Catalytic performance of La-modified Cu/SiO2 in the hydrogenation of methyl acetate
-
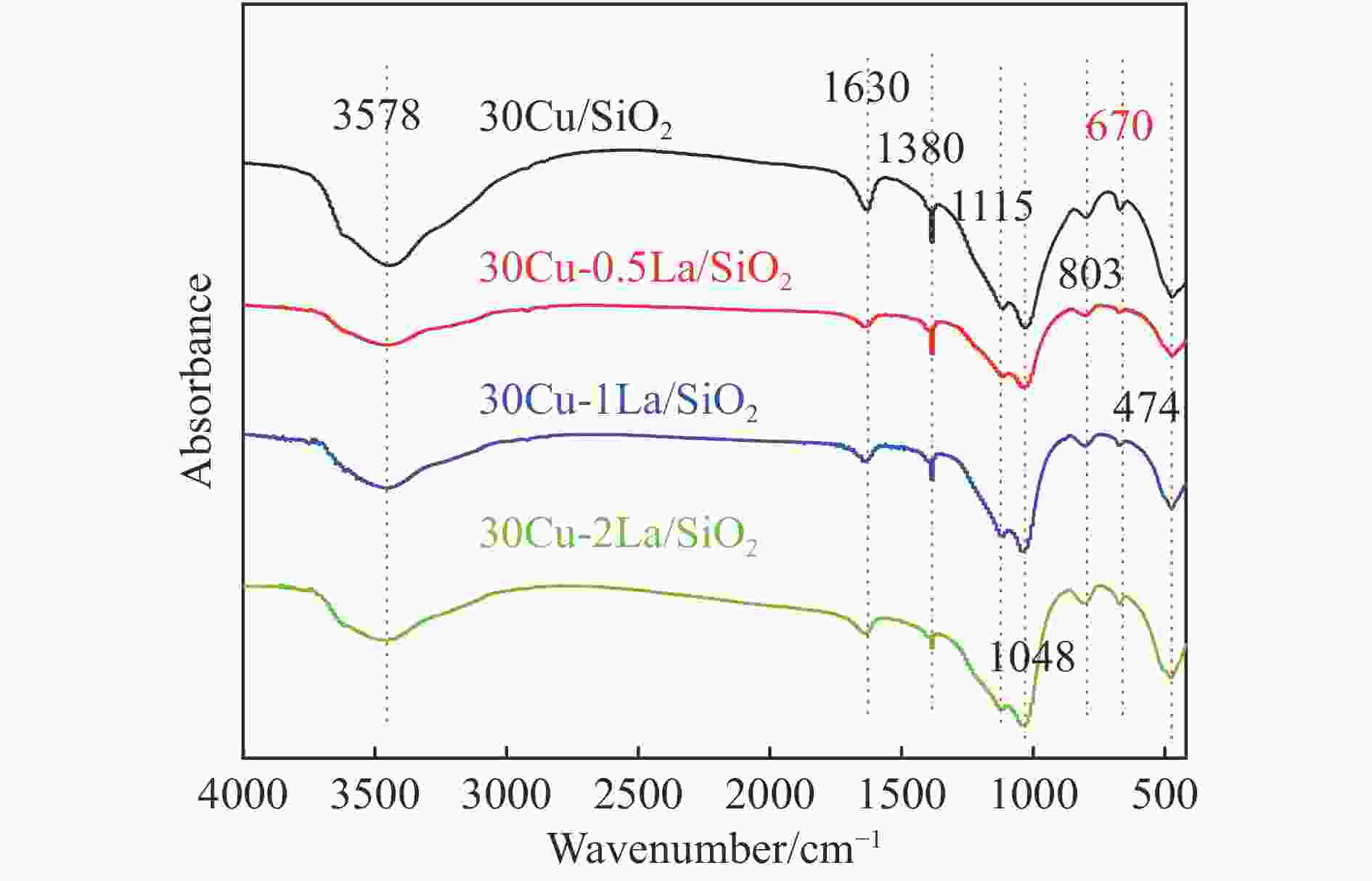
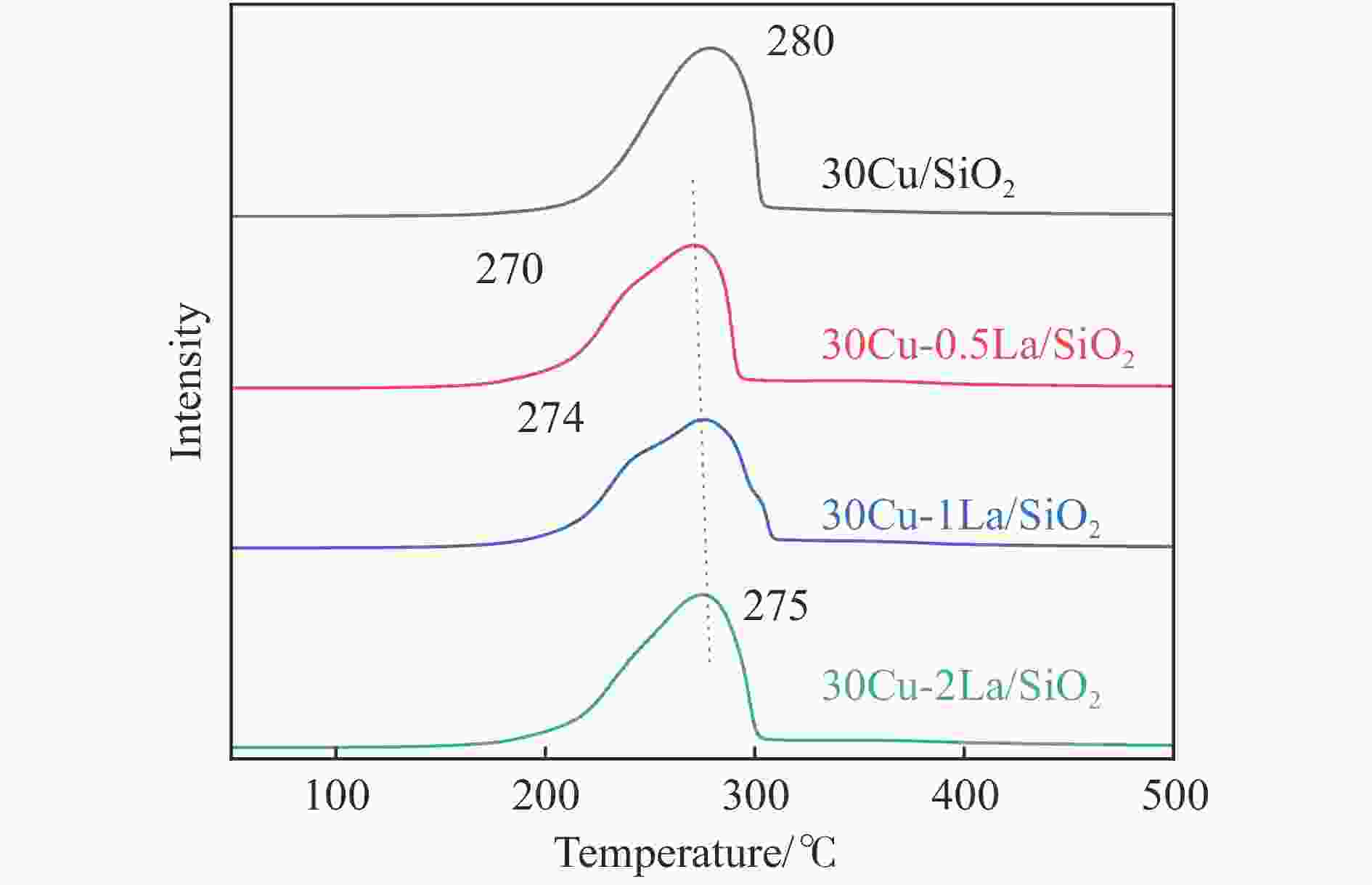
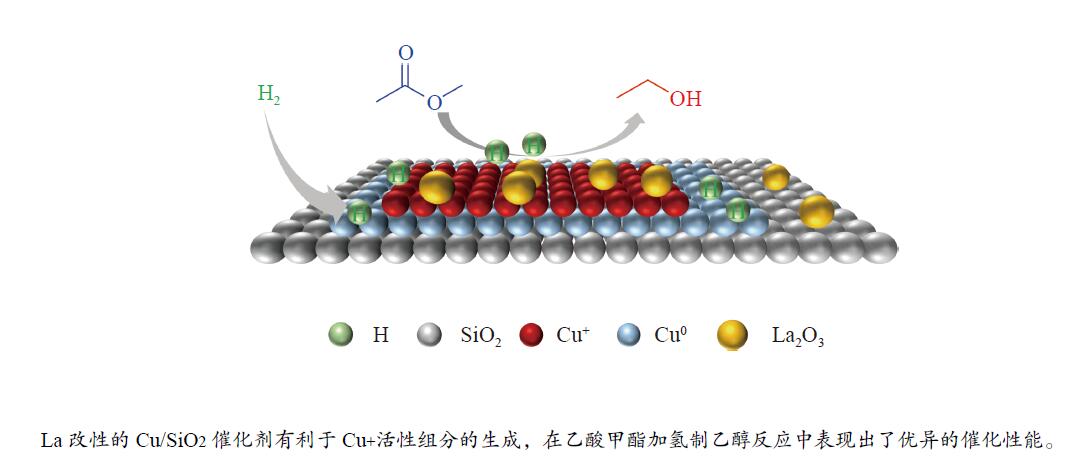
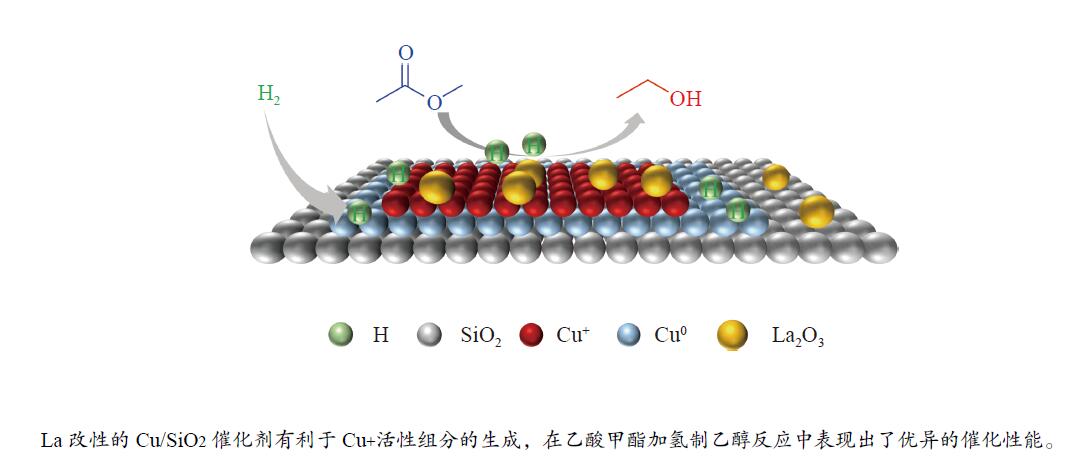
摘要: 采用蒸氨法制备了镧(La)改性的负载型铜硅(Cu/SiO2)催化剂,并对其乙酸甲酯(MeOAc)气相加氢制乙醇(EtOH)的催化性能进行了研究。采用N2吸附-脱附(N2 adsorption-desorption)、X射线粉末衍射(XRD)、电感耦合等离子体发射光谱(ICP-OES)、氢气程序升温还原(H2-TPR)、傅里叶红外光谱(FT-IR)、高分辨透射电镜(HRTEM)、光电子能谱(XPS)和原子发射光谱仪(AES)等手段对催化剂进行了的表征,发现La物种的加入产生了较多的层状硅酸铜,增强了Cu和La物种之间的相互作用。La物种的加入在结构方面提高了催化剂的比表面积,降低了铜物种的粒径,提高了铜物种的分散度;在电子还原调控方面提高了Cu+的含量,增强了催化剂吸附酰基和甲氧基的能力。与未改性的Cu/SiO2催化剂相比,镧改性后Cu/SiO2催化剂的乙酸甲酯加氢性能得到大幅提升;其中La掺杂量0.5%的Cu/SiO2催化剂表现出最佳的催化性能,乙酸甲酯转化率达98.5%,乙醇的总收率为97.0%。Abstract: A series of Cu/SiO2 catalysts modified with lanthanum (La) (30Cu-nLa/SiO2, n=0, 0.5, 1 and 2) were synthesized using the ethanol (EtOH)-assisted ammonia-evaporation method; their catalytic performance in the gas-phase hydrogenation of methyl acetate (MeOAc) to produce ethanol (EtOH) was investigated. The results indicate that the catalytic performance of Cu/SiO2 can be greatly enhanced by La modification. In particular, the 30Cu-0.5La/SiO2 catalyst exhibits excellent performance in the MeOAc hydrogenation; under 230 °C, 2 MPa H2, an LHSV of 2 h−1 and an H2/MeOAc molar ratio of 20, the MeOAc conversion reaches 98.5%, with a total EtOH yield of 97.0%. The N2-sorption, XRD, ICP-OES, H2-TPR, FT-IR, TEM, XPS, and AES characterization results reveal that the introduced La metal has a strong interaction with Cu, which can promote the dispersion of the copper species on the SiO2 support. Moreover, the content of Cu+ is increased significantly, which can enhance the electronic interaction with MeOAc via the acyl and methoxide groups and thus promote the hydrogenation of MeOAc to EtOH.
-
Key words:
- methyl acetate /
- hydrogenation /
- ethanol /
- Cu/SiO2 /
- La modification
-
表 1 30Cu-nLa/SiO2、30Cu/SiO2、2La/SiO2、SiO2催化乙酸甲酯加氢反应制乙醇的性能
Table 1 Catalytic reaction test results for the hydrogenation of MeOAc to EtOH
Catalyst MeOAc
conversion/
%EtOH
selectivity/
%EtOAc
selectivity/
%EtOH
yield/
%30Cu/SiO2 85.6 77.3 22.7 66.2 30Cu-0.5La/SiO2 98.5 98.5 1.5 97.0 30Cu-1La/SiO2 94.8 92.6 7.4 87.8 30Cu-2La/SiO2 81.5 73.4 26.6 59.8 2La/SiO2 1.40 68.7 31.3 1.0 SiO2 0.10 − − − Reaction conditions: 230 ℃, 2 MPa, LHSV=2 h−1, and H2/MeOAc=20. 表 2 不同La掺杂量的催化剂的组织结构特征
Table 2 Textural properties of the 30Cu-nLa/SiO2 catalysts with different La loadings
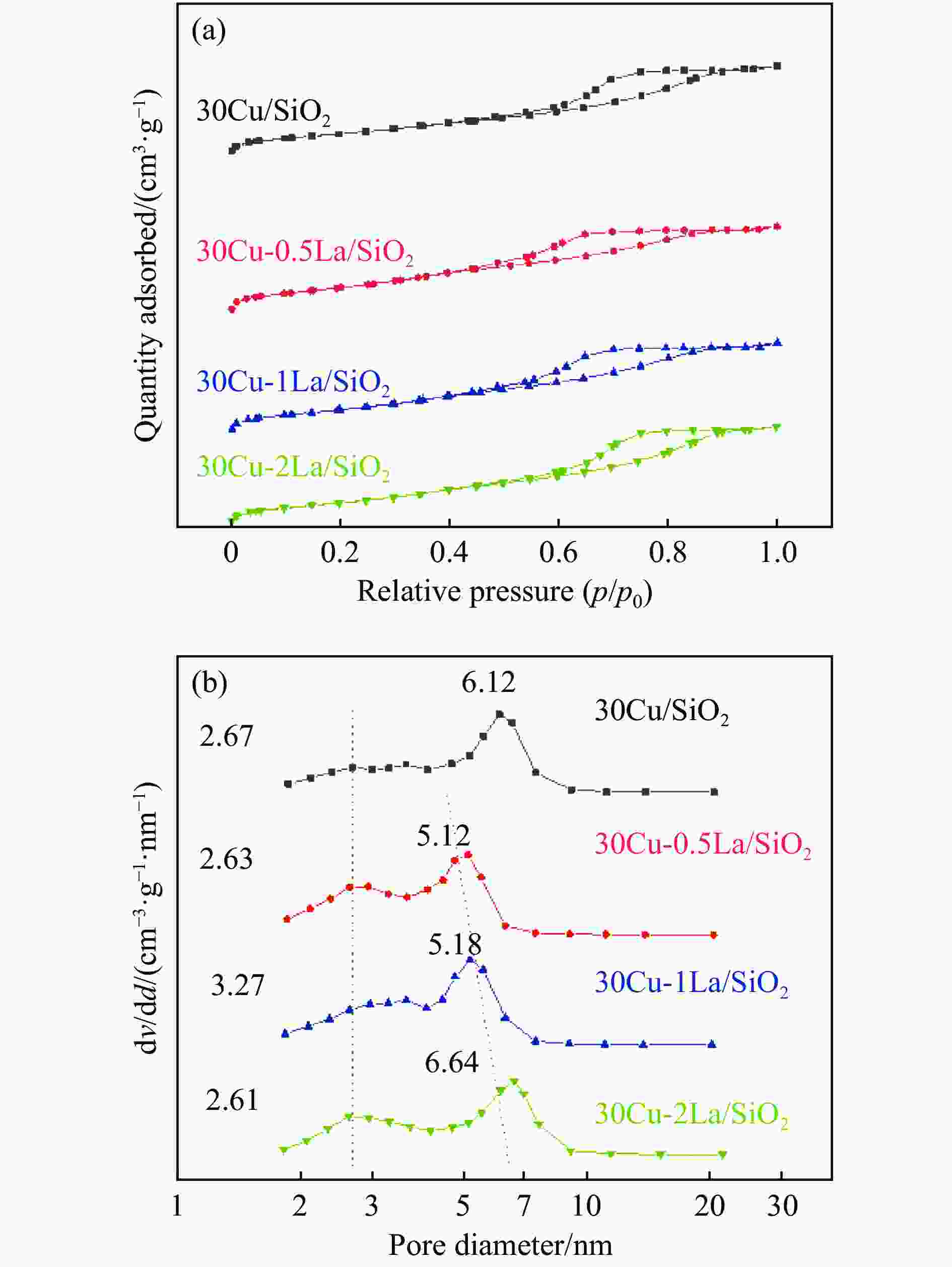
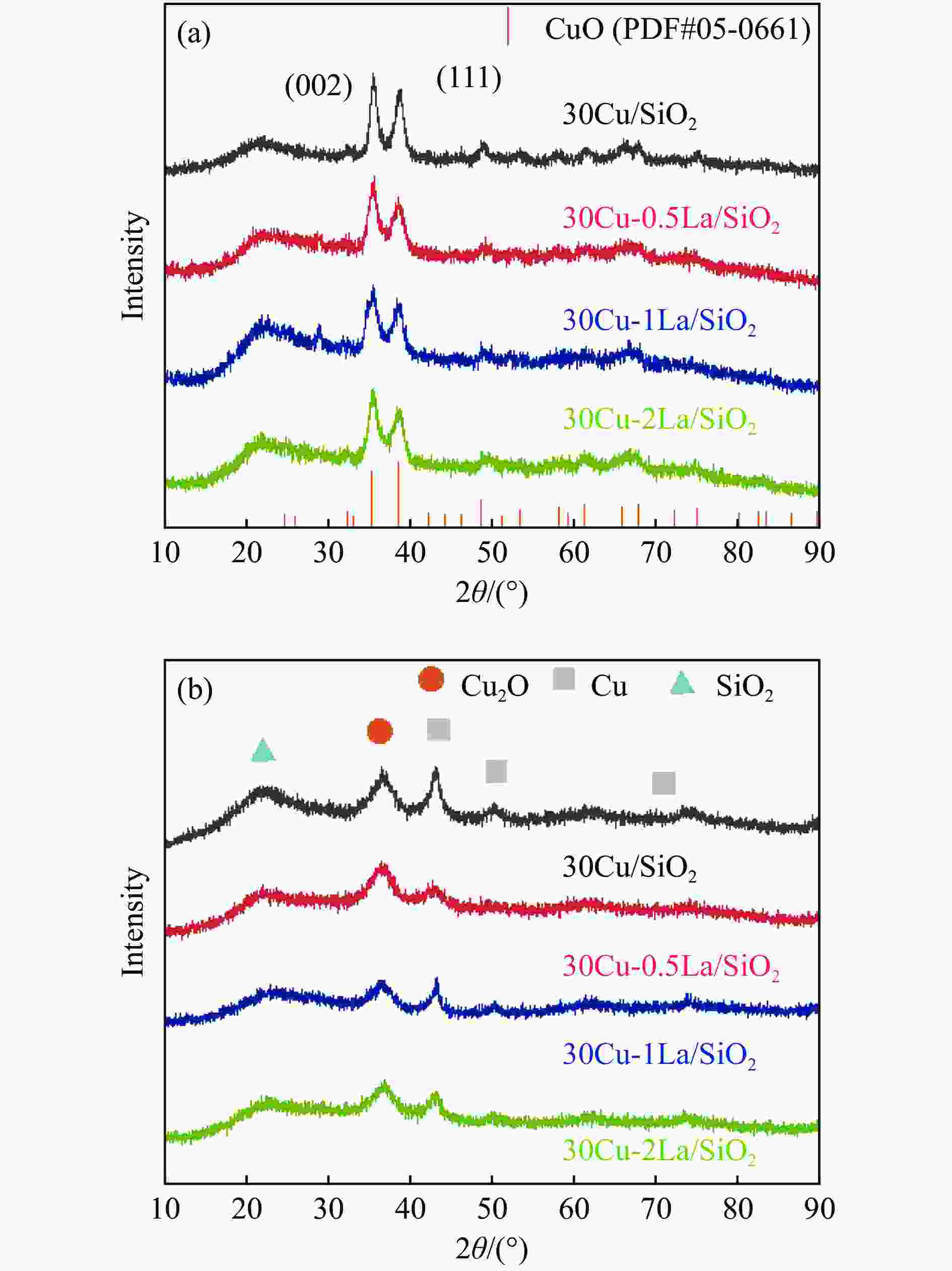
Catalyst Cu loading w/% La loading w/% SBET/(m2·g−1) dpore/(cm3·g−1) vpore/(cm3·g−1) dCu-XRD /nm dCu-TEM /nm 30Cu/SiO2 29.6 0.0 295.3 6.0 0.44 5.4 5.4 30Cu-0.5La/SiO2 29.0 0.47 350.4 4.9 0.43 4.8 4.5 30Cu-1La/SiO2 27.9 1.01 336.2 5.4 0.45 5.7 5.5 30Cu-2La/SiO2 28.9 1.91 323.7 5.9 0.49 6.2 5.8 Note: Cu and Cu loadings were measured by ICP-OES; Specific surface area (SBET), averaged pore diameter (dpore), and averaged pore volume (vpore) are determined by N2 sorption; dCu-XRD and dCu-TEM for the Cu particle sizes are obtained from XRD using the Debye-Scherrer formula and TEM measurement, respectively. 表 3 不同La掺杂量的催化剂还原后的XPS和Cu LMM XAES反卷积结果
Table 3 Summary of the Cu XPS and Cu-LMM results
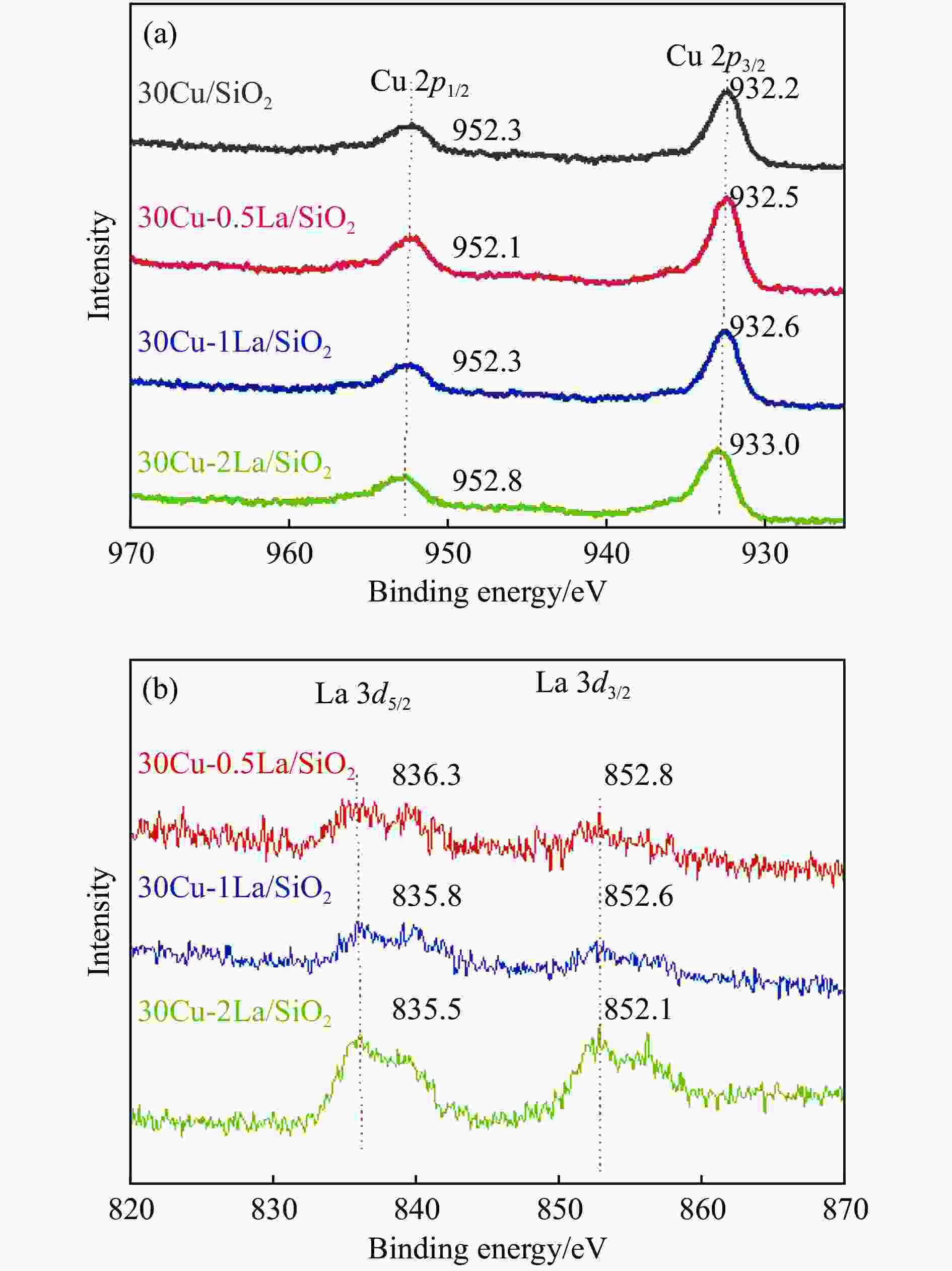
Catalyst Binding energy/eV Kinetic energy/eV xCu+/% Cu 2p1/2 Cu 2p3/2 Cu+ Cu0 30Cu 952.3 932.2 914.0 918.0 38.2 30Cu-0.5La 952.1 932.5 914.0 918.0 49.4 30Cu-1La 952.3 932.6 914.0 918.0 46.7 30Cu-2La 952.8 933.0 914.0 918.0 44.8 -
[1] ZHOU W, KANG J, CHENG K, et al. Direct conversion of syngas into methyl acetate, ethanol, and ethylene by relay catalysis via the intermediate dimethyl ether[J]. Angew Chem, Int Ed,2018,57(37):12012−12016. doi: 10.1002/anie.201807113 [2] 王辉, 吴志连, 邰志军, 等. 合成气经二甲醚羰基化及乙酸甲酯加氢制无水乙醇的研究进展[J]. 化工进展,2019,38(10):4497−4503. doi: 10.16085/j.issn.1000-6613.2019-0161WANG Hui, WU Zhilian, TAI Zhijun, et al. Advances in synthesis of anhydrous ethanol from syngas via carbonylation of dimethyl ether and hydrogenation of methyl acetate[J]. Chem Ind Eng Prog,2019,38(10):4497−4503. doi: 10.16085/j.issn.1000-6613.2019-0161 [3] WEHNER P, GUSTAFSON B. Catalytic hydrogenation of esters over Pd/ZnO[J]. J Catal,1992,135(2):420−426. doi: 10.1016/0021-9517(92)90043-H [4] WANG L, NIU X, CHEN J. SiO2 supported Ni-In intermetallic compounds: Efficient for selective hydrogenation of fatty acid methyl esters to fatty alcohols[J]. Appl Catal B: Environ,2020,278:119293. doi: 10.1016/j.apcatb.2020.119293 [5] WANG Y, LIAO J, ZHANG J, et al. Hydrogenation of methyl acetate to ethanol by Cu/ZnO catalyst encapsulated in SBA-15[J]. AIChE J,2017,63(7):2839−2849. [6] KUMMER R, TAGLIBER V, SCHNEIDER H-W. Continuous production of ethanol and plural stage distillation of the same, US4454358[P]. 1984-06-12. [7] VANDENBERG R, PARMERTIER T E, ELKJÆR C F, et al. Support functionalization to retard Ostwald ripening in copper methanol synthesis catalysts[J]. ACS Catal,2015,5(7):4439−4448. [8] ZHANG X, WILSON K, LEE A. Heterogeneously catalyzed hydrothermal processing of C5–C6 sugars[J]. Chem Rev,2016,116(19):12328−12368. [9] ALONSO D M, WERRSREIN S G, DUMESIC J A. Bimetallic catalysts for upgrading of biomass to fuels and chemicals[J]. Chem Soc Rev,2012,41(24):8075−8098. [10] BRANDS D S, POELS E K, BLIEK A. Ester hydrogenolysis over promoted Cu/SiO2 catalysts[J]. Appl Catal A: Gen,1999,184(2):279−289. [11] ZHANG Y, YE C, GUO C, et al. In2O3-modified Cu/SiO2 as an active and stable catalyst for the hydrogenation of methyl acetate to ethanol[J]. Chin J Catal,2018,39(1):99−108. [12] YE C L, GUO C L, ZHANG J L. Highly active and stable CeO2-SiO2 supported Cu catalysts for the hydrogenation of methyl acetate to ethanol[J]. Fuel Process Technol,2016,143:219−224. [13] REN Z, YOUNIS M N, ZHAO H, et al. Silver modified Cu/SiO2 catalyst for the hydrogenation of methyl acetate to ethanol[J]. Chin J Chem Eng,2020,28(6):1612−1622. [14] LI F, LU C S, LI X N. The effect of the amount of ammonia on the Cu0/Cu+ ratio of Cu/SiO2 catalyst for the hydrogenation of dimethyl oxalate to ethylene glycol[J]. Chin Chem Lett,2014,25(11):1461−1465. [15] YIN A, GUO X, DAI W L, et al. Highly active and selective copper-containing HMS catalyst in the hydrogenation of dimethyl oxalate to ethylene glycol[J]. Appl Catal A: Gen,2008,349(1/2):91−99. [16] ZHAO Y, ZHANG Y, WANG Y, et al. Structure evolution of mesoporous silica supported copper catalyst for dimethyl oxalate hydrogenation[J]. Appl Catal A: Gen,2017,539:59−69. [17] HUANG Z, CUI F, XUE J, et al. Synthesis and structural characterization of silica dispersed copper nanomaterials with unusual thermal stability prepared by precipitation-gel method[J]. J Phys Chem C,2010,114(39):16104−16113. [18] VANDERGTIFT C J G, ELBERSE P A, MULDER A, et al. Preparation of silica-supported copper-catalysts by means of deposition precipitation[J]. Appl Catal,1990,59(2):275−289. [19] 李竹霞, 钱志刚, 赵秀阁, 等. 草酸二甲酯加氢Cu/SiO2催化剂前体的研究[J]. 华东理工大学学报(自然科学版),2004,30(6):613−617. doi: 10.3969/j.issn.1006-3080.2004.06.001LI Zhuxia, QIAN Zhigang, ZHAO Xiuge, et al. Research on the precursor of catalyst Cu/SiO2 for hydrogenation of dimethyl oxalate[J]. J East China Univ Sci Technol, Nat Sci Ed,2004,30(6):613−617. doi: 10.3969/j.issn.1006-3080.2004.06.001 [20] TOUPANCE T, KERMAREC M, LOUIS C. Metal particle size in silica-supported copper catalysts. Influence of the conditions of preparation and of thermal pretreatments[J]. J Phys Chem B,2000,104(5):965−972. [21] VANDERGTIFT C J G, WIELERS A F H, JOGHI B P J, et al. Effect of the reduction treatment on the structure and reactivity of silica-supported copper particles[J]. J Catal,1991,131(1):178−189. [22] 杨文龙, 赵玉军, 王胜平, 等. 铜硅催化剂中层状硅酸铜的形成过程[J]. 化学工业与工程,2016,33(1):1−5. doi: 10.13353/j.issn.1004.9533.20141120YANG Wenlong, ZHAO Yujun, WANG Shengping, et al. Formation of copper phyllosilicate in silica supported copper catalyst[J]. Chem Ind Eng,2016,33(1):1−5. doi: 10.13353/j.issn.1004.9533.20141120 [23] SING K S W. Reporting physisorption data for gas solid systems - with special reference to the determination of surface-area and porosity[J]. Pure Appl Chem,1982,54(11):2201−2218. doi: 10.1351/pac198254112201 [24] 刘淑芝, 徐培强, 李瑞达. 助剂La对Ni/γ-Al2O3催化剂加氢性能的影响[J]. 青岛科技大学学报(自然科学版),2016,37(4):388−391.LIU Shuzhi, XU Peiqiang, LI Ruida. Effect of La on Ni/γ-Al2O3 Catalyst in Benzene Hydrogenation[J]. J Qingdao Univ Sci Technol, Nat Sci Ed,2016,37(4):388−391. [25] LEI H, HOU Z, XIE J. Hydrogenation of CO2 to CH3OH over CuO/ZnO/Al2O3 catalysts prepared via a solvent-free routine[J]. Fuel,2016,164:191−198. [26] WANG Z L, LIU Q S, YU J F, et al. Surface structure and catalytic behavior of silica-supported copper catalysts prepared by impregnation and sol-gel methods[J]. Appl Catal A: Gen,2003,239(1/2):87−94. [27] MARCHI A J, FIERRO J L G, SANTAMARIA J, et al. Dehydrogenation of isopropylic alcohol on a Cu/SiO2 catalyst: A study of the activity evolution and reactivation of the catalyst[J]. Appl Catal A: Gen,1996,142(2):375−386. [28] ZHENG X, LIN H, ZHENG J, et al. Lanthanum oxide-modified Cu/SiO2 as a high-performance catalyst for chemoselective hydrogenation of dimethyl oxalate to ethylene glycol[J]. ACS Catal,2013,3(12):2738−2749. [29] GONG J, YUE H, ZHAO Y, et al. Synthesis of ethanol via syngas on Cu/SiO2 catalysts with balanced Cu0-Cu+ sites[J]. J Am Chem Soc,2012,134(34):13922−13925. [30] HUANG J J, DING T, MA K, et al. Modification of Cu/SiO2 catalysts by La2O3 to quantitatively tune Cu+-Cu0 dual sites with improved catalytic activities and stabilities for dimethyl ether steam reforming[J]. ChemCatChem,2018,10(17):3862−3871. [31] ZHU Y, KONG X, CAO D B, et al. The rise of calcination temperature enhances the performance of Cu catalysts: Contributions of support[J]. ACS Catal,2014,4(10):3675−3681. [32] XI Y, WANG Y, YAO D, et al. Impact of the oxygen vacancies on copper electronic state and activity of Cu-based catalysts in the hydrogenation of methyl acetate to ethanol[J]. ChemCatChem,2019,11(11):2607−2614. [33] AI P, TAN M, YAMANE N, et al. Synergistic effect of a boron-doped carbon-nanotube-supported Cu catalyst for selective hydrogenation of dimethyl oxalate to ethanol[J]. Chem Eur J,2017,23(34):8252−8261. [34] GONG X, WANG M, FANG H, et al. Copper nanoparticles socketed in situ into copper phyllosilicate nanotubes with enhanced performance for chemoselective hydrogenation of esters[J]. Chem Commun,2017,53(51):6933−6936. doi: 10.1039/C7CC02093G [35] YUE H, ZHAO Y, ZHAO S, et al. A copper-phyllosilicate core-sheath nanoreactor for carbon-oxygen hydrogenolysis reactions[J]. Nat Commun,2013,4(1):2339. doi: 10.1038/ncomms3339 [36] WANG Z Q, XU Z N, PENG S Y, et al. High-performance and long-lived Cu/SiO2 nanocatalyst for CO2 hydrogenation[J]. ACS Catal,2015,5(7):4255−4259. doi: 10.1021/acscatal.5b00682 -




 下载:
下载: