Regulation of the Lewis acidity on matrix and their performance in the catalytic cracking of light hydrocarbons
-
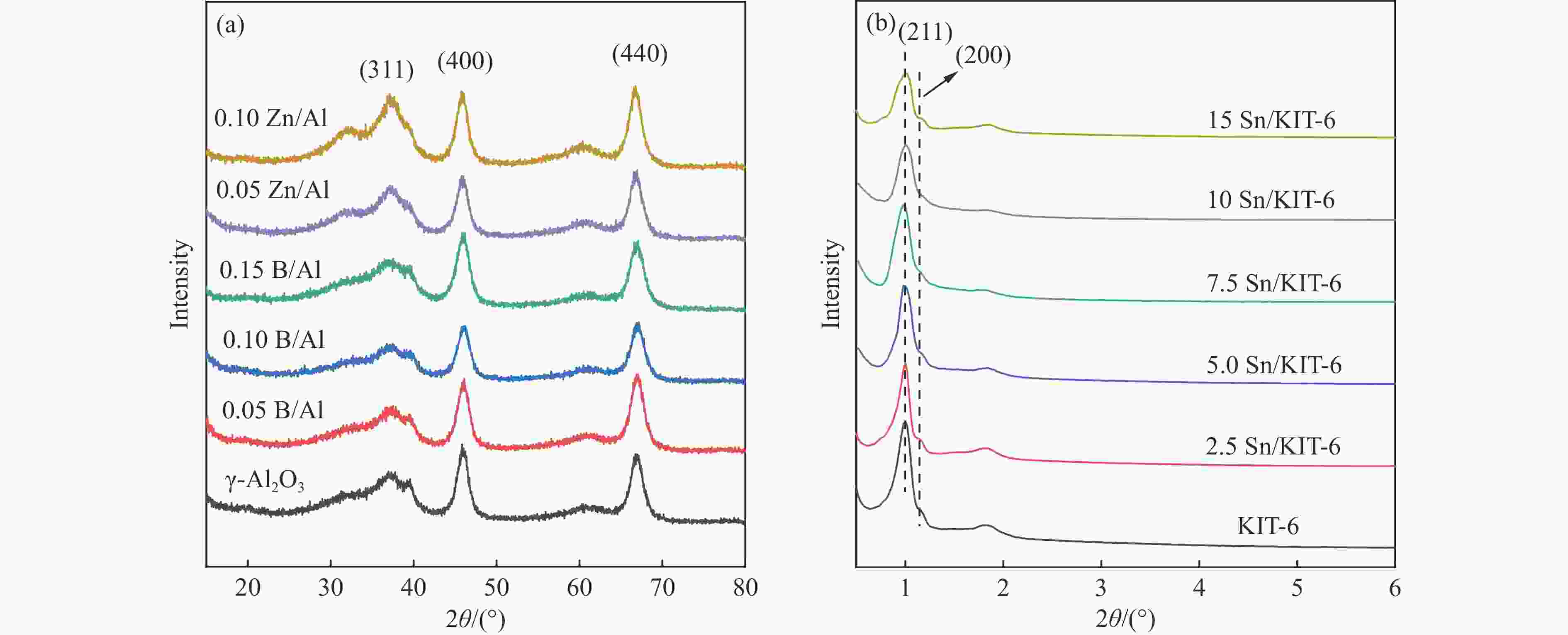
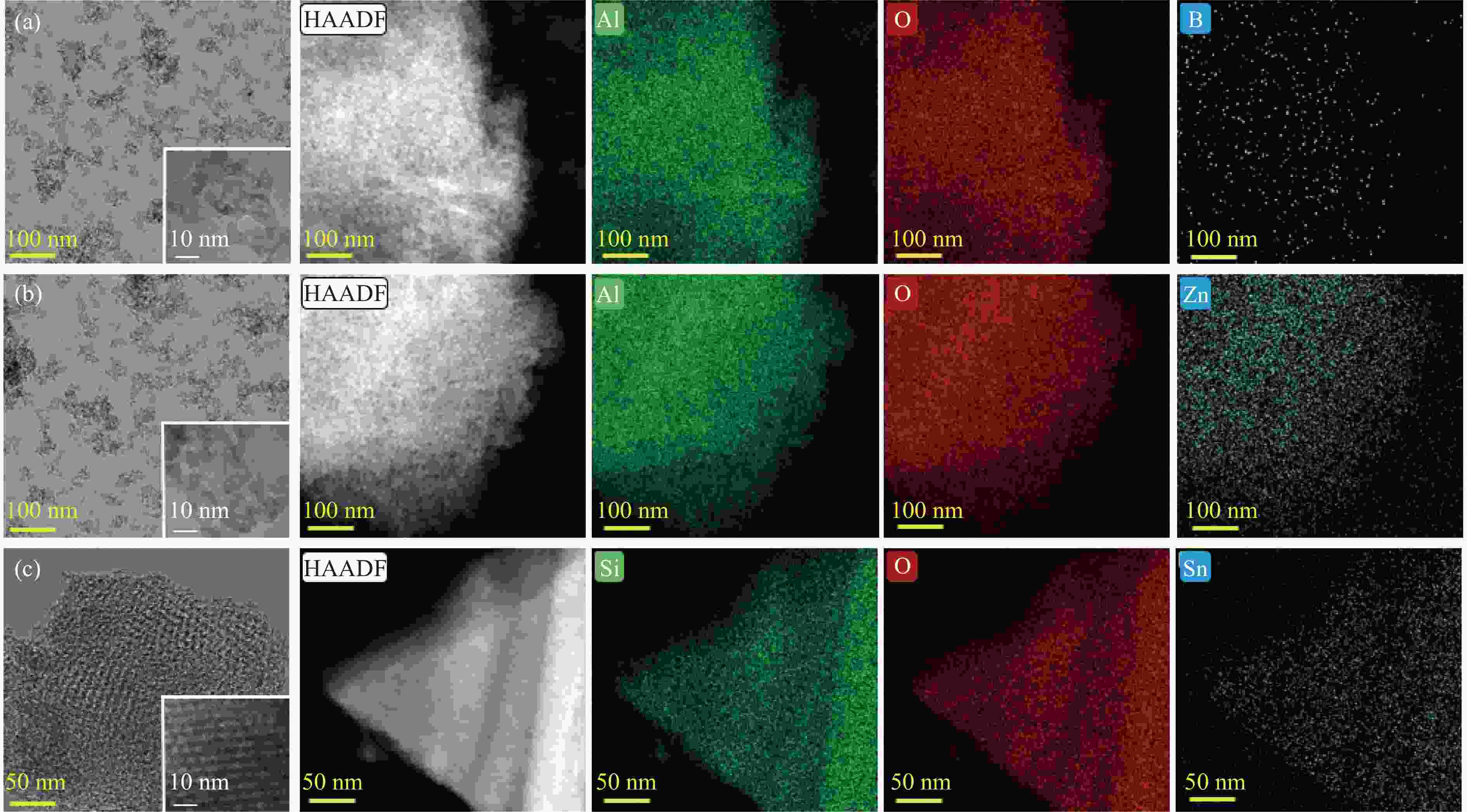
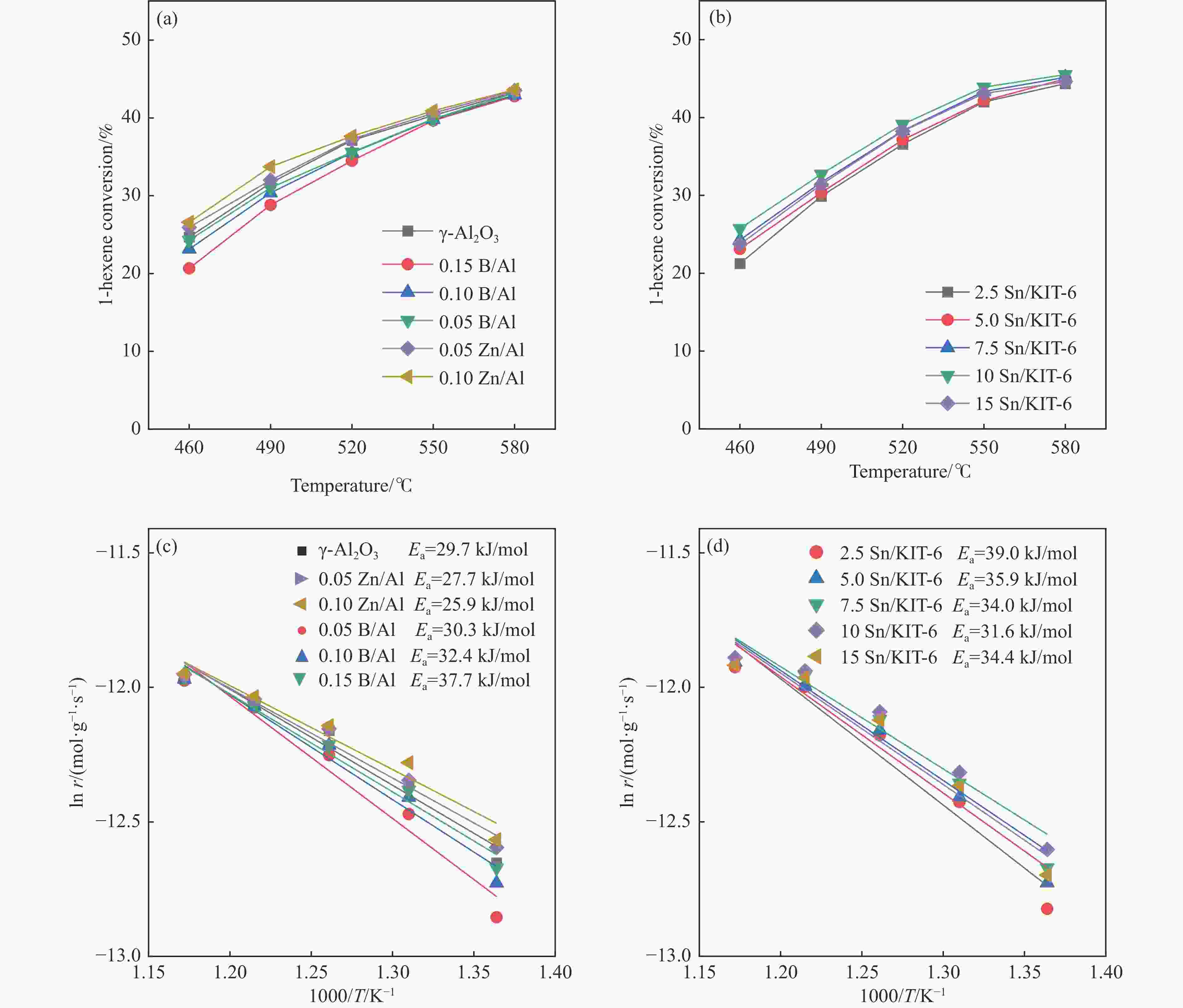
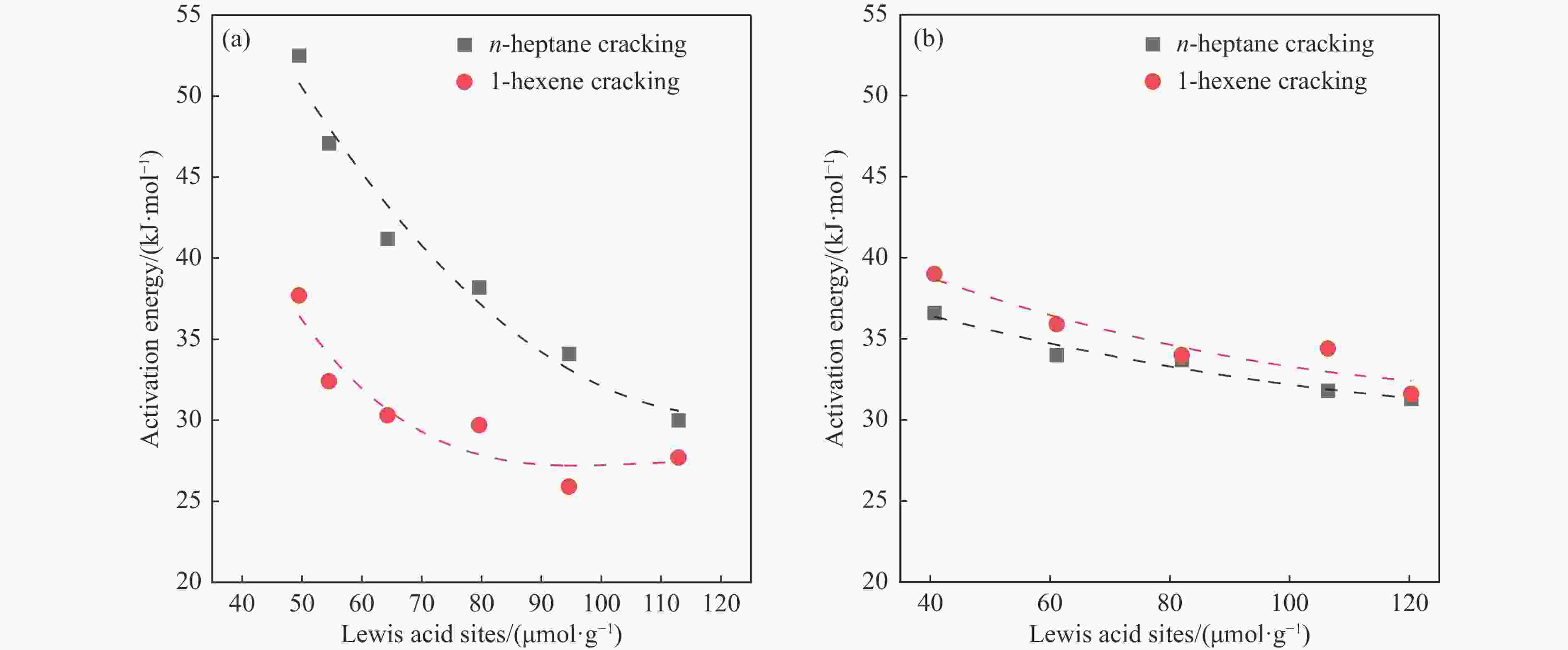
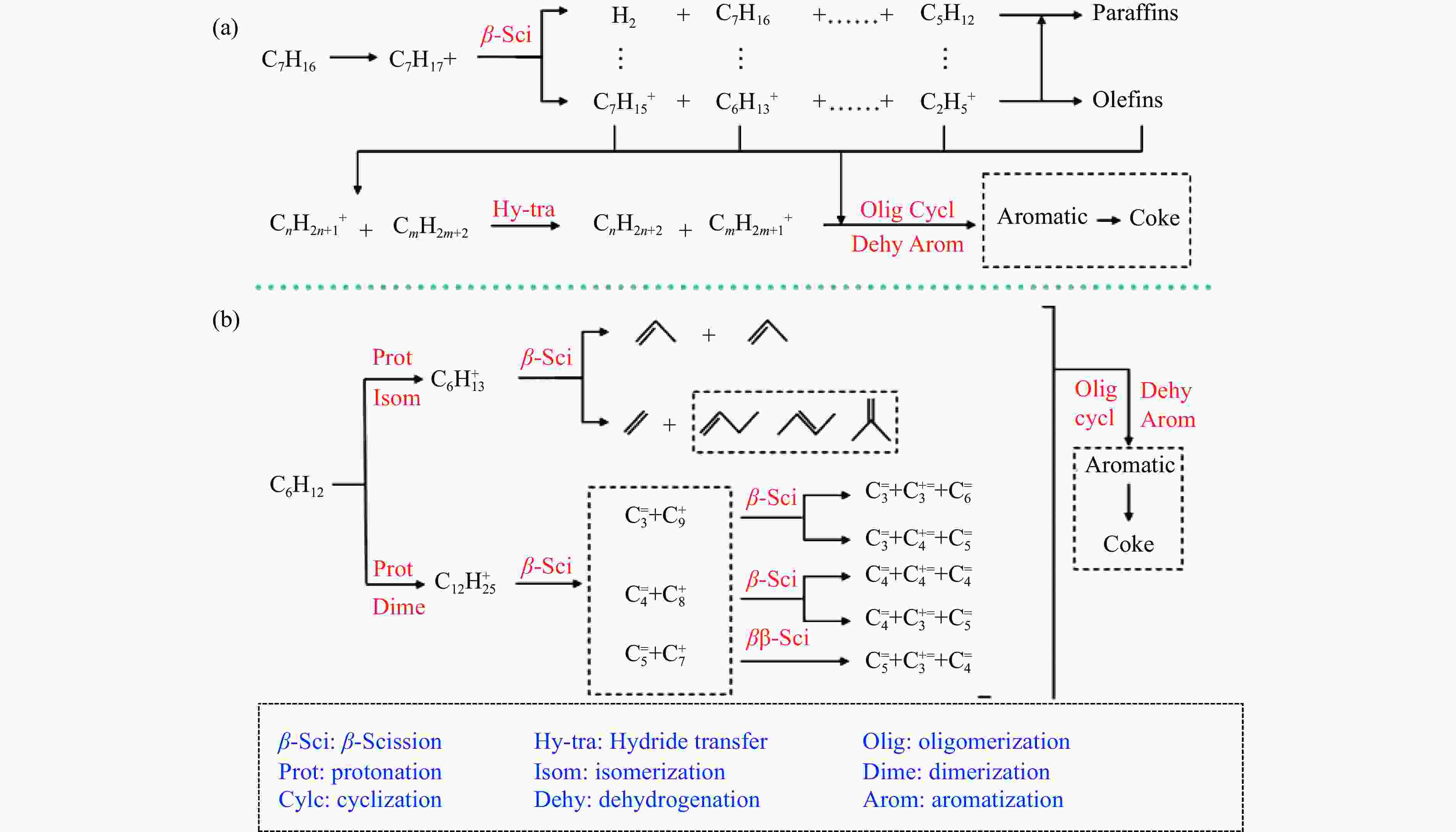
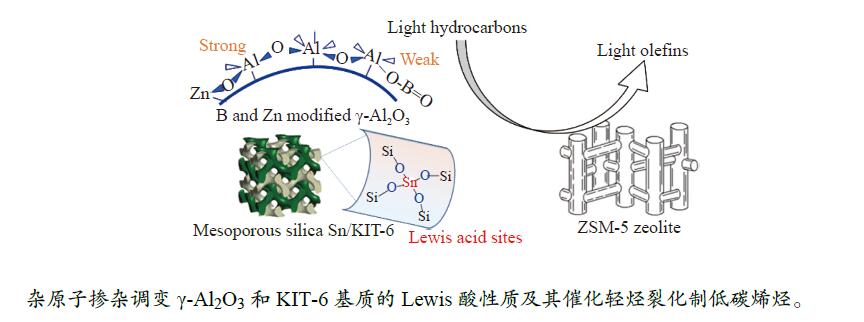
摘要: 催化剂分子筛和基质的合理匹配是提高石脑油催化裂化制低碳烯烃产量的最有效路径之一,但是基质表面Lewis酸性对裂化反应的影响尚未明确。本研究通过硼和锌改性γ-Al2O3和锡改性KIT-6介孔氧化硅材料调变表面的Lewis酸,研究基质及其与ZSM-5分子筛配合使用时催化正庚烷和1-己烯裂化制低碳烯烃的性能。采用XRD、TEM、N2物理吸附-脱附以及NH3-TPD等方法探讨了改性γ-Al2O3和KIT-6的结构性质和表面酸性质。结果表明,B可以降低γ-Al2O3的表面Lewis酸性(酸量和酸强度),而Zn可以增强其表面酸性;此外,Sn可以提高有序介孔KIT-6表面Lewis酸性。催化裂化反应结果表明,当基质单独使用时,随基质表面Lewis酸性增强,轻烃反应活化能降低且转化率升高;当基质与ZSM-5配合使用时,基质在上分子筛在下的双床层排布方式对应的转化率最高,且随基质Lewis酸性增强,轻烃转化率升高,但Lewis酸性过强会加速氢转移反应,降低低碳烯烃的选择性。Abstract: The reasonable matching of zeolite and matrix is one of the most effective strategies to increase the yield of light olefins in naphtha catalytic cracking. However, the influence of the surface Lewis acidity within the matrix on the cracking reactions has remained ambiguous. Therefore, in present study, boron and zinc co-modified γ-Al2O3 and tin modified mesoporous silica KIT-6 with tuned surface Lewis acidity were applied to evaluate the cracking reactivity of n-heptane and 1-hexene to light olefins, in which the matrix was used alone and coupled with ZSM-5 zeolite in different packed modes. The effects of the modifiers on the textural properties and surface acidity of γ-Al2O3 and KIT-6 were investigated by XRD, TEM, N2 physical absorption-desorption, and NH3-TPD. The results showed that B doping reduced the Lewis acidity (both in the amount and acid strength) of γ-Al2O3, while the incorporation of Zn doping led to increased Lewis acidity. In addition, the Lewis acidity of ordered mesoporous KIT-6 increased as Sn doping rose. While for pure matrix, the ascend in conversions of n-heptane and 1-hexene was consistent with the increased Lewis acidity of the B and Zn co-modified γ-Al2O3 and xSn/KIT-6 rose, along with decreased activation energy. In contrast, when coupled with ZSM-5 zeolite, the highest conversion was achieved in the dual-bed manner of matrix and zeolite, and the conversion increased concomitantly with the increase in the Lewis acidity of the matrix. However, excessive Lewis acidity can accelerate the hydrogen transfer rate while diminishing the selectivity of light olefins.
-
Key words:
- Lewis acid /
- matrix /
- catalytic cracking /
- light olefins
-
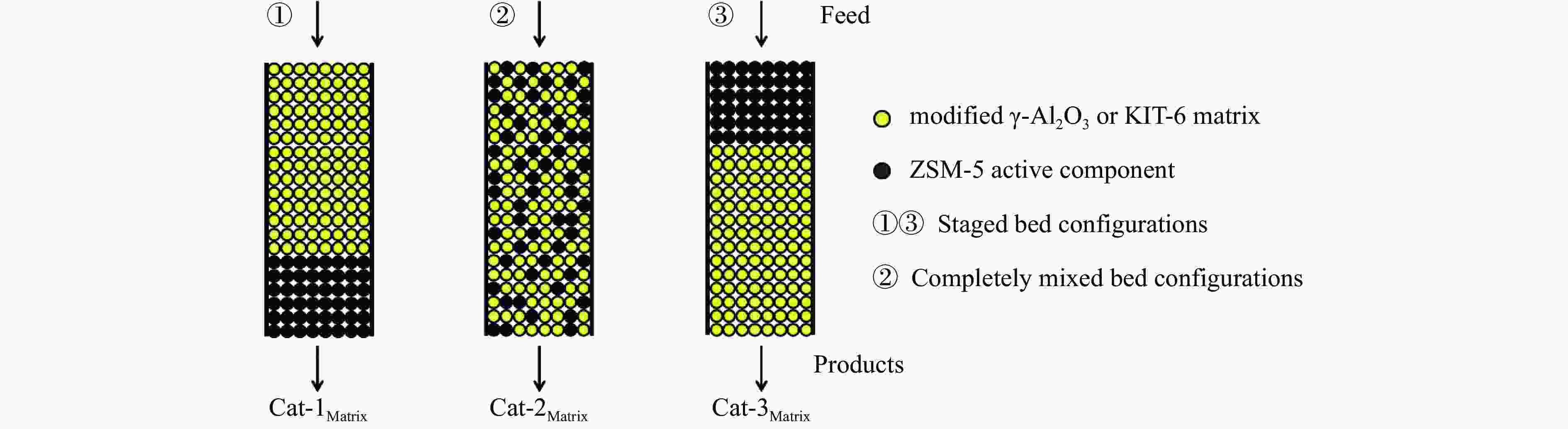
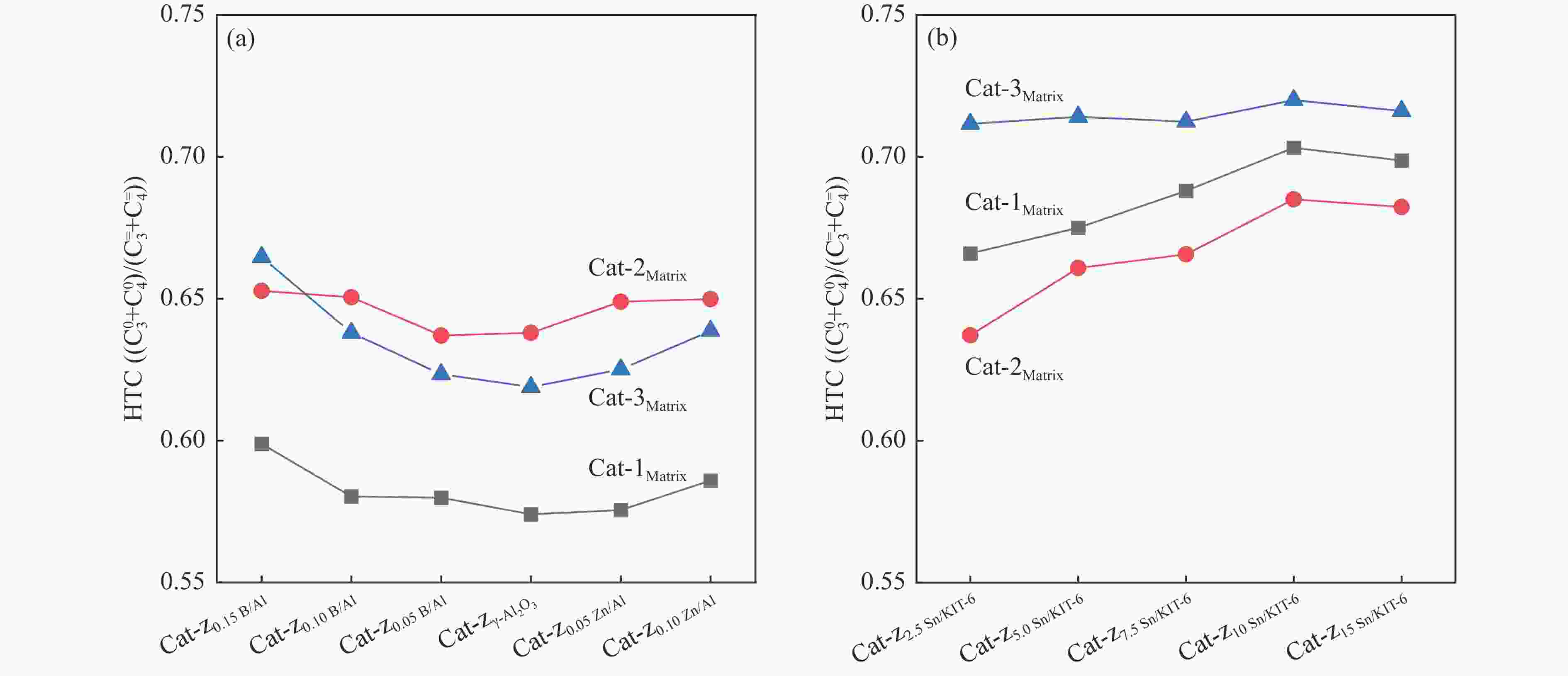
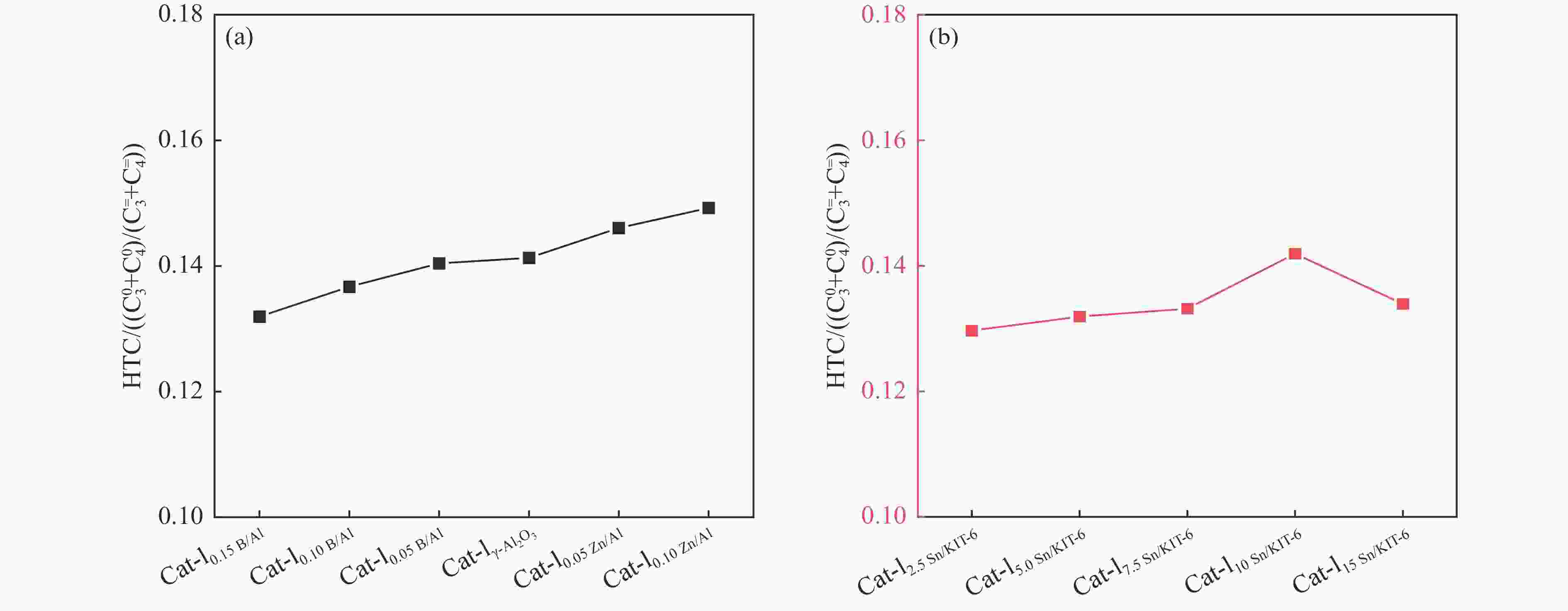
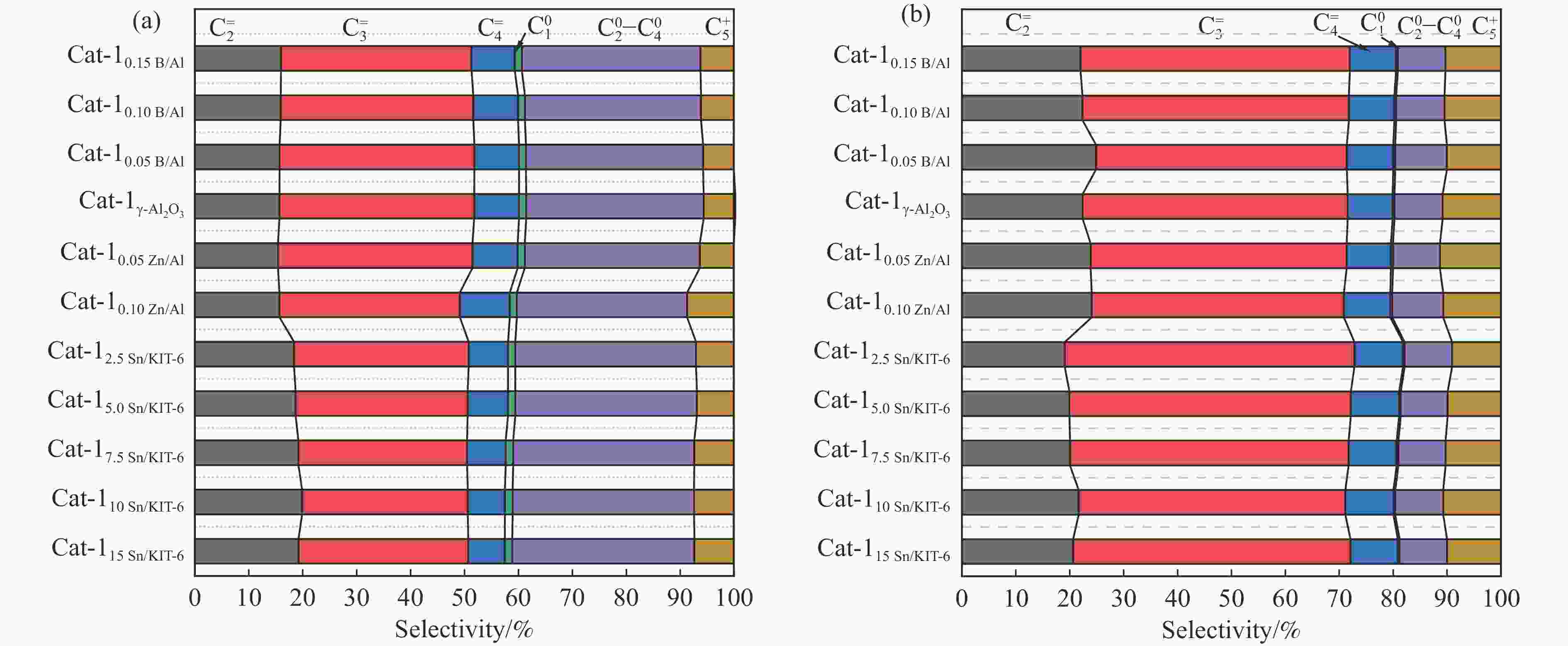
Figure 10 Conversion of n-heptane (a) &( c) and the selectivity of light olefins (b) & (d) over different kinds of catalyst beds
Reaction conditions: 0.1 g catalyst, t=550 °C, WHSV of feed was 0.34 h−1, 50 mL/min Argon as carrier gas, the results were the average value of 5 times detection within time-on-stream of 3 h.
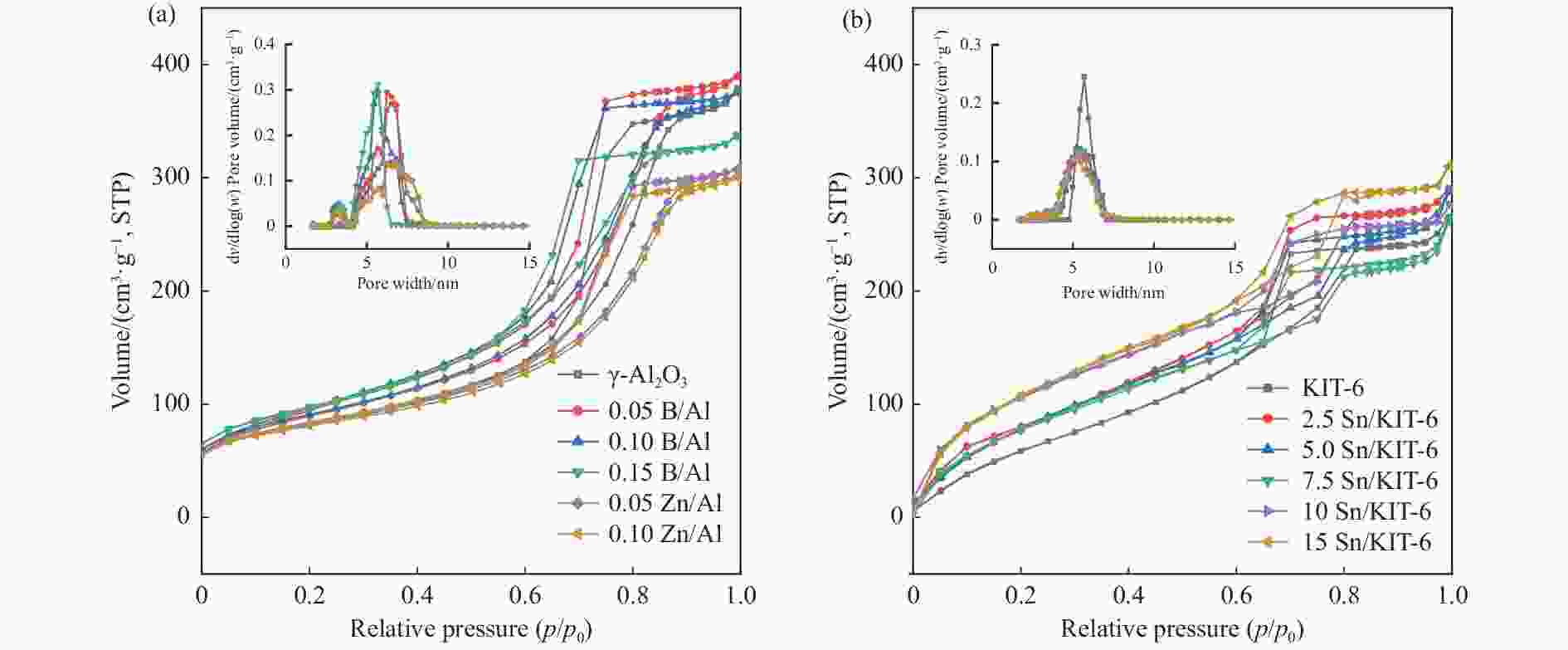
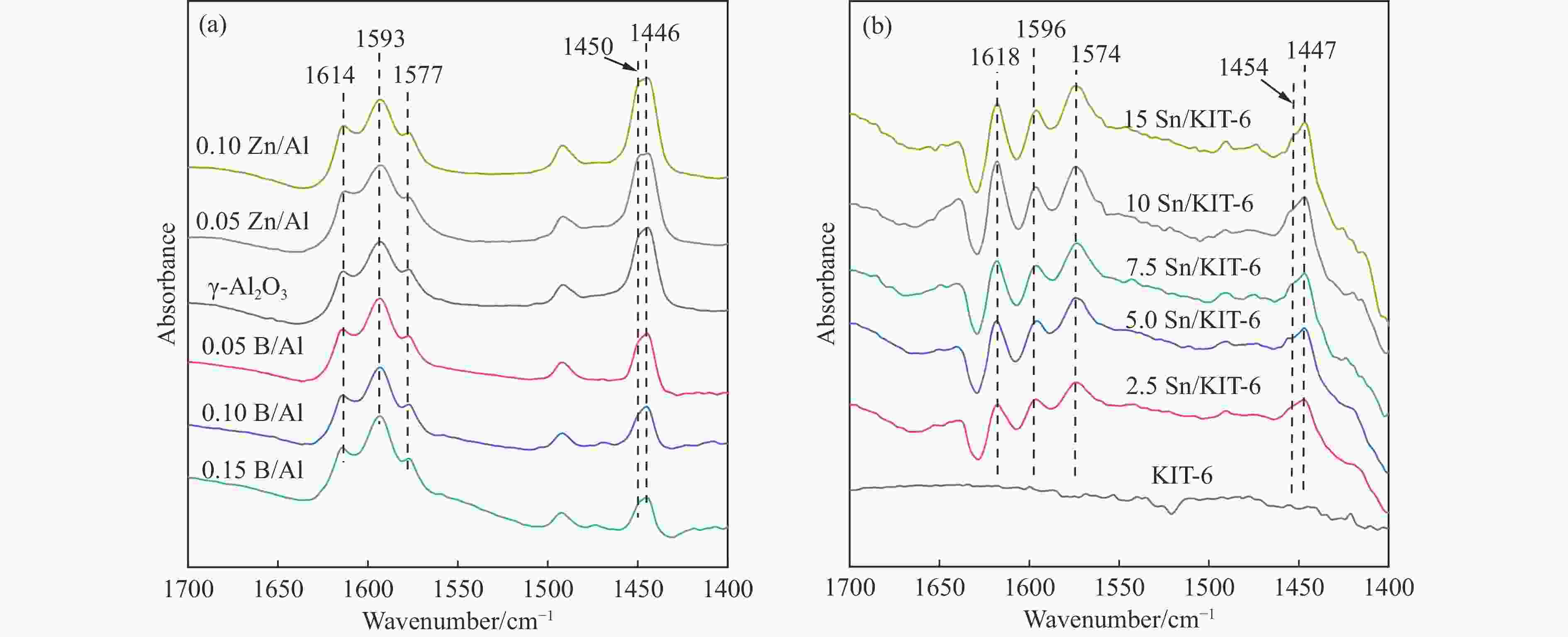
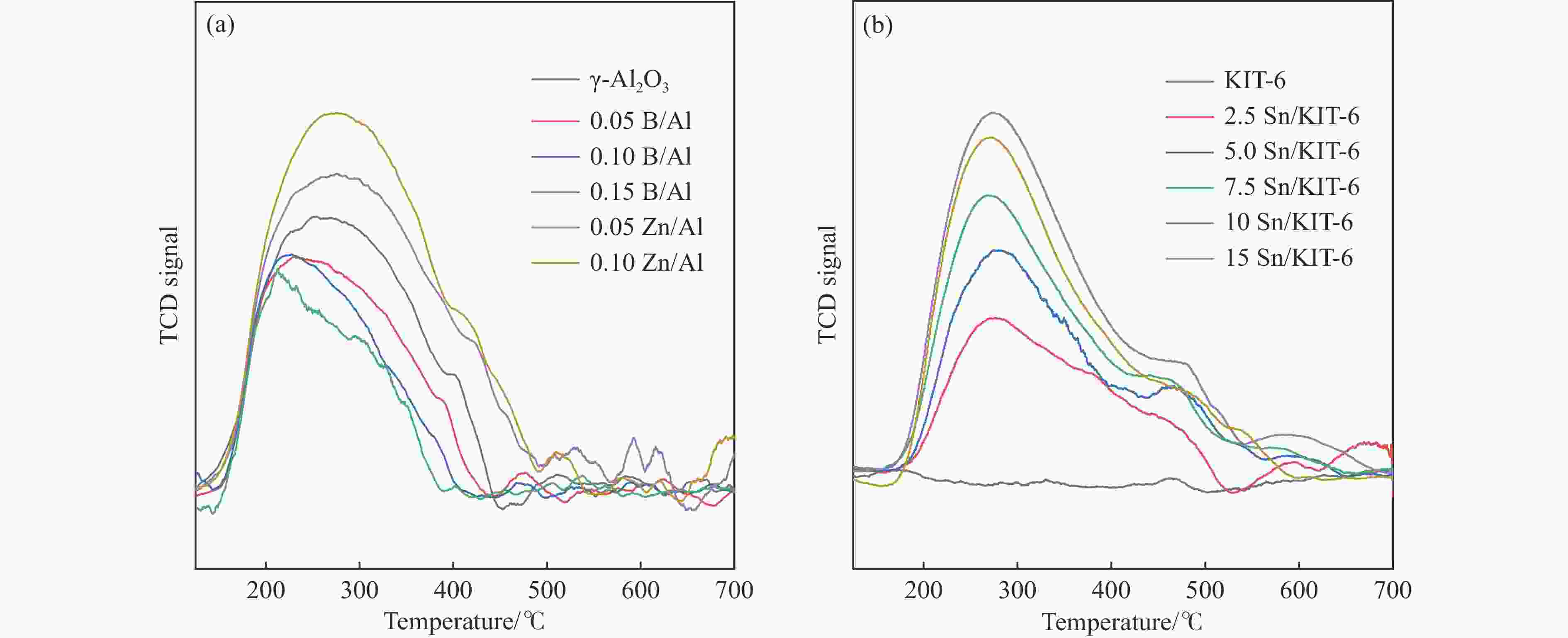
Table 1 Textural properties and surface acidity of B and Zn modified γ-Al2O3 and xSn/KIT-6 catalysts
Sample BET surface area S/(m2·g−1) Total pore volume v/(cm3·g−1) Average pore size d/nm Weak acid sites
/(μmol·g−1)Strong acid sites
/(μmol·g−1)Total acid sites
/(μmol·g−1)γ-Al2O3 228.7 0.556 9.7 56.9 22.7 79.6 0.05B/Al 254.4 0.573 9.0 47.9 16.4 64.3 0.10B/Al 256.0 0.551 8.6 44.2 10.3 54.5 0.15B/Al 278.8 0.492 7.1 41.6 7.9 49.5 0.05Zn/Al 191.0 0.431 9.0 64.7 29.9 94.6 0.10Zn/Al 184.6 0.418 9.1 78.6 34.3 112.9 KIT-6 573.5 0.756 5.7 ‒ ‒ ‒ 2.5Sn/KIT-6 566.7 0.740 5.6 28.8 11.9 40.7 5.0Sn/KIT-6 560.0 0.721 5.6 44.8 16.3 61.1 7.5Sn/KIT-6 540.1 0.695 5.5 61.6 20.4 82.0 10Sn/KIT-6 523.9 0.651 5.4 92.5 27.8 120.3 15Sn/KIT-6 502.4 0.622 5.1 83.1 21.3 106.4 -
[1] BLAY V, LOUIS B, MIRAVALLES R, et al. Engineering zeolites for catalytic cracking to light olefins[J]. ACS Catal,2017,7(10):6542−6566. doi: 10.1021/acscatal.7b02011 [2] ABBAS-ABADI M S, UREEL Y, ESCHENBACHER A, et al. Challenges and opportunities of light olefin production via thermal and catalytic pyrolysis of end-of-life polyolefins: Towards full recyclability[J]. Prog Energy Combust Sci,2023,96:101046. doi: 10.1016/j.pecs.2022.101046 [3] XIAO X, SUN B, WANG P, et al. Tuning the density of Brønsted acid sites on mesoporous ZSM-5 zeolite for enhancing light olefins selectivity in the catalytic cracking of n-octane[J]. Microporous Mesoporous Mater,2022,330:111621. doi: 10.1016/j.micromeso.2021.111621 [4] FAKHROLESLAM M, SADRAMELI S M. Thermal/catalytic cracking of hydrocarbons for the production of olefins; a state-of-the-art review III: Process modeling and simulation[J]. Fuel,2019,252:553−566. doi: 10.1016/j.fuel.2019.04.127 [5] GAO M, ZHANG G, ZHAO L, et al. Research progress of basic catalyst used in catalytic cracking for olefin production and heavy oil utilization[J]. Ind Eng Chem Res,2023,62(3):1215−1226. doi: 10.1021/acs.iecr.2c03939 [6] WANG B, HAN C, ZHANG Q, et al. Studies on the preliminary cracking of heavy oils: The effect of matrix acidity and a proposal of a new reaction route. Energ Fuel, 2015, 29(9): 5701-5713. [7] GAO X, QIN Z, WANG B, et al. High silica REHY zeolite with low rare earth loading as high-performance catalyst for heavy oil conversion[J]. Appl Catal A: Gen,2012,413–414:254−260. doi: 10.1016/j.apcata.2011.11.015 [8] MIZUNO T, YAMAZAKI H, HASEGAWA H, et al. Effects of the FCC catalyst binder type on propylene production during catalytic cracking of VGO[J]. Appl Catal A: Gen,2023,661:119214. doi: 10.1016/j.apcata.2023.119214 [9] HAUFE L A, TIMOSHEV V, SEIFERT M, et al. Crucial role of silica-alumina binder mixtures for hydrocarbon cracking with ZSM-5 additives[J]. ACS Omega,2022,7(49):44892−44902. doi: 10.1021/acsomega.2c05003 [10] ZHANG Y, WU Q, ZHANG K, et al. Synergetic regulation of the microstructure and acidity of HZSM-5/MCM-41 for efficient catalytic cracking of n-decane[J]. Langmuir,2023,39(9):3494−3501. doi: 10.1021/acs.langmuir.3c00028 [11] XU S, ZHANG Q, FENG Z, et al. A high-surface-area silicoaluminophosphate material rich in Brönsted acid sites as a matrix in catalytic cracking[J]. J Nat Gas Chem,2012,21(6):685−693. doi: 10.1016/S1003-9953(11)60420-9 [12] WANG B, ZHANG Q, HAN D, et al. Effects of acid strength of matrix in catalyst on the yield of small olefins during the catalytic cracking process[J]. Acta Pet Sin (Pet Process Sect),2016,32:666−673. [13] FENG R, YAN X, HU X, et al. High performance of H3BO3 modified USY and equilibrium catalyst with tailored acid sites in catalytic cracking[J]. Microporous Mesoporous Mater,2017,243:319−330. doi: 10.1016/j.micromeso.2017.02.041 [14] FENG R, LIU S, BAI P, et al. Preparation and characterization of γ-Al2O3 with rich Brönsted acid sites and its application in the fluid catalytic cracking process[J]. J Phys Chem C,2014,118(12):6226−6234. doi: 10.1021/jp411405r [15] WANG G, LI Z, LIU Y, et al. FCC-catalyst coking: Sources and estimation of their contribution during coker gas oil cracking process[J]. Ind Eng Chem Res,2012,51(5):2247−2256. doi: 10.1021/ie2012328 [16] ALOTAIBI F M, GONZALEZ-CORTES S, ALOTIBI M F, et al. Enhancing the production of light olefins from heavy crude oils: Turning challenges into opportunities[J]. Catal Today,2018,317:86−98. doi: 10.1016/j.cattod.2018.02.018 [17] AHREM L, SCHOLZ G, GUTMANN T, et al. Direct observation of coordinatively unsaturated sites on the surface of a fluoride-doped alumina catalyst[J]. J Phys Chem C,2017,121(22):12206−12213. doi: 10.1021/acs.jpcc.7b02535 [18] GORA-MAREK K, DEREWINSKI M, SARV P, et al. IR and NMR studies of mesoporous alumina and related aluminosilicates[J]. Catal Today,2005,101(2):131−138. doi: 10.1016/j.cattod.2005.01.010 [19] HENSEN E J M, PODUVAL D G, DEGIRMENCI V, et al. Acidity characterization of amorphous silica-alumina[J]. J Phys Chem C,2013,116(40):21416−21429. [20] CAN F, TRAVERT A, RUAUX V, et al. FCC gasoline sulfur reduction additives: Mechanism and active sites[J]. J Catal,2007,249(1):79−92. doi: 10.1016/j.jcat.2007.04.001 [21] MUDDADA N B, OLSBYE U, FUGLERUD T, et al. The role of chlorine and additives on the density and strength of Lewis and Brønsted acidic sites of γ-Al2O3 support used in oxychlorination catalysis: A FTIR study[J]. J Catal,2011,284(2):236−246. doi: 10.1016/j.jcat.2011.08.014 [22] FENG R, YAN X, HU X. Effects of boron and fluorine modified gamma-Al2O3 with tailored surface acidity on catalytic ethanol dehydration to ethylene[J]. J Porous Mater,2018,25(4):1105−1114. doi: 10.1007/s10934-017-0522-y [23] VATUTINA Y V, KLIMOV O V, NADEINA K A, et al. Influence of boron addition to alumina support by kneading on morphology and activity of HDS catalysts[J]. Appl Catal B: Environ,2016,199:23−32. doi: 10.1016/j.apcatb.2016.06.018 [24] TANG B, DAI W, WU G, et al. Improved postsynthesis strategy to Sn-beta zeolites as Lewis acid catalysts for the ring-opening hydration of epoxides[J]. ACS Catal,2014,4(8):2801−2810. doi: 10.1021/cs500891s [25] CHEN W, LIN M, ZHOU J, et al. The regulation of electron distribution on Fe Lewis acidic sites within silicon skeleton and its contribution to Ketoprofen ozonation[J]. Sep Purif Technol,2023,309:123113. doi: 10.1016/j.seppur.2023.123113 [26] FERRINI P, DIJKMANS J DE CLERCQ R, et al. Lewis acid catalysis on single site Sn centers incorporated into silica hosts[J]. Coord Chem Rev,2017,343:220−255. doi: 10.1016/j.ccr.2017.05.010 [27] LIAO W, LIN H, ZHANG J, et al. Au/Sn-Beta catalyst with metal-Lewis acid cooperative sites steers aerobic oxidation of 5-hydroxymethylfurfural[J]. Appl Surf Sci,2023,608:155154. doi: 10.1016/j.apsusc.2022.155154 [28] RAJALAKSHMI R, MAHESWARI R, RAMANATHAN A. Characterization and activity of novel tin incorporated ordered cubic mesoporous silicate, Sn-KIT-6[J]. Mater Res Bull,2016,75:224−229. doi: 10.1016/j.materresbull.2015.11.046 [29] SHINTAKU H, NAKAJIMA K, KITANO M, et al. Lewis acid catalysis of TiO4 tetrahedra on mesoporous silica in water[J]. ACS Catal,2014,4(4):1198−1204. doi: 10.1021/cs401149n [30] LUO H Y, LEWIS J D, ROMAN-LESHKOV Y. Lewis acid zeolites for biomass conversion: Perspectives and challenges on reactivity, synthesis, and stability[J]. Annu Rev Chem Biomol Eng,2016,7(1):663−692. doi: 10.1146/annurev-chembioeng-080615-034551 [31] KIM T W, KLEITZ F, PAUL B, et al. MCM-48-like large mesoporous silicas with tailored pore structure: Facile synthesis domain in a ternary triblock copolymer-butanol-water system[J]. J Am Chem Soc,2005,127(20):7601−7610. doi: 10.1021/ja042601m [32] ULLAH R, ZHANG Z, BAI P, et al. One-pot cation-anion double hydrolysis derived Ni/ZnO-Al2O3 absorbent for reactive adsorption desulfurization[J]. Ind Eng Chem Res,2016,55(13):3751−3758. doi: 10.1021/acs.iecr.5b04421 [33] SILES-QUESADA S, PARLETT C M A, LAMB A C, et al. Synthesis and catalytic advantage of a hierarchical ordered macroporous KIT-6 silica[J]. Mater Today Chem,2023,30:101574. doi: 10.1016/j.mtchem.2023.101574 [34] LIU X. DRIFTS study of surface of γ-alumina and its dehydroxylation[J]. J Phys Chem C,2008,112(13):5066−5073. doi: 10.1021/jp711901s [35] SCHMIDT S A, PEURLA M, KUMAR N, et al. Preparation of selective ZnCl2/alumina catalysts for methyl chloride synthesis: Influence of pH, precursor and zinc loading[J]. Appl Catal A: Gen,2015,490:117−127. doi: 10.1016/j.apcata.2014.11.008 [36] CHEN C J, RANGARAJAN S, HILL I M, et al. Kinetics and thermochemistry of C4–C6 olefin cracking on H-ZSM-5[J]. ACS Catal,2014,4(7):2319−2327. doi: 10.1021/cs500119n [37] CNUDDE P, DE WISPELAERE K, VANDUYFHUYS L, et al. How chain length and branching influence the alkene cracking reactivity on H-ZSM-5[J]. ACS Catal,2018,8(10):9579−9595. doi: 10.1021/acscatal.8b01779 [38] LEITCH D C, LABINGER J A, BERCAW J E. Scope and mechanism of homogeneous tantalum/iridium tandem catalytic alkane/alkene upgrading using sacrificial hydrogen acceptors[J]. Organometallics,2014,33(13):3353−3365. doi: 10.1021/om500231t [39] ZHANG L, HU Q, QIN Y, et al. Optimized zeolite distribution of FCC catalysts for promoting heavy-oil catalytic cracking. Ind Eng Chem Res, 2022, 61(32): 11628-11635. [40] CHEN J, YAN H, GONG H, et al. Tetrahedrally coordinated W(VI) species induced Lewis acid for stable catalytic cracking of 1-hexene to propene[J]. Chem Eng J,2022,448:137504. doi: 10.1016/j.cej.2022.137504 [41] LIU W, LIU X, GU Y, et al. A new composite consisting of Y zeolite and ZrO2 for fluid catalytic cracking reaction[J]. Compos Part B-Eng,2020,200:108317. doi: 10.1016/j.compositesb.2020.108317 [42] CRESPO I, HERTZOG J, CARRE V, et al. Alumina-embedded HZSM-5 with enhanced behavior for the catalytic cracking of biomass pyrolysis bio-oil: Insights into the role of mesoporous matrix in the deactivation by coke[J]. J Anal Appl Pyrolysis,2023,172:106009. doi: 10.1016/j.jaap.2023.106009 [43] KOTREL S, KNOZINGER H, GATES B C. The Haag-Dessau mechanism of protolytic cracking of alkanes[J]. Microporous Mesoporous Mater,2000,35–36:11−20. doi: 10.1016/S1387-1811(99)00204-8 [44] HAAG W O, DESSAU R M, LAGO R M, et al. Kinetics and mechanism of paraffin cracking with zeolite catalysts[J]. In Stud Surf Sci Catal,1991,60:255−265. [45] ZHAO R, HALLER G L, LERCHER J A. Akene adsorption and cracking on acidic zeolites–A gradual process of understanding[J]. Microporous Mesoporous Mater,2023,358:112390. doi: 10.1016/j.micromeso.2022.112390 [46] SUN H, CAO L, ZHANG Y, et al. Effect of catalyst acidity and reaction temperature on hexene cracking reaction to produce propylene[J]. Energy Fuels,2021,35(4):3295−3306. doi: 10.1021/acs.energyfuels.0c03544 -





 下载:
下载: