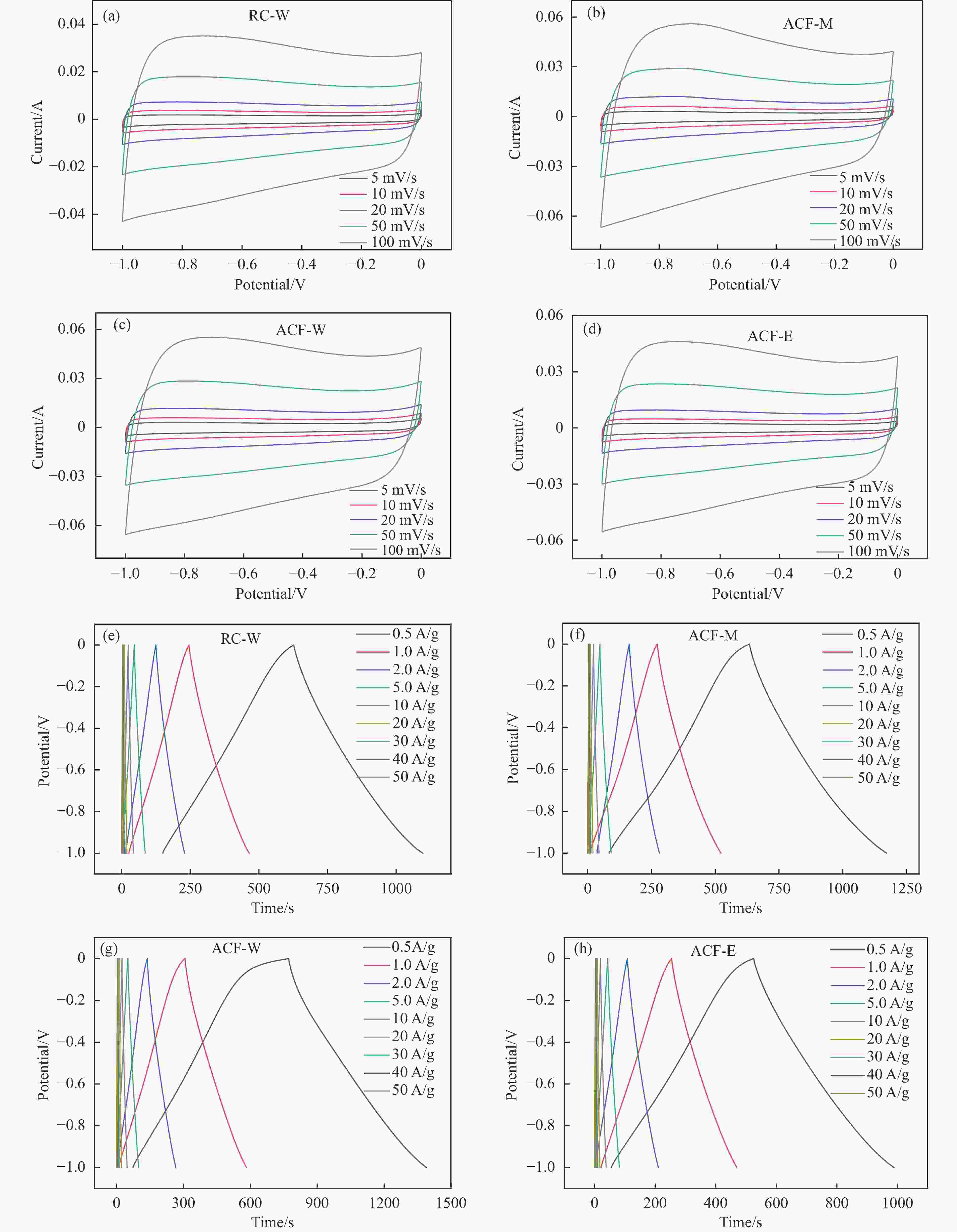
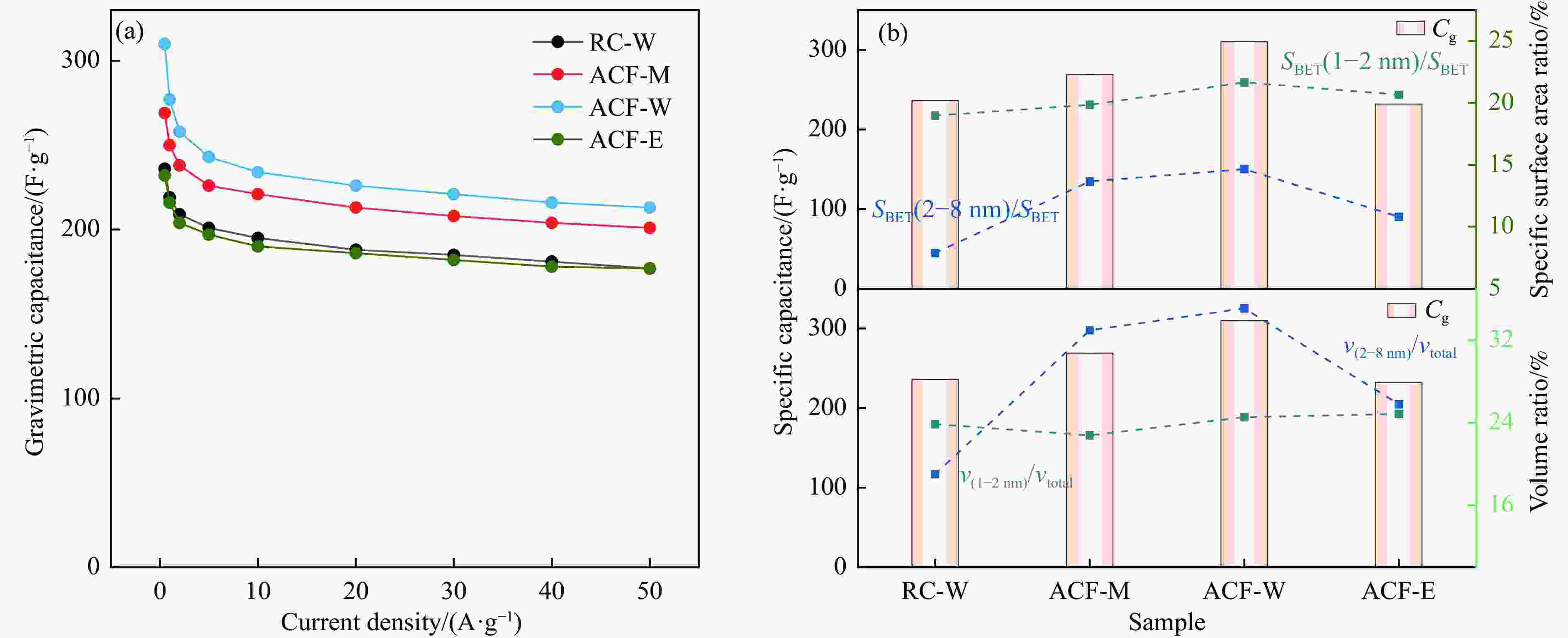
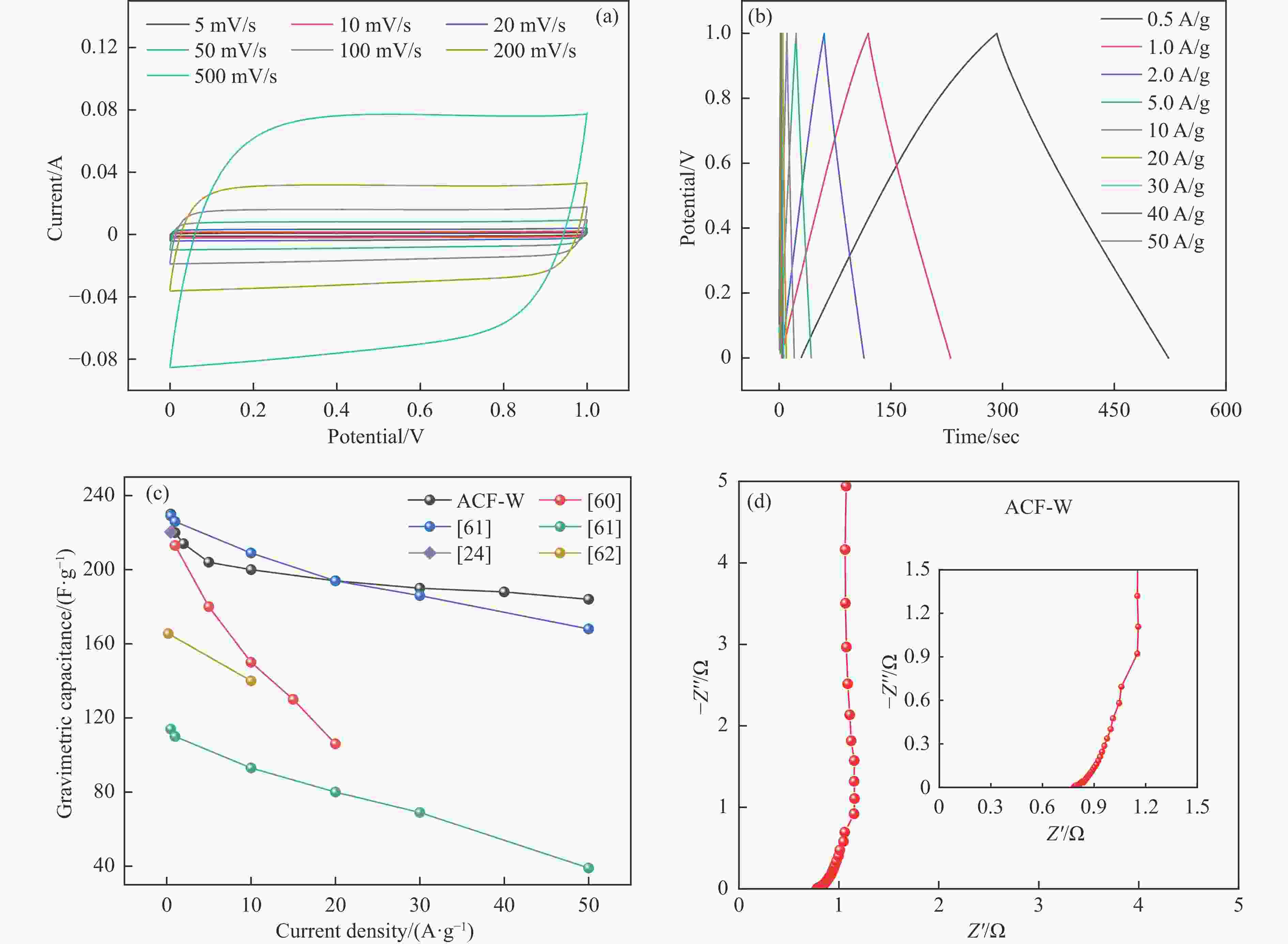
图 10 三电极体系下HPCs电极的电化学性能((a)−(d)在5−100 mV/s扫描速率下的CV曲线;(e)−(h)在0.5−50 A/g电流密度下的GCD曲线)
Figure 10 Electrochemical performances of HPC as supercapacitor electrode in the three-electrode configuration ((a)−(d) CV curves at the scan rates of 5−100 mV/s; (e)−(h) GCD curves at different current densities of 0.5−50 A/g)
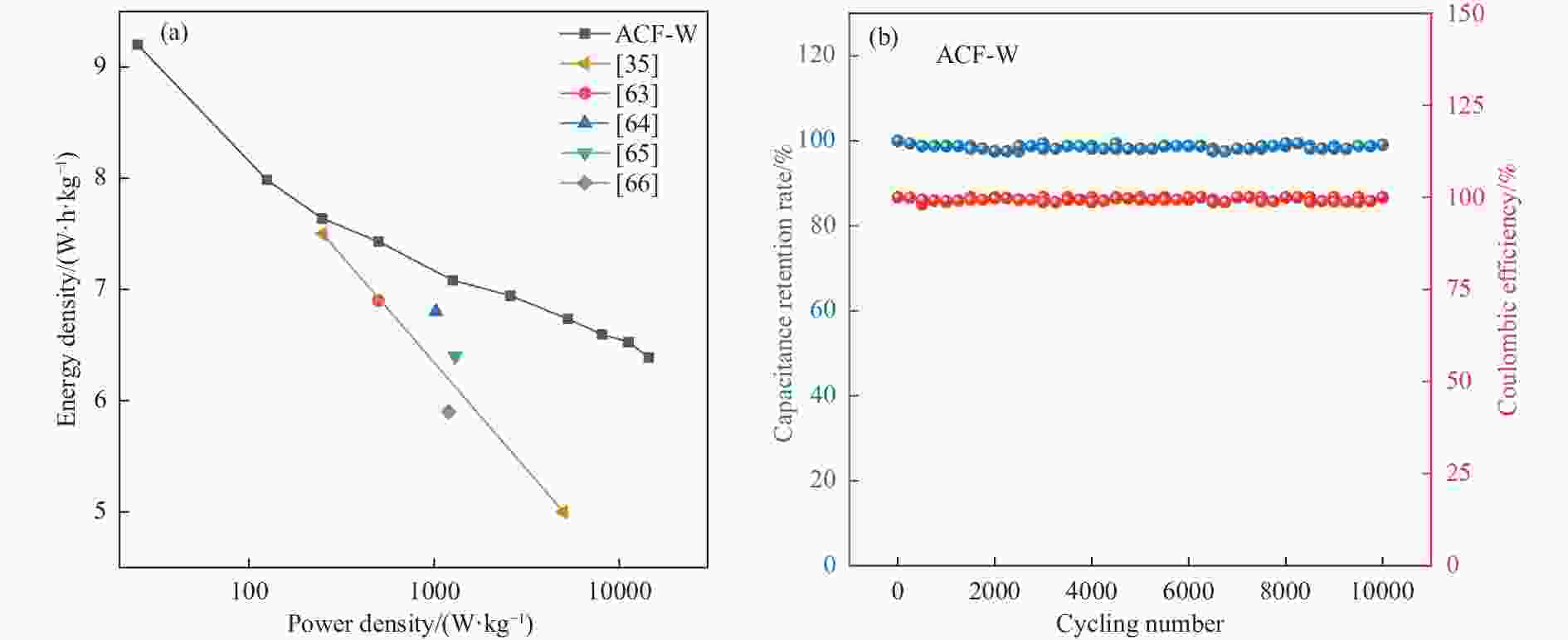
图 13 两电极体系下ACF-W作为超级电容器电极的电化学性能((a)在5−500 mV/s扫描速率下的CV曲线;(b)在0.5−50 A/g电流密度下的GCD曲线;(c)比电容与电流密度的关系;(d)Nyquist图)
Figure 13 Electrochemical performances of ACF-Ws as supercapacitor electrode in the two-electrode configuration ((a) CV curves at the scan rates of 5−500 mV/s; (b) GCD curves at different current densities of 0.5−50 A/g; (c) Dependence of specific capacitance (Cg) on various current densities; (d) Nyquist plots)
表 1 原料煤的煤质分析
Table 1 Proximate and ultimate analyses of coal
Proximate analysis w/% Ultimate analysis wdaf/% G Mad Ad Vdaf FCdaf C H N O* S 0.63 10.52 29.18 70.82 77.85 4.48 1.36 15.17 1.14 95 Note: ad is air-dried basis; d is dry basis; daf is dried and ash-free basis; G is caking index;
*: by difference.表 2 不同样品的孔结构
Table 2 Pore structure parameters of different samples
Sample SBET/
(m2·g−1)Smic/
(m2·g−1)vtotal/
(cm3·g−1)vmic/
(cm3·g−1)vmes/
(cm3·g−1)vmes/vtotal dave/
nmYield/% RC 2.23 1.21 3.14 × 10−3 4.79 × 10−4 2.66 × 10−3 84.76 5.65 − NCF 1.43 0.99 1.54 × 10−3 3.56 × 10−4 1.18 × 10−3 76.87 4.31 − RC-W 1739.32 1419.21 0.85 0.61 0.24 28.43 1.96 36.23 ACF-M 3023.46 1755.84 1.59 0.75 0.84 52.56 2.11 48.26 ACF-W 3098.35 1628.27 1.68 0.68 0.99 59.13 2.16 50.50 ACF-E 2850.44 1921.94 1.44 0.81 0.63 43.74 2.02 50.75 表 3 不同样品的孔隙结构特征
Table 3 Pore structure characteristics of different samples
Sample SBET(0.5−1 nm)/
(m2·g−1)SBET(1−2 nm)/
(m2·g−1)SBET(2−8 nm)/
(m2·g−1)v(0.5−1 nm)/
(cm3·g−1)v(1−2 nm)/
(cm3·g−1)v(2−8 nm)/
(cm3·g−1)RC-W 752.89 329.77 137.33 0.21 0.20 0.16 ACF-M 900.24 600.44 412.97 0.27 0.36 0.52 ACF-W 789.09 670.78 454.46 0.22 0.41 0.59 ACF-E 926.41 588.60 308.11 0.26 0.36 0.37 表 4 KOH水溶液与KOH乙醇溶液的黏度对比
Table 4 Viscosity comparison between KOH aqueous solution and KOH ethanol solution
Sample KOH aqueous solution KOH ethanol solution Viscosity/(MPa·s) 0.53 1.58 表 5 样品的微晶结构参数
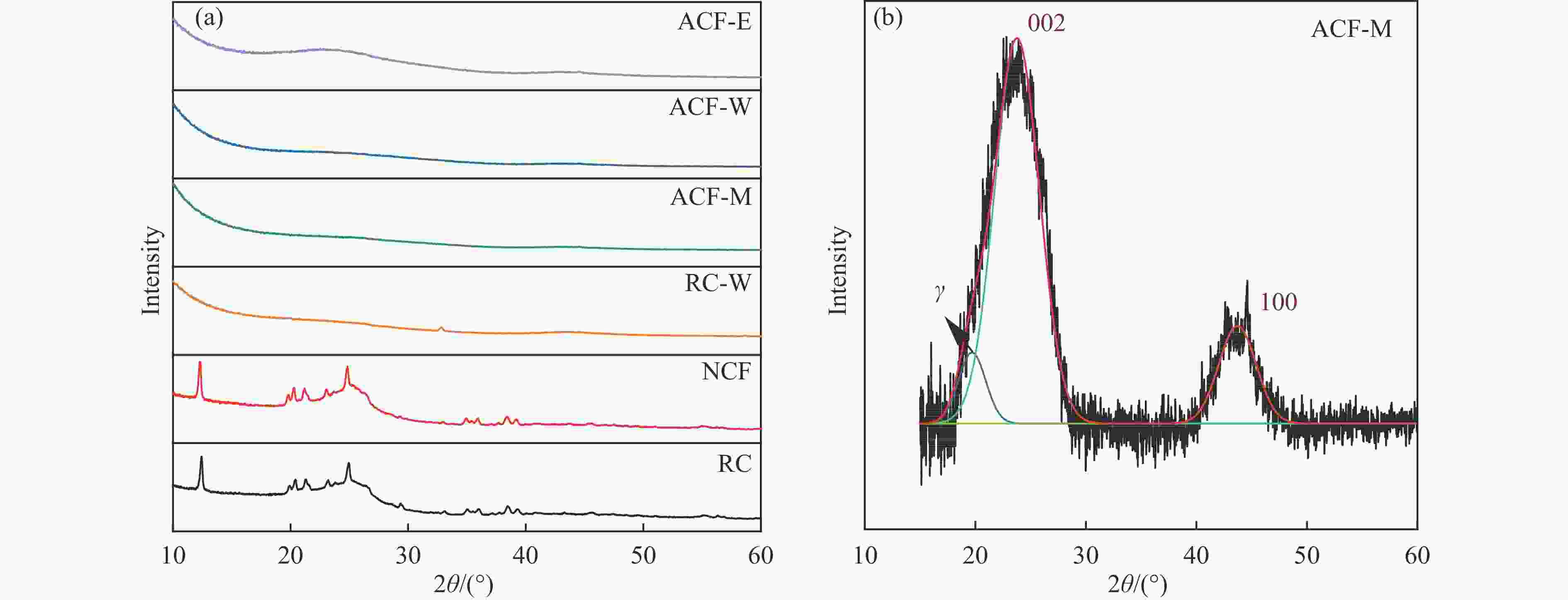
Table 5 Crystal structure parameters of samples
Sample Aγ 2θ002/(°) 2θ100/(°) A002 d002/nm La/nm Lc/nm fa N RC 5036.69 25.21 43.92 11133.75 0.3531 2.02 2.55 0.6885 7.21 NCF 3957.89 25.19 43.89 10925.60 0.3535 2.23 2.81 0.7341 7.94 RC-W 443.75 25.71 43.75 1842.42 0.3464 3.58 1.49 0.8059 4.31 ACF-M 174.69 25.88 43.50 1045.83 0.3443 3.67 1.56 0.8569 4.54 ACF-W 188.38 25.39 43.03 1232.29 0.3507 3.79 1.58 0.8674 4.51 ACF-E 416.79 23.77 43.71 3702.12 0.3744 4.39 1.70 0.8988 4.55 表 6 XPS光谱所得样品表面元素相对含量
Table 6 Related concentration of the chemical element on the surface of samples from XPS spectra
Sample Concentration of chemical element/% C O N S RC 75.02 21.48 2.74 0.76 NCF 90.60 6.48 2.16 0.77 RC-W 86.26 12.34 1.11 0.30 ACF-M 90.43 8.01 1.36 0.20 ACF-W 87.44 11.40 0.96 0.20 ACF-E 87.64 10.86 1.13 0.37 表 7 样品的C 1s和O 1s光谱分峰拟合
Table 7 Contribution of the components in the area of C 1s and O 1s XPS spectra
Sample Relative content of different types of
oxygen on the surface
/%Relative content of different types of
carbon on the surface
/%C=O IO C−O sp2-C sp3-C C−O C=O RC 32.79 2.75 64.46 61.92 12.30 3.73 2.44 NCF 39.10 3.43 57.48 84.66 9.07 3.61 2.66 RC-W 68.03 2.41 29.56 57.59 26.61 7.57 8.23 ACF-M 80.99 − 19.01 60.41 24.03 7.02 8.53 ACF-W 74.82 − 25.18 61.27 22.92 6.87 8.93 ACF-E 77.34 − 22.66 61.85 22.54 6.91 8.70 表 8 三电极体系中不同炭材料比容量(Cg)对比
Table 8 Specific capacitance (Cg) comparison of different carbon materials under the three-electrode configuration
Precursor SBET/
(m2·g−1)Current density/
(A·g−1)Cg/
(F·g−1)Electrolyte Ref. Bituminous coal 3098.4 0.5 310 6 mol/L KOH this work Agricultural wastes 952.0 1.0 160 6 mol/L KOH [38] Pitaya peel 1872.0 1.0 255 6 mol/L KOH [51] Lignite 2728.0 1.0 246 6 mol/L KOH [52] Bituminous coal 2784.0 0.5 293 6 mol/L KOH [17] Coal 877.0 1.0 260 6 mol/L KOH [53] Zhundong coal 1872.0 1.0 211 6 mol/L KOH [35] Taixi anthracite 984.6 1.0 199 6 mol/L KOH [22] Anthracite coal 2455.0 0.05 104 6 mol/L KOH [54] Qitaihe bituminous 2985.9 1.0 194 6 mol/L KOH [55] -
[1] ZHONG C, DENG Y D, HU W B, et al. A review of electrolyte materials and compositions for electrochemical supercapacitors[J]. Chem Soc Rev,2015,44(21):7484−7539. doi: 10.1039/C5CS00303B [2] LU X H, YU M H, WANG G M, et al. Flexible solid-state supercapacitors: Design, fabrication and applications[J]. Energy Environ Sci,2014,7(7):2160−2181. doi: 10.1039/c4ee00960f [3] ABIOYE A M, ANI F N. Recent development in the production of activated carbon electrodes from agricultural waste biomass for supercapacitors: a review[J]. Renewable Sustainable Energy Rev,2015,52:1282−1293. doi: 10.1016/j.rser.2015.07.129 [4] ZHANG Q, HAN K H, LI S J, et al. Synthesis of garlic skin-derived 3D hierarchical porous carbon for high-performance supercapacitors[J]. Nanoscale,2018,10(5):2427−2437. doi: 10.1039/C7NR07158B [5] LI X Y, LIU K T, LIU Z Z, et al. Hierarchical porous carbon from hazardous waste oily sludge for all-solid-state flexible supercapacitor[J]. Electrochim Acta,2017,240:43−52. doi: 10.1016/j.electacta.2017.04.061 [6] SIMON P, GOGOTSI Y, DUNN B. Where do batteries and supercapacitors begin?[J]. Science,2014,343(6176):1210−1211. doi: 10.1126/science.1249625 [7] YAN J, WANG Q, WEI T, et al. Recent advances in design and fabrication of electrochemical supercapacitors with high energy densities[J]. Adv Energy Mater,2014,4(4):1300816. doi: 10.1002/aenm.201300816 [8] BURKE A. Ultracapacitors: Why, how, and where is the technology[J]. J Power Sources,2000,91(1):37−50. doi: 10.1016/S0378-7753(00)00485-7 [9] LONG C L, WEI T, YAN J, et al. Supercapacitors based on graphere-supported iron nanosheets as negative electrode materials[J]. ACS Nano,2013,7(12):11325−11332. doi: 10.1021/nn405192s [10] CHEN L F, LU Y, YU L, et al. Designed formation of hollow particle-based nitrogen-doped carbon nanofibers for high-performance supercapacitors[J]. Energy Environ Sci,2017,10(8):1777−1783. doi: 10.1039/C7EE00488E [11] XIAO Z Y, MEI Y J, YUAN S, et al. Controlled hydrolysis of metal-organic frameworks: Hierarchical Ni/Co-layered double hydroxide microspheres for high-performance supercapacitors[J]. ACS Nano,2019,13(6):7024−7030. doi: 10.1021/acsnano.9b02106 [12] 侯彩霞, 孔碧华, 樊丽华, 等. 超级电容器用煤基活性炭研究[J]. 洁净煤技术,2017,23(5):56−61. doi: 10.13226/j.issn.1006-6772.2017.05.011HOU Caixia, KONG Bihua, FAN Lihua, et al. Study on coal-based activated carbon for supercapacitor[J]. Clean Coal Technol,2017,23(5):56−61. doi: 10.13226/j.issn.1006-6772.2017.05.011 [13] YANG H, KANNAPPAN S, PANDIAN A S, et al. Graphene supercapacitor with both high power and energy density[J]. Nanotechnology,2017,28(44):445401. doi: 10.1088/1361-6528/aa8948 [14] ZHAI Y P, DOU Y Q, ZHAO D Y, et al. Carbon materials for chemical capacitive energy storage[J]. Adv Mater,2011,23(42):4828−4850. doi: 10.1002/adma.201100984 [15] 陈航. 煤基活性泡沫炭制备及用于超级电容器性能研究[D]. 北京: 中国矿业大学, 2016.CHEN Hang. Study on preparation of coal-based active carbon foam and performance of supercapacitor[D]. Beijing: China University of Mining and Technology, 2016. [16] CHONG L W, WONG Y W, RAJKUMAR R K, et al. An adaptive learning control strategy for standalone PV system with battery-supercapacitor hybrid energy storage system[J]. J Power Sources,2018,394:35−49. doi: 10.1016/j.jpowsour.2018.05.041 [17] 刘宇昊. 煤基活性炭的制备及其电化学性能研究[D]. 河南: 河南理工大学, 2020.LIU Yuhao. Study on preparation and electrochemical properties of coal-based activated carbon[D]. Henan: Henan Polytechnic University, 2020. [18] DONG D, ZHANG Y S, XIAO Y, et al. High performance aqueous supercapacitor based on nitrogen-doped coal-based activated carbon electrode materials[J]. J Colloid Interf Sci,2020,580:77−87. doi: 10.1016/j.jcis.2020.07.018 [19] SHI M, XIN Y F, CHEN X X, et al. Coal-derived porous activated carbon with ultrahigh specific surface area and excellent electrochemical performance for supercapacitors[J]. J Alloy Compd,2021,859:157856. doi: 10.1016/j.jallcom.2020.157856 [20] YUE X M, AN Z Y, YE M, et al. Preparation of porous activated carbons for high performance supercapacitors from Taixi anthracite by multi-stage activation[J]. Molecules,2019,24(19):3588. doi: 10.3390/molecules24193588 [21] JIANG Y T, JIANG Z M, SHI M J, et al. Enabling high surface and space utilization of activated carbon for supercapacitors by homogeneous activation[J]. Carbon,2021,182:559−563. doi: 10.1016/j.carbon.2021.06.039 [22] 王勋, 曾丹林, 陈诗渊, 等. 生物质活性炭的研究进展[J]. 化工新型材料,2018,46(6):27−30.WANG Xun, ZENG Danlin, CHEN Shiyuan, et al. Research progress of biomass activated carbon[J]. New Chem Mater,2018,46(6):27−30. [23] 高奇, 项洪中, 倪良萌, 等. 超级电容器用生物质基活性炭电极材料研究进展[J]. 化工新型材料,2022,50(3):12−17. doi: 10.19817/j.cnki.issn1006-3536.2022.03.003GAO Qi, XIANG Hongzhong, NI Liangmeng, et al. Research progress of biomass based activated carbon electrode materials for supercapacitors[J]. New Chem Mater,2022,50(3):12−17. doi: 10.19817/j.cnki.issn1006-3536.2022.03.003 [24] 吴雅俊. 物理化学活化法制备煤基活性炭及其电化学性能研究[D]. 北京: 中国矿业大学, 2018.WU Yajun. Preparation of coal-based activated carbon by physicochemical activation and its electrochemical properties[D]. Beijing: China University of Mining and Technology, 2018. [25] 刘俊科. 无灰煤基活性炭孔结构调控及其电化学性能[D]. 河北: 华北理工大学, 2020.LIU Junke. Pore structure regulation and electrochemical performance of ash-free coal-based activated carbon[D]. Hebei: North China University of Science and Technology, 2020. [26] ELMOUWAHIDI A, BAILÓN-GARCÍA E, PÉREZ-CADENAS A, et al. Activated carbons from KOH and H3PO4 activation of olive residues and its application as supercapacitor electrodes[J]. Electrochim Acta,2017,229:219−228. doi: 10.1016/j.electacta.2017.01.152 [27] MORENO-CASTILLA C, CARRASCO-MARÍN F, LÓPEZ-RAMÓN M V, et al. Chemical and physical activation of olive-mill waste to produce activated carbons[J]. Carbon,2001,39(9):1415−1420. doi: 10.1016/S0008-6223(00)00268-2 [28] UBAGO-PÉREZ R, CARRASCO-MARÍN F, FAIRÉN-JIMÉNEZ D, MORENO-CASTILLA C. Granular and monolithic activated carbons from KOH-activation of olive stones[J]. Microporous Mesoporous Mater,2006,92(1/3):64−70. [29] 王美君, 杨暖暖, 任秀蓉, 等. 基于常压自发泡制备煤基泡沫炭的方法: 中国, CN111547703A[P]. 2020-08-18.WANG Meijun, YANG Nuannuan, REN Xiurong, et al. Preparation of coal-based foam carbon based on atmospheric self-foaming: China, CN111547703A[P]. 2020-08-18. [30] LU H Y, LI Q W, GUO J H, et al. Hierarchically porous carbon with high-speed ion transport channels for high performance supercapacitors[J]. Appl Surf Sci,2018,427:992−999. doi: 10.1016/j.apsusc.2017.09.065 [31] 王海洋, 朱洪喆, 王守凯, 等. 煤沥青基多孔炭材料的研究进展[J]. 功能材料,2019,50(8):8032−8039. doi: 10.3969/j.issn.1001-9731.2019.08.006WANG Haiyang, ZHU Hongzhe, WANG Shoukai, et al. Research progress of coal bitumen based porous carbon materials[J]. J Funct Mater,2019,50(8):8032−8039. doi: 10.3969/j.issn.1001-9731.2019.08.006 [32] YANG N N, JI L, FU H C, et al. Hierarchical porous carbon derived from coal-based carbon foam for high-performance supercapacitors[J]. Chin Chem Lett,2022,33(8):3961−3967. doi: 10.1016/j.cclet.2022.03.037 [33] 刘冬冬. 不定型煤基活性炭结构演变机制及调控方法研究[D]. 哈尔滨: 哈尔滨工业大学, 2017.LIU Dongdong. Study on structure evolution mechanism and regulation method of amorphous coal-based activated carbon[D]. Harbin: Harbin Institute of Technology, 2017. [34] SONIBARE O O, HAEGER T, FOLEY S F. Structural characterization of nigerian coals by X-ray diffraction, Raman and FTIR spectroscopy[J]. Energy,2010,35(12):5347−5353. doi: 10.1016/j.energy.2010.07.025 [35] WANG L J, SUN F, GAO J H, et al. A novel melt infiltration method promoting porosity development of low-rank coal derived activated carbon as supercapacitor electrode materials[J]. J Taiwan Inst Chem E,2018,91:588−596. doi: 10.1016/j.jtice.2018.06.014 [36] ZHANG Y D, KANG X J, TAN J L, et al. Influence of calcination and acidification on structural characterization of Anyang anthracites[J]. Energy Fuels,2013,27(11):7191−7197. doi: 10.1021/ef401658p [37] SEKINE Y, ISHIKAWA K, KIKUCHI E, et al. Reactivity and structural change of coal char during steam gasification[J]. Fuel,2006,85(2):122−126. doi: 10.1016/j.fuel.2005.05.025 [38] WEI H G, WANG H, LI A, et al. Advanced porous hierarchical activated carbon derived from agricultural wastes toward high performance supercapacitors[J]. J Alloy Compd,2020,820:153111. doi: 10.1016/j.jallcom.2019.153111 [39] RAYMUNDO-PIÑERO E, LEROUX F, BÉGUIN F. A high-performance carbon for supercapacitors obtained by carbonization of a seaweed biopolymer[J]. Adv mater,2006,18(14):1877−1882. doi: 10.1002/adma.200501905 [40] CAI X Y, REN Q Y, SUN W, et al. High-performance activated carbons for supercapacitor: Effects of porous structures, heteroatom doping, and morphology[J]. Int J Energy Res,2021,45(15):21414−21434. doi: 10.1002/er.7191 [41] LI P, FENG C N, LI H P, et al. Facile fabrication of carbon materials with hierarchical porous structure for high-performance supercapacitors[J]. J Alloy Compd,2021,851:156922. doi: 10.1016/j.jallcom.2020.156922 [42] BEDIN K C, MARTINS A C, CAZETTA A L, et al. KOH-activated carbon prepared from sucrose spherical carbon: Adsorption equilibrium, kinetic and thermodynamic studies for methylene blue removal[J]. Chem Eng J,2016,286:476−484. doi: 10.1016/j.cej.2015.10.099 [43] FANG B, WEI Y Z, KUMAGAI M. Modified carbon materials for high-rate EDLCs application[J]. J Power Sources,2006,155(2):487−491. doi: 10.1016/j.jpowsour.2005.04.012 [44] YIN X, ZHANG J Q, YANG L, et al. Carbon electrodes with ionophobic characteristics in organic electrolyte for high-performance electric double-layer capacitors[J]. Sci China Mater,2022,65(2):383−390. doi: 10.1007/s40843-021-1751-x [45] 王心如, 刘嘉怡, 丁晗, 等. 超级电容器碳材料的制备及性能测试[J]. 广州化工,2022,50(13):76−80. doi: 10.3969/j.issn.1001-9677.2022.13.023WANG Xinru, LIU Jiayi, DING Han, et al. Preparation and performance test of carbon materials for supercapacitors[J]. Guangzhou Chem Ind,2022,50(13):76−80. doi: 10.3969/j.issn.1001-9677.2022.13.023 [46] LI Q H, WANG Y, YUN L, et al. Dual-template endowing N, O co-doped hierarchically porous carbon from potassium citrate with high capacitance and rate capability for supercapacitors[J]. Chem Eng J,2021,417:129289. [47] 苏婷, 兰新哲, 宋永辉, 等. KOH添加方式对煤基电极材料结构及性能的影响[J]. 煤炭转化,2020,43(3):73−80. doi: 10.19726/j.cnki.ebcc.202003011SU Ting, LAN Xinzhe, SONG Yonghui, et al. Effect of KOH addition method on structure and properties of coal-based electrode materials[J]. Coal Convers,2020,43(3):73−80. doi: 10.19726/j.cnki.ebcc.202003011 [48] EHRBURGER P, ADDOUN A, ADDOUN F, et al. Carbonization of coals in the presence of alkaline hydroxides and carbonates: Formation of activated carbons[J]. Fuel,1986,65(10):1447−1449. doi: 10.1016/0016-2361(86)90121-3 [49] LILLO-RÓDENAS M A, CAZORLA-AMORÓS D, LINARES-SOLANO A. Understanding chemical reactions between carbons and NaOH and KOH: an insight into the chemical activation mechanism[J]. Carbon,2003,41(2):267−275. doi: 10.1016/S0008-6223(02)00279-8 [50] SEVILLA M, DÍEZ N, FUERTES A B. More sustainable chemical activation strategies for the production of porous carbons[J]. ChemSusChem,2021,14(1):94−117. doi: 10.1002/cssc.202001838 [51] LU W J, CAO X H, HAO L N, et al. Activated carbon derived from pitaya peel for supercapacitor applications with high capacitance performance[J]. Mater Lett,2020,264:127339. doi: 10.1016/j.matlet.2020.127339 [52] 续士勇, 岳劲松, 程园, 等. 褐煤基活性炭的制备及其电化学性能研究[J]. 煤炭转化,2023,46(1):62−71.XU Shiyong, YUE Jinsong, CHENG Yuan, et al. Study on preparation and electrochemical performance of lignite based activated carbon[J]. Coal Convers,2023,46(1):62−71. [53] HE Y T, WANG L X, JIA D Z. Coal/PAN interconnected carbon nanofibers with excellent energy storage performance and electrical conductivity[J]. Electrochim Acta,2016,194:239−245. doi: 10.1016/j.electacta.2016.01.191 [54] 马卫平. 面向超级电容的煤基多孔炭微观结构调控及电化学性能研究[D]. 北京: 中国矿业大学, 2018.MA Weiping. Study on microstructure regulation and electrochemical properties of coal-based multi-pore carbon for Supercapacitor[D]. Beijing: China University of Mining and Technology, 2018. [55] 苏坤行. 煤基负极材料的调控及其构筑超级电容器储能性能研究[D]. 吉林: 东北电力大学, 2022.SU Kunxing. Study on the regulation of coal-based anode Materials and its Energy Storage Performance in Constructing Supercapacitors[D]. Jilin: Northeast Electric Power University, 2022. [56] ZHAN Y B, ZHOU H M, GUO F Q, et al. Preparation of highly porous activated carbons from peanut shells as low-cost electrode materials for supercapacitors[J]. J Energy Storage,2021,34:102180. doi: 10.1016/j.est.2020.102180 [57] PENG H R, YAO B, WEI X J, et al. Pore and Heteroatom Engineered Carbon Foams for Supercapacitors[J]. Adv Energy Mater,2019,9(19):1803665. doi: 10.1002/aenm.201803665 [58] YAGLIKCI S, GOKCE Y, YAGMUR E, et al. Does high sulphur coal have the potential to produce high performance-low cost supercapacitors?[J]. Surf Interfaces,2021,22:100899. doi: 10.1016/j.surfin.2020.100899 [59] LI Z P, CHENG A, ZHONG W H, et al. Facile fabrication of carbon nanosheets with hierarchically porous structure for high-performance supercapacitor[J]. Microporous Mesoporous Mater,2020,306:110440. doi: 10.1016/j.micromeso.2020.110440 [60] LIU H G, LI T H, SHI Y C, et al. Effect of different secondary quinoline insoluble content on the cellular structure of carbon foam derived from coal tar pitch[J]. J Anal Appl Pyrolysis,2014,108:310−315. doi: 10.1016/j.jaap.2014.03.002 [61] ALIFANOV O M, BUDNIK S A, NENAROKOMOV A V, et al. Design of thermal protection based on open cell carbon foam structure optimization[J]. Appl Therm Eng,2020,173:115252. doi: 10.1016/j.applthermaleng.2020.115252 [62] 岳晓明, 吴雅俊, 张双全, 等. 物理化学两步活化法制备煤基活性炭电极材料[J]. 中国矿业大学学报,2017,46(4):888−894. doi: 10.13247/j.cnki.jcumt.000711YUE Xiaoming, WU Yajun, ZHANG Shuangquan, et al. Preparation of coal-based activated carbon electrode materials by physicochemical two-step activation method[J]. Int Mining Sci Technol,2017,46(4):888−894. doi: 10.13247/j.cnki.jcumt.000711 [63] YAKABOYLU G A, JIANG C L, YUMAK T, et al. Engineered hierarchical porous carbons for supercapacitor applications through chemical pretreatment and activation of biomass precursors[J]. Renewable Energy,2021,163:276−287. doi: 10.1016/j.renene.2020.08.092 [64] WAHID M, PARTE G, PHASE D, et al. Yogurt: A novel precursor for heavily nitrogen doped supercapacitor carbon[J]. J Mater Chem A,2015,3(3):1208−1215. doi: 10.1039/C4TA06068G [65] REN Y M, ZHANG J M, XU Q, et al. Biomass-derived three-dimensional porous N-doped carbonaceous aerogel for efficient supercapacitor electrodes[J]. RSC Adv,2014,4(45):23412−23419. doi: 10.1039/c4ra02109f [66] SEO D H, YICK S, SU D W, et al. Sustainable process for all-carbon electrodes: Horticultural doping of natural-resource-derived nano-carbons for high-performance supercapacitors[J]. Carbon,2015,91:386−394. doi: 10.1016/j.carbon.2015.05.018 -




 下载:
下载: