Effect of Ce modification on the performance of CuLDH catalyst for CO2 hydrogenation to methanol
-
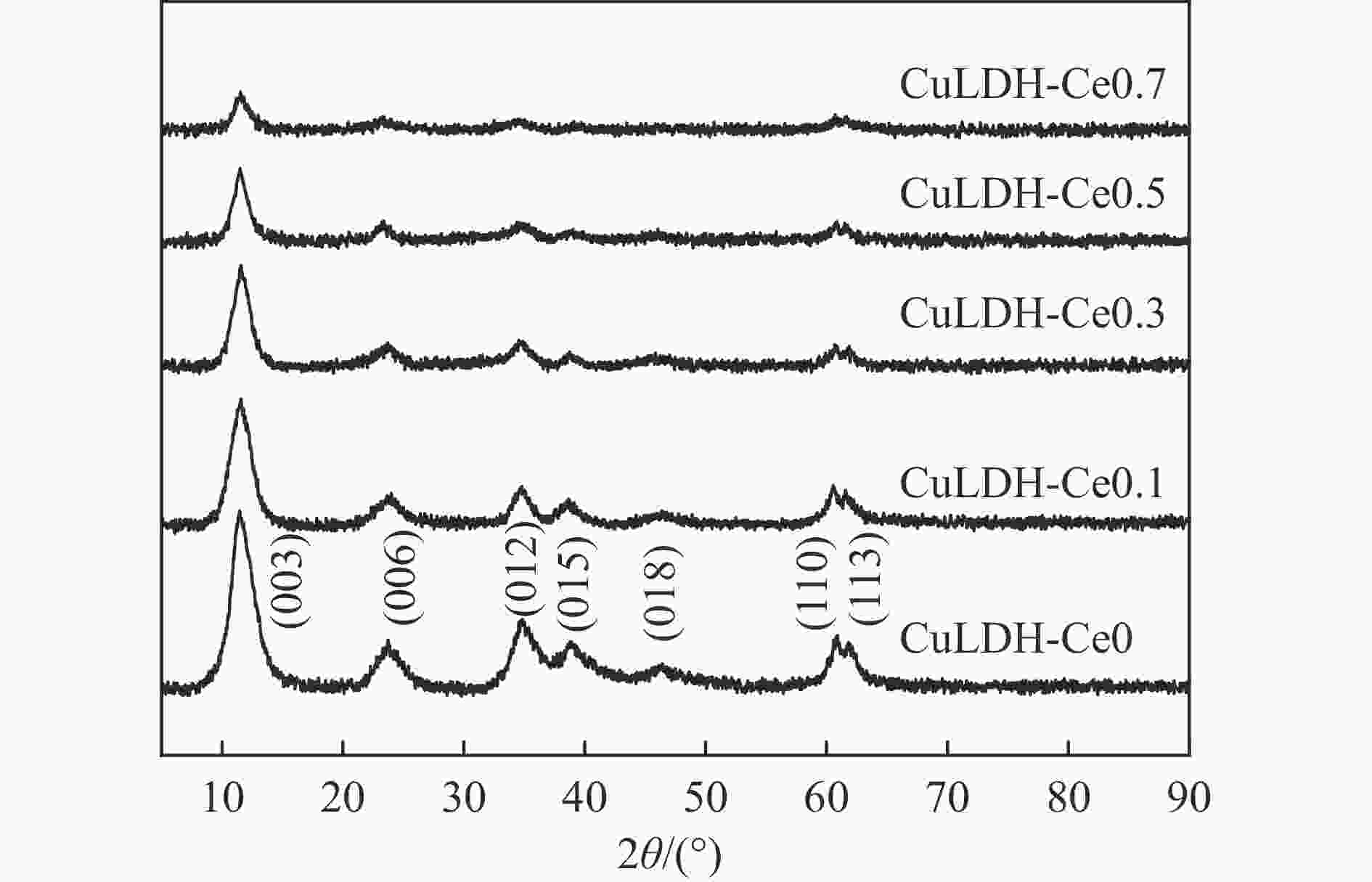
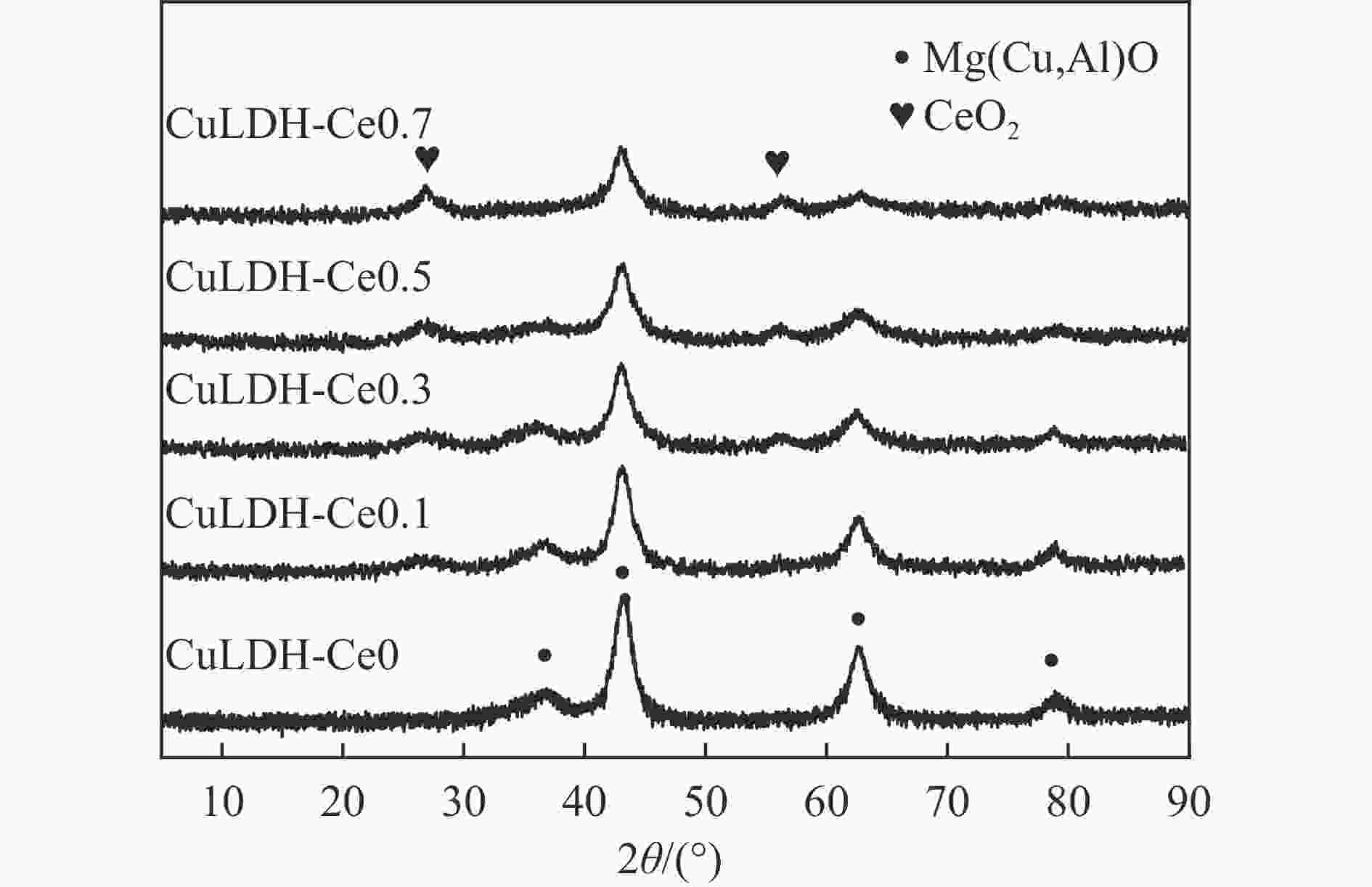
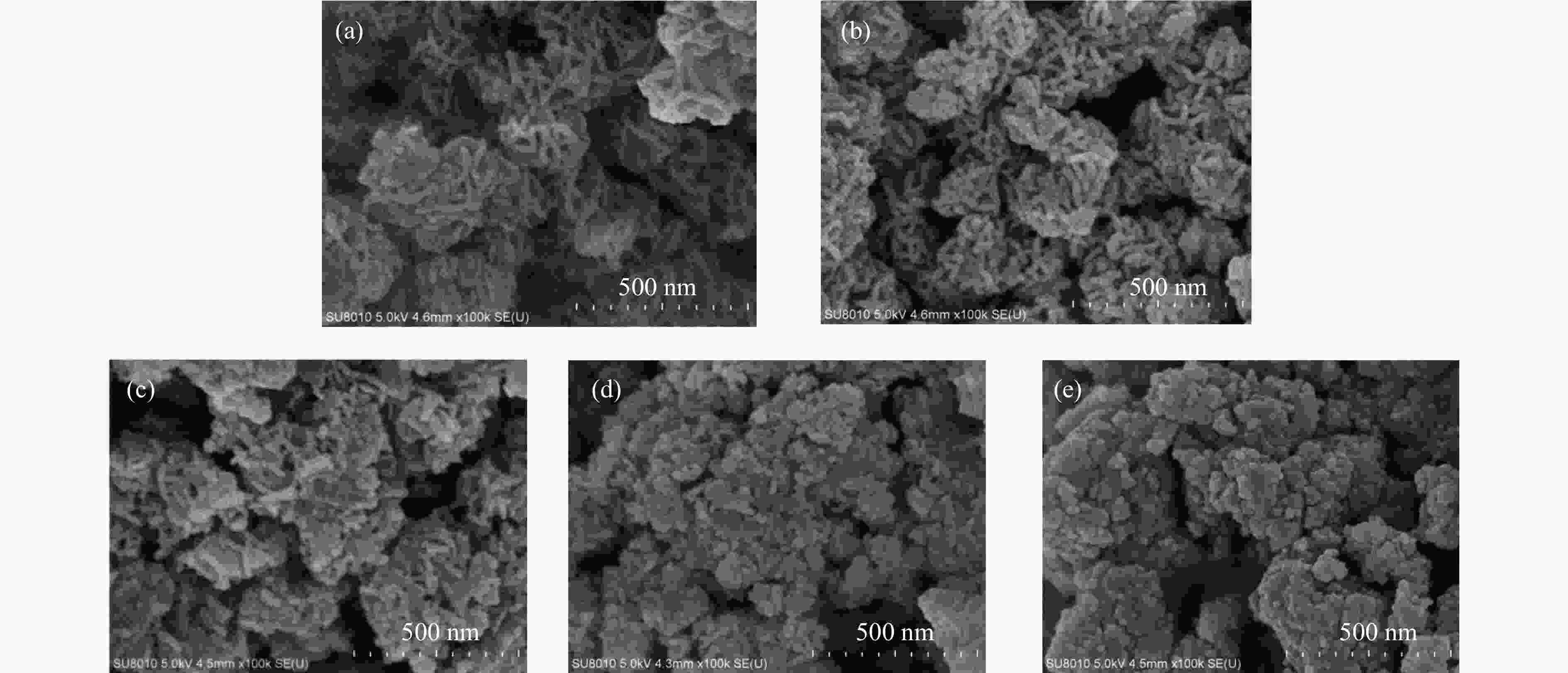
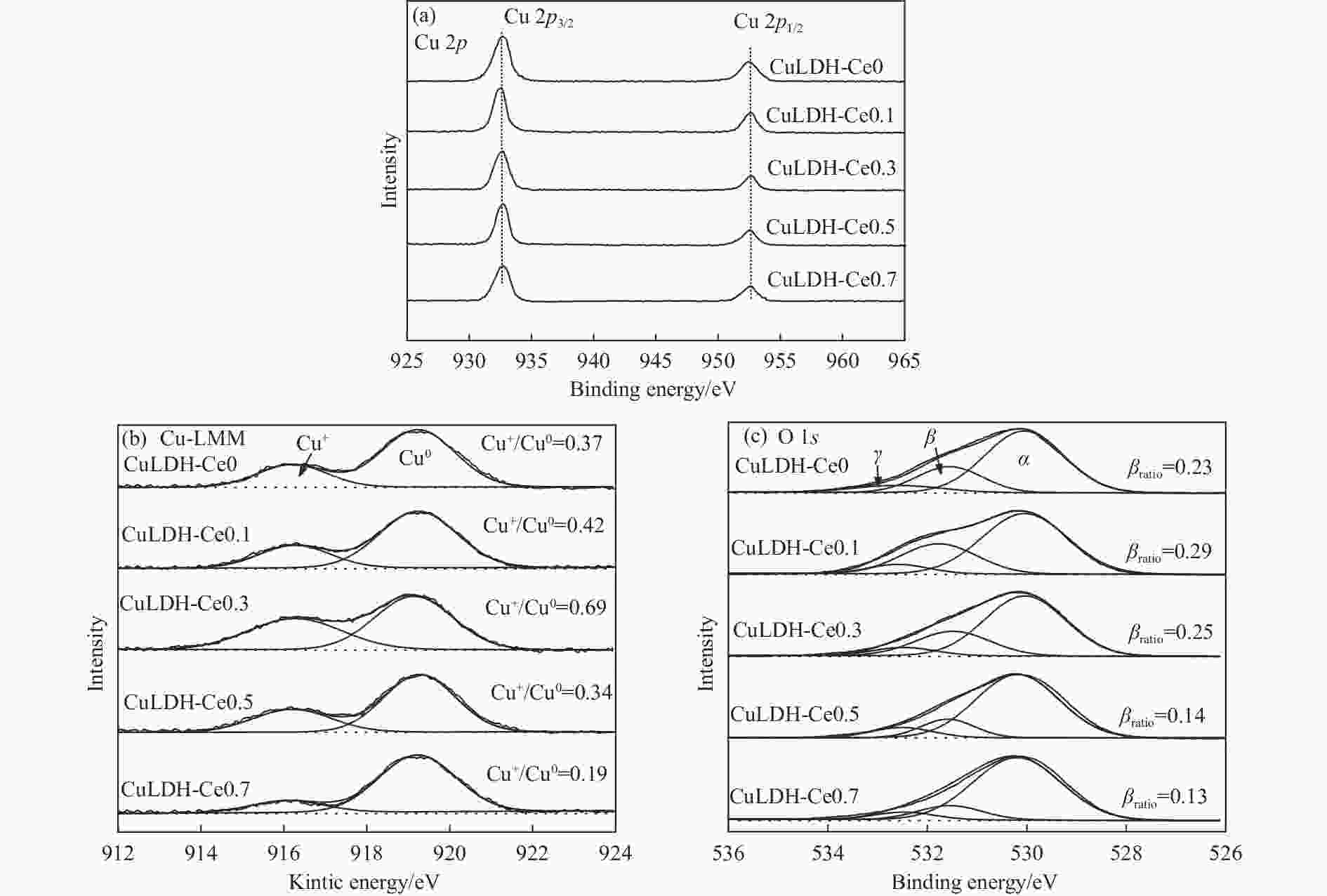
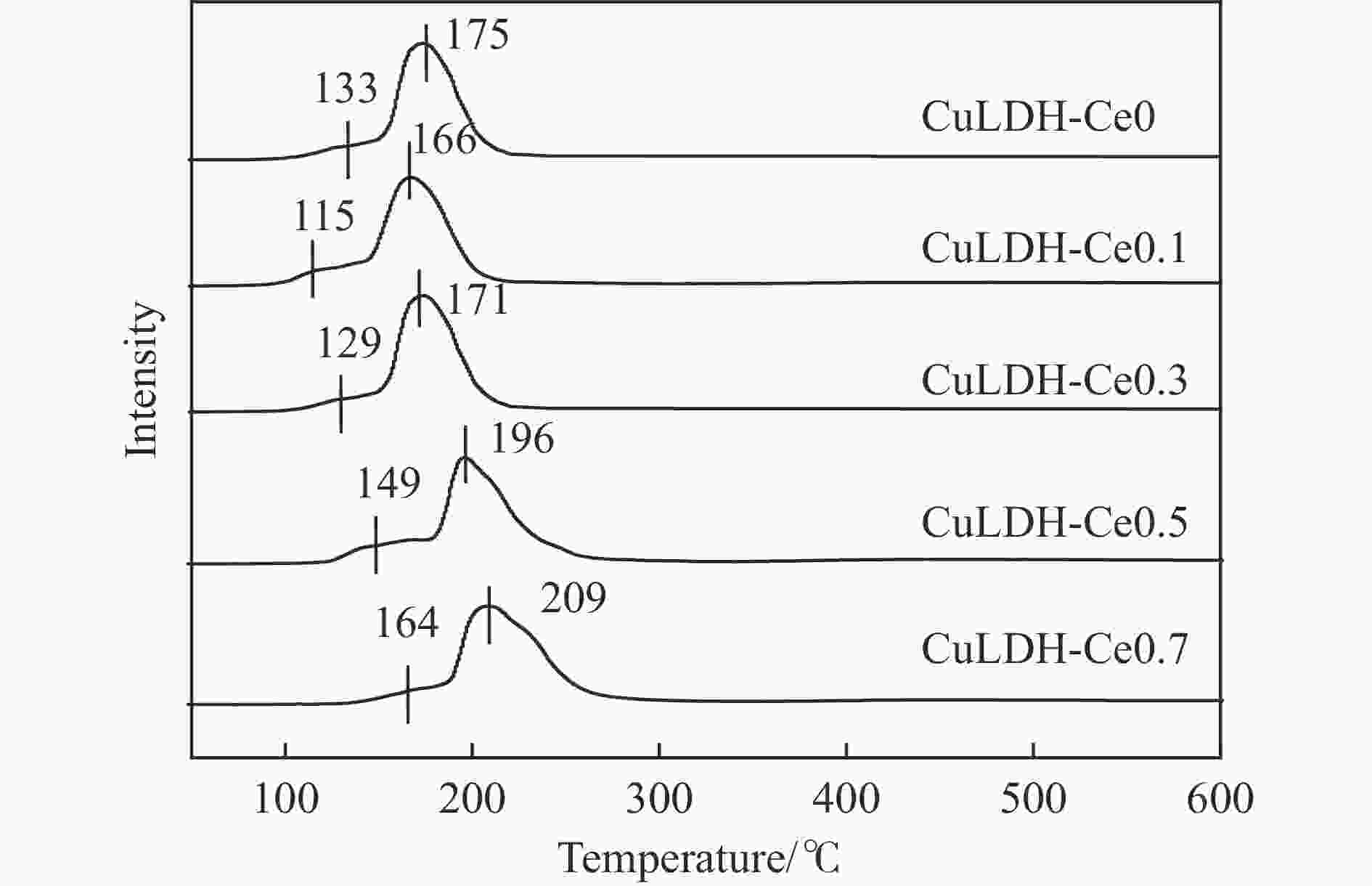
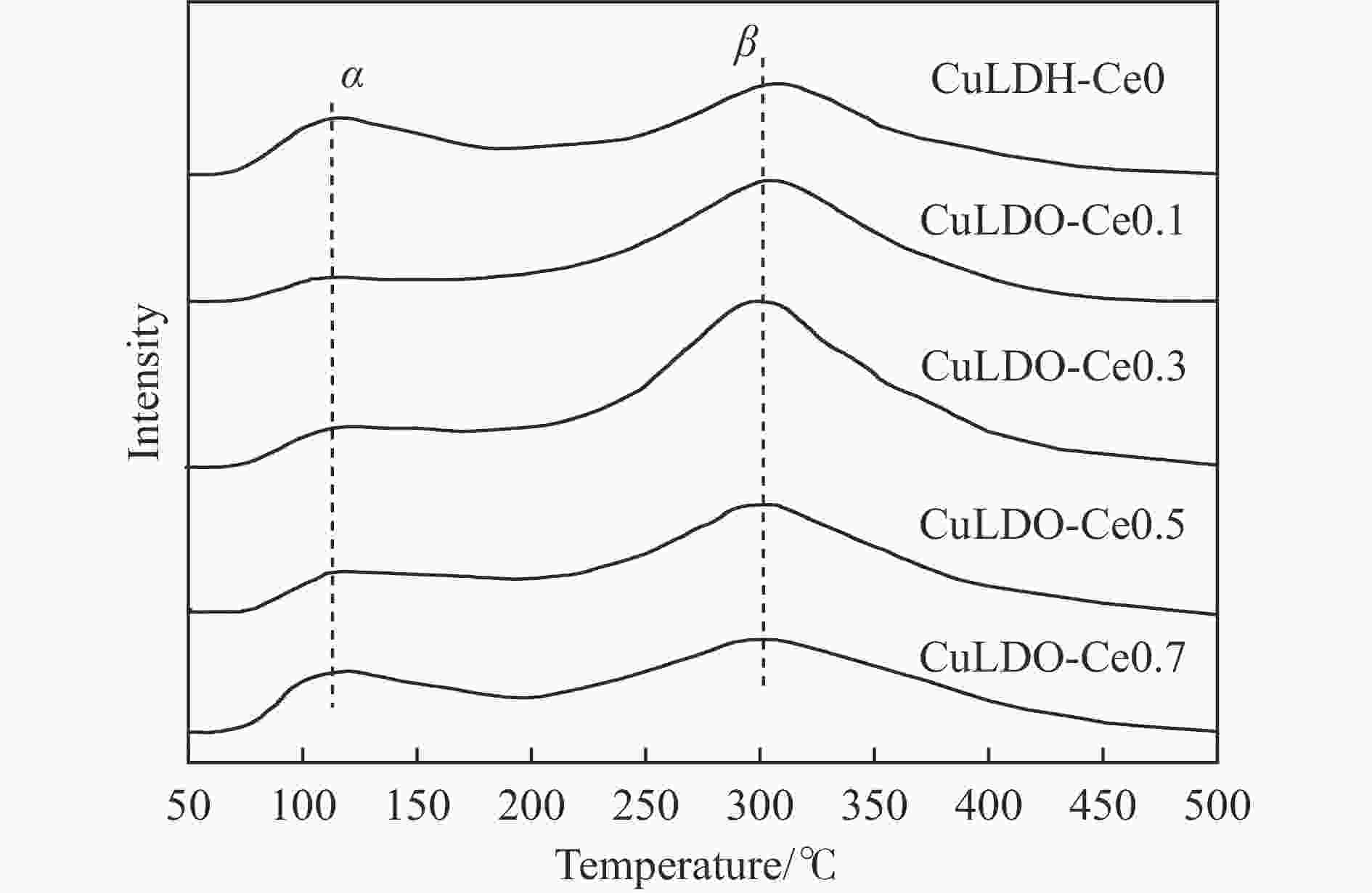
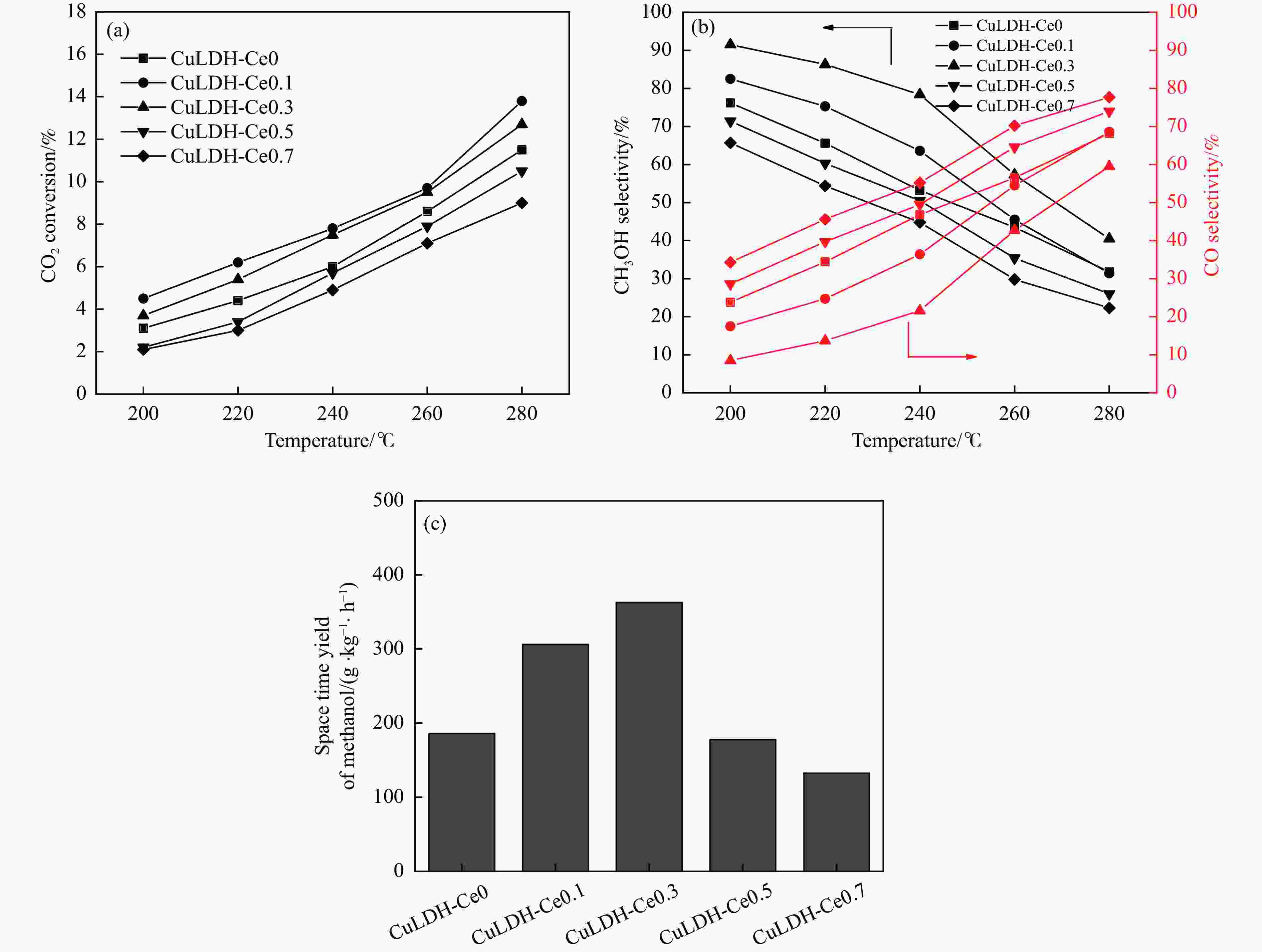
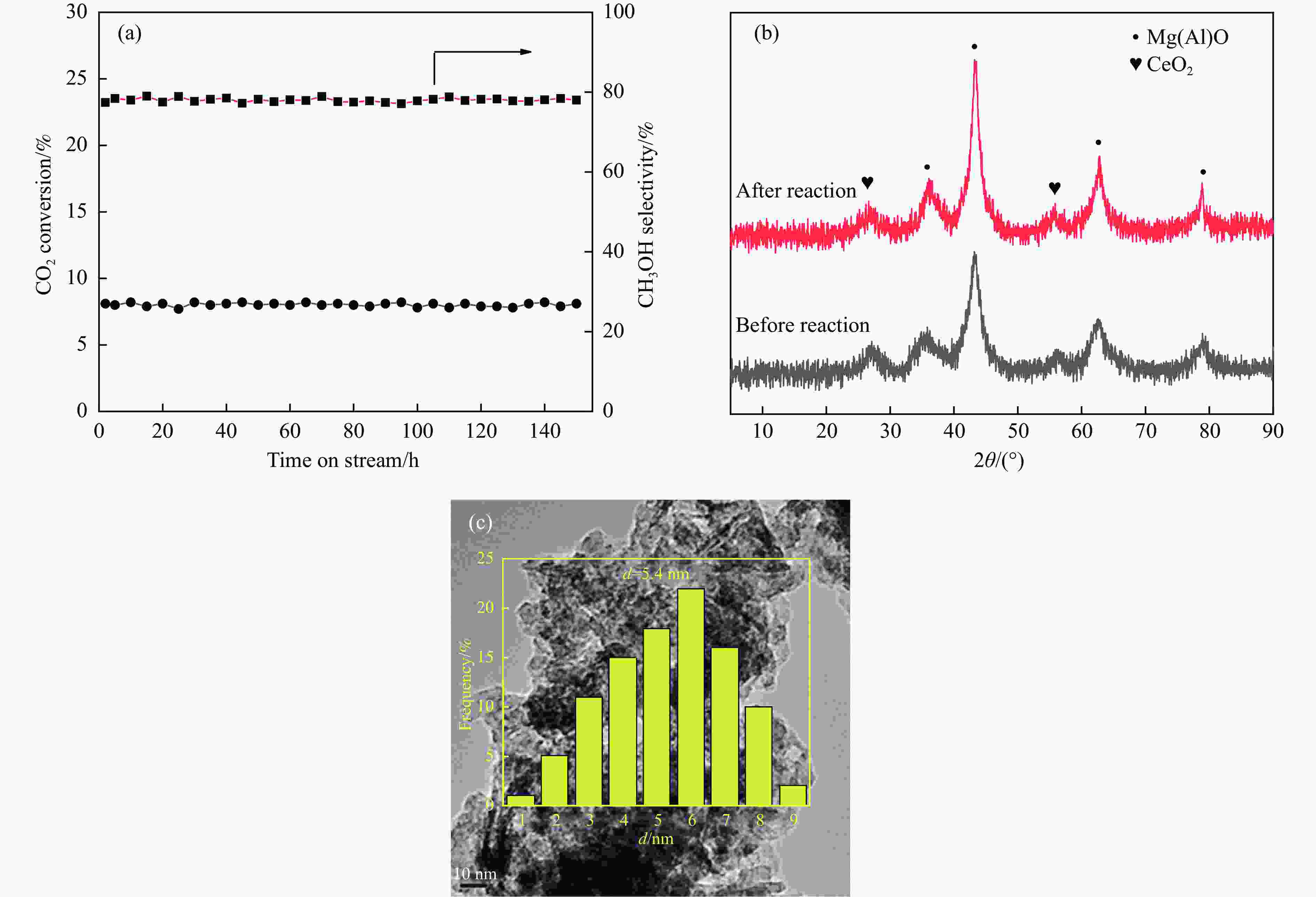
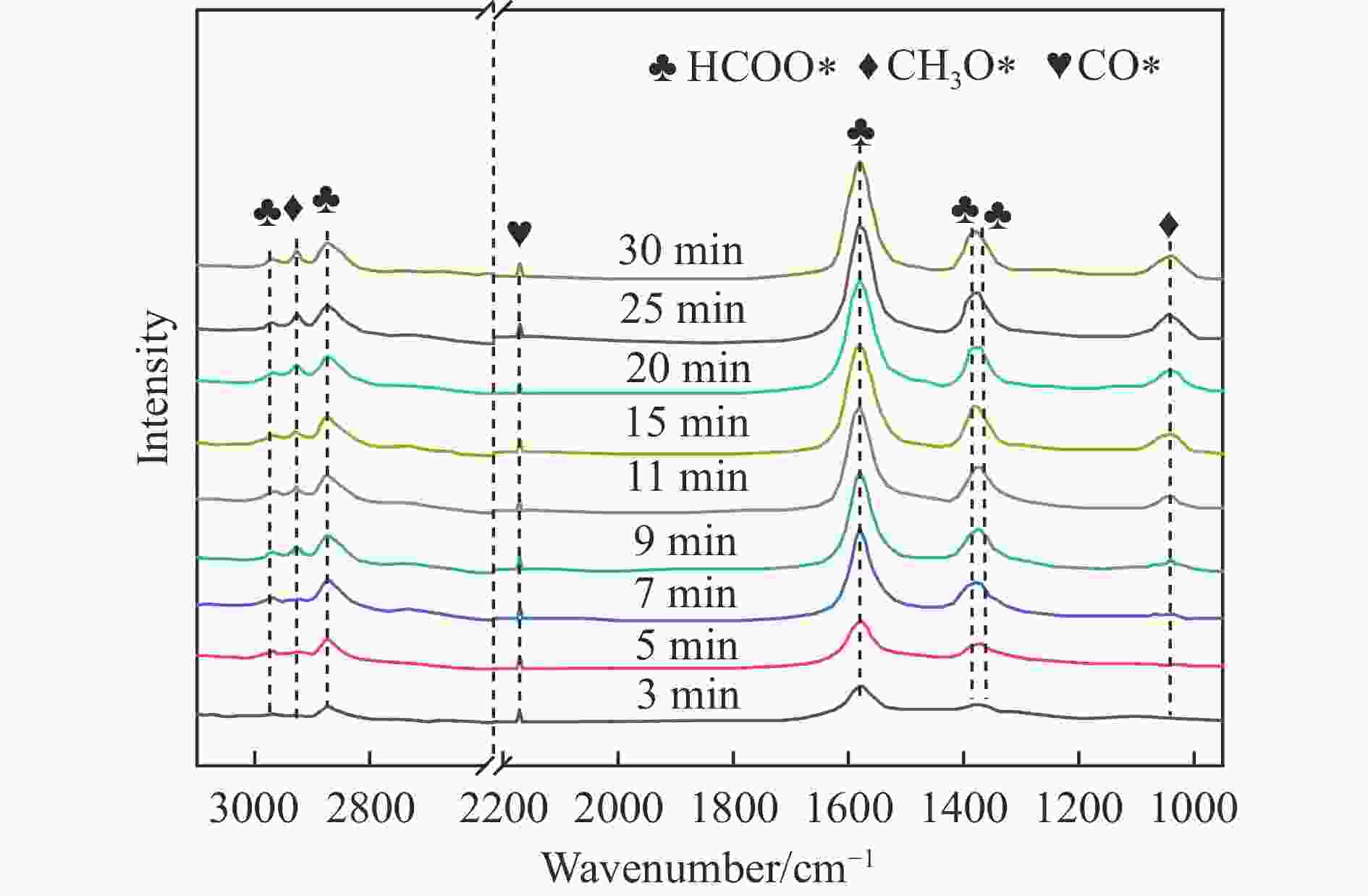
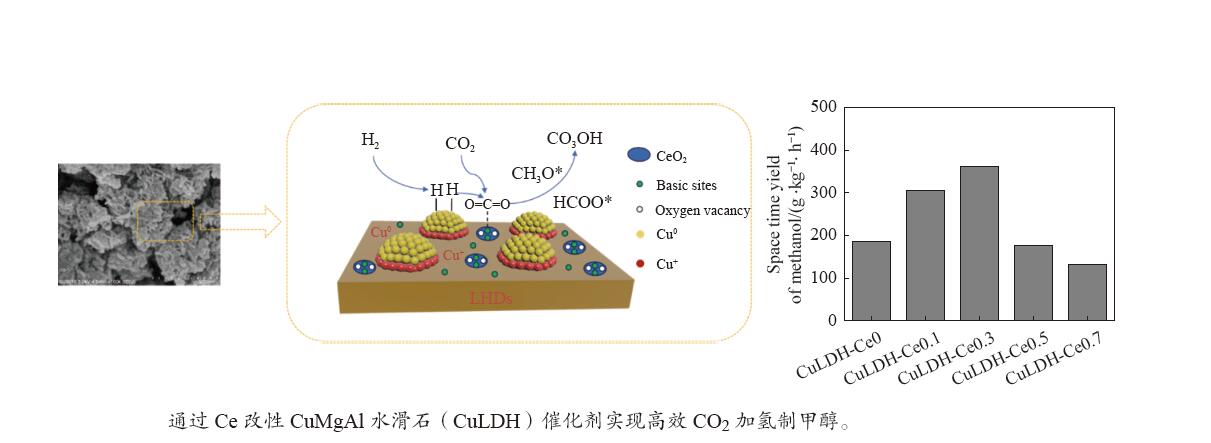
摘要: 通过向CuMgAl水滑石(CuLDH)催化剂中添加不同量的Ce,合成了一系列Ce改性的CuLDH-Cex催化剂。采用X射线衍射(XRD)、N2吸附-脱附(BET)、透射电子显微镜(TEM)、X射线光电子能谱(XPS)等分析手段对催化剂的理化性质进行表征。结果表明,添加Ce会改变Cu-LDH催化剂的水滑石结构,适量的Ce会增大催化剂的比表面积,改善了Cu颗粒的分散度。同时,适量的Ce有利于增加催化剂表面强碱性位点的密度和氧空位的数量,促进了CO2的吸附和转化。Ce有利于调变催化剂表面的Cu+/Cu0比例,较高的Cu+/Cu0比例有利于甲醇的生成。当Ce/Cu比例为0.3时,在空速为9000 mL/(g·h),温度为240 ℃,压力为2.5 MPa的条件下,催化剂的CO2的转化率为7.5%,甲醇选择性为78.4%,甲醇的时空收率最高可达362.8 g/(kg·h)。通过原位红外光谱(in-situ DRIFTS)证明CuLDH-Ce0.3催化剂在CO2加氢合成甲醇过程中遵循HCOO*反应路径。Abstract: A series of Ce modified CuLDH-Cex catalysts were synthesized by adding different amounts of Ce to CuMgAl hydrotalcite (CuLDH) catalysts. The physicochemical properties of the catalysts were characterized by X-ray diffraction (XRD), N2 adsorption-desorption (BET), transmission electron microscopy (TEM), X-ray photoelectron spectroscopy (XPS), etc. The results showed that the addition of Ce changed the hydrotalcite structure of CuLDH catalyst, and an appropriate amount of Ce increased the surface area of the catalyst and improved the dispersion of Cu particles. At the same time, an appropriate amount of Ce was beneficial for increasing the density of strong alkaline sites and the number of oxygen vacancies on the catalyst surface, promoting the adsorption and conversion of CO2. Ce was beneficial for adjusting the Cu+/Cu0 ratio on the catalyst surface, and a higher Cu+/Cu0 ratio was conducive to the formation of methanol. When the Ce/Cu ratio was 0.3, the catalyst exhibited higher activity with 7.5% CO2 conversion, 78.4% methanol selectivity and 362.8 g/(kg·h) spatiotemporal yield at 240 ℃ under 2.5 MPa with a GHSV=9000 mL/(g·h). It was proved by in-situ DRIFTS that CuLDH-Ce0.3 catalyst followed HCOO* reaction path during CO2 hydrogenation for methanol.
-
Key words:
- Ce /
- hydrotalcite /
- CO2 hydrogenation /
- alkaline sites /
- oxygen vacancies /
- methanol
-
表 1 CuLDH-Cex催化剂的物理化学性质
Table 1 Physical and chemical properties of CuLDH-Cex catalysts
Catalyst Molar ratio Cu/Mg/Al/Cea SBETb/
(m2·g−1)vpb/
(cm3·g−1)dpb/
nmdCu c/
nmdCud /
%SCud/
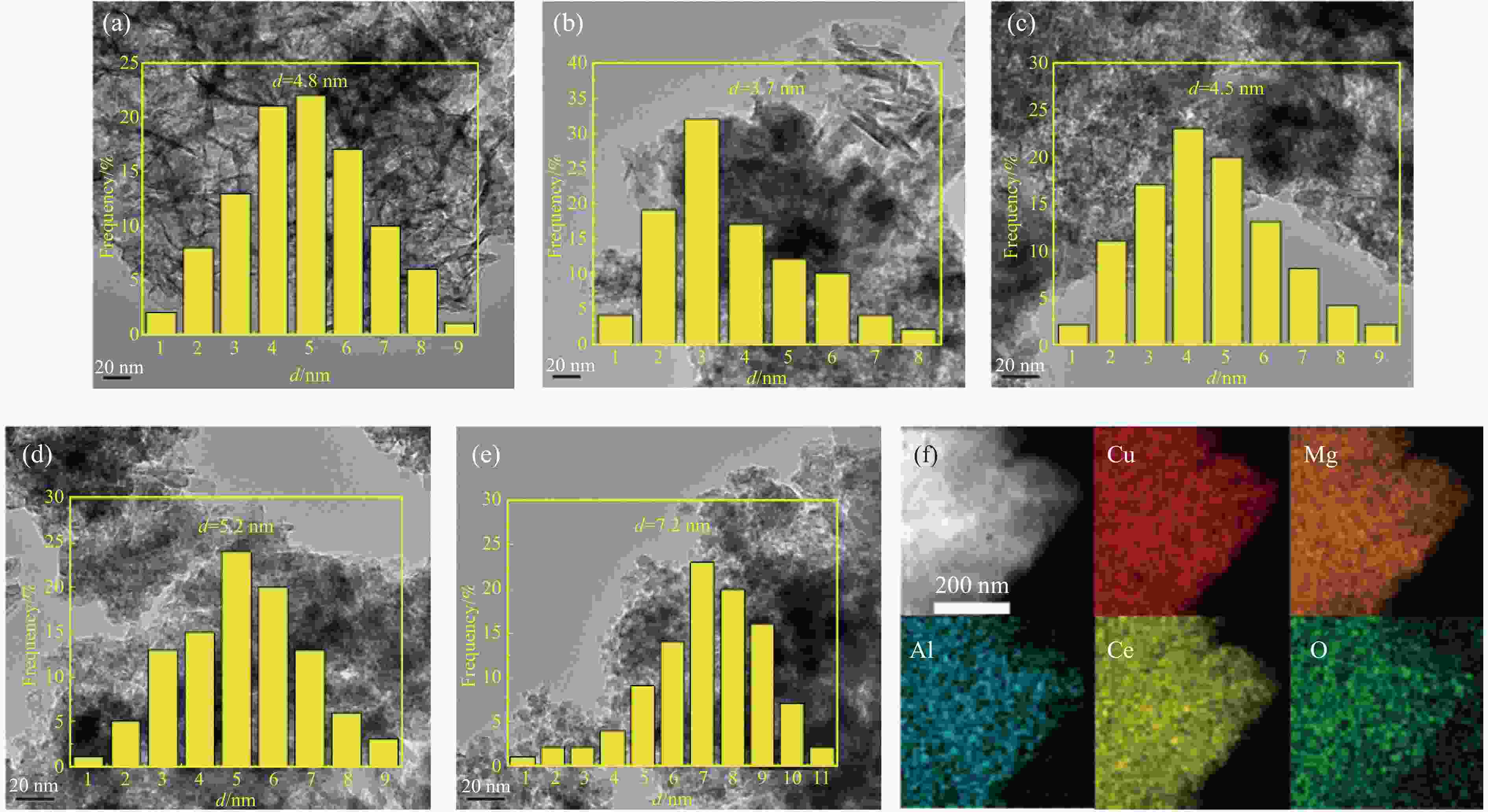
(m2·g−1)CuLDH-Ce0 9.9/29.7/10.4/0 199.5 0.41 6.2 4.9 23.5 32.1 CuLDH-Ce0.1 10.2./29.9/9.8/1.1 208.4 0.47 7.3 3.7 26.3 35.4 CuLDH-Ce0.3 10.1/30.3/9.7/2.9 205.6 0.43 6.5 4.5 24.2 33.2 CuLDH-Ce0.5 9.8/29.8/10.2/5.2 194.9 0.35 5.3 5.2 20.4 29.6 CuLDO-Ce0.7 10.3/29.7/9.9/7.1 182.3 0.31 4.4 7.2 18.1 25.3 a: Measured by ICP; b: Calculated by BET and BJH equations; c: Measured by TEM; d: Measured by N2O titration. 表 2 CuLDH-Cex催化剂碱性位点密度
Table 2 The basic sites density of CuLDH-Cex catalysts
Catalyst Total basic
sites/
(μmol·g−1)Weakly basic
sites/
(μmol·g−1)Moderately basic
sites/
(μmol·g−1)Density of moderately
basic site/
(μmol·m2)CuLDH-Ce0 232.8 55.7 177.1 0.89 CuLDH-Ce0.1 256.5 23.2 233.3 1.12 CuLDH-Ce0.3 297.2 34.5 262.7 1.28 CuLDH-Ce0.5 210.3 48.8 161.5 0.83 CuLDO-Ce0.7 198.6 64.3 134.3 0.74 -
[1] SHA F, HAN Z, TANG S, et al. Hydrogenation of carbon dioxide to methanol over non-Cu-based heterogeneous catalysts[J]. ChemSusChem,2020,13(23):6160−6181. doi: 10.1002/cssc.202002054 [2] LI S, GUO L, ISHIHARA T. Hydrogenation of CO2 to methanol over Cu/AlCeO catalyst[J]. Catal Today,2020,339:352−361. doi: 10.1016/j.cattod.2019.01.015 [3] CHANG K, ZHANG H, CHENG M J, et al. Application of ceria in CO2 conversion catalysis[J]. ACS Catal,2019,10(1):613−631. [4] JIA X, SUN K, WANG J, et al. Selective hydrogenation of CO2 to methanol over Ni/In2O3 catalyst[J]. J Energy Chem,2020,50:409−415. doi: 10.1016/j.jechem.2020.03.083 [5] TAN Q, SHI Z, WU D. CO2 hydrogenation to methanol over a highly active Cu-Ni/CeO2-nanotube catalyst[J]. Ind Eng Chem Res,2018,57(31):10148−10158. doi: 10.1021/acs.iecr.8b01246 [6] GAO P, ZHANG L N, LI S G, et al. Novel heterogeneous catalysts for CO2 hydrogenation to liquid fuels[J]. ACS Cent Sci,2020,6(10):1657−1670. doi: 10.1021/acscentsci.0c00976 [7] ZHAN F, FAN L, XU K, et al. Hierarchical sheet-like Cu/Zn/Al nanocatalysts derived from LDH/MOF composites for CO2 hydrogenation to methanol[J]. J CO2 Util,2019,33:222−232. doi: 10.1016/j.jcou.2019.05.021 [8] LU Z, WANG J, SUN K H, et al. CO2 hydrogenation to methanol over Rh/In2O3-ZrO2 catalyst with improved activity[J]. Green Chem Eng ,2022,3(2):165−170. [9] LI M M J, CHEN C P, AYVALI T, et al. CO2 Hydrogenation to methanol over catalysts derived from single cationic layer CuZnGa LDH precursors[J]. ACS Catal,2018,8(5):4390−4401. doi: 10.1021/acscatal.8b00474 [10] SHI Z, TAN Q, WU D. Enhanced CO2 hydrogenation to methanol over TiO2 nanotubes-supported CuO-ZnO-CeO2 catalyst[J]. Appl Catal A: Gen,2019,581:58−66. doi: 10.1016/j.apcata.2019.05.019 [11] CHENG S Y, KOU J W, GAO Z H, et al. Preparation of complexant-modified Cu/ZnO/Al2O3 catalysts via hydrotalcite-like precursors and its highly efficient application in direct synthesis of isobutanol and ethanol from syngas[J]. Appl Catal A: Gen,2018,556:113−220. doi: 10.1016/j.apcata.2018.02.027 [12] ZHANG F, ZHANG Y L, LIU Y, et al. Synthesis of Cu/Zn/Al/Mg catalysts on methanol production by different precipitation methods[J]. Mol Catal,2017,441:190−198. doi: 10.1016/j.mcat.2017.08.015 [13] XIAO S, ZHANG Y, GAO P, et al. Highly efficient Cu-based catalysts via hydrotalcite-like precursors for CO2 hydrogenation to methanol[J]. Catal Today,2017,281:327−36. doi: 10.1016/j.cattod.2016.02.004 [14] LI S Z, WANG Y, YANG B, et al. A highly active and selective mesostructured Cu/AlCeO catalyst for CO2 hydrogenation to methanol[J]. Appl Catal A: Gen,2019,571:51−60. doi: 10.1016/j.apcata.2018.12.008 [15] ZHANG C, YANG H Y, GAO P, et al. Preparation and CO2 hydrogenation catalytic properties of alumina microsphere supported Cu-based catalyst by deposition-precipitation method[J]. J CO2 Util,2017,17:263−272. doi: 10.1016/j.jcou.2016.11.015 [16] OUYANG B, TAN W L, LIU B. Morphology effect of nanostructure ceria on the Cu/CeO2 catalysts for synthesis of methanol from CO2 hydrogenation[J]. Catal Commun,2017,95:36−39. doi: 10.1016/j.catcom.2017.03.005 [17] GAO P, XIE R Y, WANG H, et al. Cu/Zn/Al/Zr catalysts via phase-pure hydrotalcite-like compounds for methanol synthesis from carbon dioxide[J]. J CO2 Util,2015,11:41−48. doi: 10.1016/j.jcou.2014.12.008 [18] FANG X, MEN Y H, WU F, et al. Moderate-pressure conversion of H2 and CO2 to methanol via adsorption enhanced hydrogenation[J]. Int J Hydrogen Energy,2019,44(39):21913−21925. doi: 10.1016/j.ijhydene.2019.06.176 [19] GAO P, ZHONG L S, ZHANG L N, et al. Yttrium oxide modified Cu/ZnO/Al2O3 catalysts via hydrotalcite-like precursors for CO2 hydrogenation to methanol[J]. Catal Sci Technol,2015,5(9):4365−4377. [20] GAO P, YANG H Y, ZHANG L N, et al. Fluorinated Cu/Zn/Al/Zr hydrotalcites derived nanocatalysts for CO2 hydrogenation to methanol[J]. J CO2 Util,2016,16:32−41. doi: 10.1016/j.jcou.2016.06.001 [21] GAO P, LI F, ZHAO N, et al. Influence of modifier (Mn, La, Ce, Zr and Y) on the performance of Cu/Zn/Al catalysts via hydrotalcite-like precursors for CO2 hydrogenation to methanol[J]. Appl Catal A: Gen,2013,468:442−452. doi: 10.1016/j.apcata.2013.09.026 [22] LIM A, YEO J W, ZENG H C. Preparation of CuZn-doped MgAl-layered double hydroxide catalysts through the memory effect of hydrotalcite for effective hydrogenation of CO2 to Methanol[J]. ACS Appl Energy Mater,2023,6(2):782−794. doi: 10.1021/acsaem.2c03045 [23] CORED J, MAZARIO J, CERDA-MORENO C, et al. Enhanced methanol production over non-promoted Cu-MgO-Al2O3 Materials with ex-solved 2 nm Cu particles: Insights from an operando spectroscopic study[J]. ACS Catal,2022,12(7):3845−3857. doi: 10.1021/acscatal.1c06044 [24] FANG X, MEN Y H, WU F, et al. Improved methanol yield and selectivity from CO2 hydrogenation using a novel Cu-ZnO-ZrO2 catalyst supported on Mg-Al layered double hydroxide (LDH)[J]. J CO2 Util,2019,29:57−64. doi: 10.1016/j.jcou.2018.11.006 [25] WANG X, ALABSI M H, ZHENG P, et al. PdCu supported on dendritic mesoporous CexZr1-xO2 as superior catalysts to boost CO2 hydrogenation to methanol[J]. J Colloid Interface Sci,2022,611:739−751. doi: 10.1016/j.jcis.2021.11.172 [26] YOO C J, LEE D W, KIM M S, et al. The synthesis of methanol from CO/CO2/H2 gas over Cu/Ce1-xZrxO2 catalysts[J]. J Mol Catal A: Chem,2013,378:255−262. doi: 10.1016/j.molcata.2013.06.023 [27] LI N, WANG W W, SONG L X, et al. CO2 hydrogenation to methanol promoted by Cu and metastable tetragonal CexZryOz interface[J]. J Energy Chem,2022,68:771−779. doi: 10.1016/j.jechem.2021.12.053 [28] HU X S, ZHAO C Y, GUAN Q X, et al. Selective hydrogenation of CO2 over a Ce promoted Cu-based catalyst confined by SBA-15[J]. Inorg Chem Front,2019,6(7):1799−1812. doi: 10.1039/C9QI00397E [29] WANG W W, QU Z P, SONG L X, et al. CO2 hydrogenation to methanol over Cu/CeO2 and Cu/ZrO2 catalysts: Tuning methanol selectivity via metal-support interaction[J]. J Energy Chem,2020,40:22−30. doi: 10.1016/j.jechem.2019.03.001 [30] ZHANG J P, SUN X H, WU C Y, et al. Engineering Cu+/CeZrO interfaces to promote CO2 hydrogenation to methanol[J]. J Energy Chem,2023,77:45−53. doi: 10.1016/j.jechem.2022.10.034 [31] SHAO Y W, WANG J Z, DU H N, et al. Importance of magnesium in Cu-based catalysts for selective conversion of biomass-derived furan compounds to diols[J]. ACS Sustainable Chem Eng,2020,8(13):5217−5228. doi: 10.1021/acssuschemeng.9b07841 [32] GOSWAMI K, ANANTHAKRISHNAN R. Ce-doped CuMgAl oxide as a redox couple mediated catalyst for visible light aided photooxidation of organic pollutants[J]. ACS Appl Nano Mater,2019,2(9):6030−6039. doi: 10.1021/acsanm.9b01557 [33] LI D L, CAI Y B, CHEN C Q, et al. Magnesium-aluminum mixed metal oxide supported copper nanoparticles as catalysts for water-gas shift reaction[J]. Fuel,2016,184:382−389. doi: 10.1016/j.fuel.2016.06.131 [34] ZHU J D, CIOLCA D, LIU L, et al. Flame synthesis of Cu/ZnO-CeO2 catalysts: Synergistic metal-support interactions promote CH3OH selectivity in CO2 hydrogenation[J]. ACS Catal,2021,11(8):4880−4892. doi: 10.1021/acscatal.1c00131 [35] CHEN G Q, YU J, LI G H, et al. Cu+-ZrO2 interfacial sites with highly dispersed copper nanoparticles derived from Cu@UiO-67 hybrid for efficient CO2 hydrogenation to methanol[J]. Int J Hydrogen Energy,2023,48(7):2605−2616. doi: 10.1016/j.ijhydene.2022.10.172 [36] NIU J T, LIU H Y, JIN Y, et al. Comprehensive review of Cu-based CO2 hydrogenation to CH3OH: Insights from experimental work and theoretical analysis[J]. Int J Hydrogen Energy,2022,47(15):9183−9200. doi: 10.1016/j.ijhydene.2022.01.021 [37] XU Y N, GAO Z H, PENG L, et al. A highly efficient Cu/ZnOx/ZrO2 catalyst for selective CO2 hydrogenation to methanol[J]. J Catal,2022,414:236−244. doi: 10.1016/j.jcat.2022.09.011 [38] XU Y M, DING Z L, QIU R, et al. Effect of support and reduction temperature in the hydrogenation of CO2 over the Cu-Pd bimetallic catalyst with high Cu/Pd ratio[J]. Int J Hydrogen Energy,2022,47(65):27973−27985. doi: 10.1016/j.ijhydene.2022.06.110 [39] WANG Y D, YU H R, HU Q, et al. Application of microimpinging stream reactor coupled with ultrasound in Cu/CeZrOx solid solution catalyst preparation for CO2 hydrogenation to methanol[J]. Renewable Energy,2023,202:834−843. doi: 10.1016/j.renene.2022.11.075 [40] SUN X C, JIN Y F, CHENG Z Z, et al. Dual active sites over Cu-ZnO-ZrO2 catalysts for carbon dioxide hydrogenation to methanol[J]. J Environ Sci,2023,131:162−172. doi: 10.1016/j.jes.2022.10.002 [41] ZHANG C C, WANG L T, ETIM U J, et al. Oxygen vacancies in Cu/TiO2 boost strong metal-support interaction and CO2 hydrogenation to methanol[J]. J Catal,2022,413:284−296. doi: 10.1016/j.jcat.2022.06.026 [42] SHEN C Y, BAO Q Q, XUE W J, et al. Synergistic effect of the metal-support interaction and interfacial oxygen vacancy for CO2 hydrogenation to methanol over Ni/In2O3 catalyst: A theoretical study[J]. J Energy Chem,2022,65:623−629. doi: 10.1016/j.jechem.2021.06.039 [43] LI J, DU T, LI Y N, et al. Novel layered triple hydroxide sphere CO2 adsorbent supported copper nanocluster catalyst for efficient methanol synthesis via CO2 hydrogenation[J]. J Catal,2022,409:24−32. doi: 10.1016/j.jcat.2022.03.020 [44] GUO T, GUO Q, LI S Z, et al. Effect of surface basicity over the supported Cu-ZnO catalysts on hydrogenation of CO2 to methanol[J]. J Catal,2022,407:312−321. doi: 10.1016/j.jcat.2022.01.035 [45] COLLINS S E, BALTANAS M A, DELGDO J J, et al. CO2 hydrogenation to methanol on Ga2O3-Pd/SiO2 catalysts: Dual oxide-metal sites or (bi)metallic surface sites?[J]. Catal Today,2021,381:154−62. doi: 10.1016/j.cattod.2020.07.048 [46] SHA F, TANG S, TANG C Z, et al. The role of surface hydroxyls on ZnZrOx solid solution catalyst in CO2 hydrogenation to methanol[J]. Chin J Catal,2023,45:162−173. doi: 10.1016/S1872-2067(22)64176-7 [47] DASIREDDY V D B C, LIKOZAR B. The role of copper oxidation state in Cu/ZnO/Al2O3 catalysts in CO2 hydrogenation and methanol productivity[J]. Renewable Energy,2019,140:452−460. doi: 10.1016/j.renene.2019.03.073 [48] HAN X Y, LI M S, CHANG X, et al. Hollow structured Cu@ZrO2 derived from Zr-MOF for selective hydrogenation of CO2 to methanol[J]. J Energy Chem,2022,71:277−287. doi: 10.1016/j.jechem.2022.03.034 -




 下载:
下载: