Catalytic decomposition of N2O over Mg-Co composite oxides hydrothermally prepared by using carbon sphere as template
-
摘要: 用自制的碳球为模板剂,尿素为沉淀剂,120℃水热合成尖晶石型Mg-Co复合氧化物(MgCo2O4),在其表面浸渍K2CO3溶液制得K改性催化剂,用于催化分解N2O。用X射线衍射(XRD)、N2物理吸附-脱附、扫描电镜(SEM)、H2程序升温还原(H2-TPR)、O2程序升温脱附(O2-TPD)、X射线光电子能谱(XPS)等技术对催化剂进行结构表征,考察了钴镁离子/碳球的质量比、尿素/钴镁离子的物质的量比等制备参数对催化剂活性的影响。结果表明,钴镁离子/碳球的质量比为0.192、尿素/钴镁离子的物质的量比为2,制得的MgCo2O4催化剂活性较高。K改性MgCo2O4催化剂在400℃有氧无水、有氧有水条件下连续反应50 h,N2O转化率分别保持在91%和62%,稳定性较好。
-
关键词:
- N2O催化分解 /
- Mg-Co复合氧化物 /
- K改性催化剂 /
- 催化活性
Abstract: MgCo2O4 composite oxides with spinel structure were hydrothermally prepared at 120℃ by using carbon sphere as template and urea as precipitant. K2CO3 solution was impregnated on MgCo2O4 and the K-modified catalyst was obtained. These catalysts were applied in catalytic decomposition of N2O and characterized by X-ray diffraction(XRD), nitrogen physisorption, scanning electron microscopy (SEM), temperature-programmed reduction of hydrogen (H2-TPR), temperature-programmed desorption of oxygen (O2-TPD), and X-ray photoelectron spectroscopy (XPS). Effect of catalysts preparation parameters such as mass ratio of cobalt and magnesium to carbon sphere, molar ratio of urea to metallic cations, on their catalytic activity was investigated. It is shown that the catalyst prepared with mass ratio 0.192 of cobalt and magnesium to carbon sphere, molar ratio 2 of urea to cobalt and magnesium cations, exhibits higher catalytic activity than others. Furthermore, 91% and 62% conversions of N2O could be reached over 0.02 K/MgCo2O4 catalyst at 400℃ after continuous running for 50 h under the atmosphere of oxygen-alone and oxygen-steam together, respectively, revealing that K-modified MgCo2O4 catalyst is stable under both reaction atmospheres. -
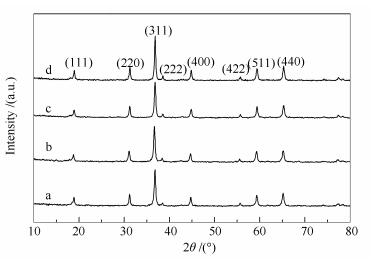
Table 1 Crystallite size and BET surface area of MgCo2O4 prepared by changing the mass ratio of cobalt and magnesium to carbon sphere
Catalyst Crystallite size d/nma BET surface area A/(m2·g-1) MgCo2O4-0.149 33.2 32.1 MgCo2O4-0.192 32.1 25.8 MgCo2O4-0.234 33.3 21.1 MgCo2O4-0.320 47.6 25.4 a: calculated by scherrer equation on the basis of (311) crystallographic plane data in XRD patterns Table 2 XPS data of K-modified MgCo2O4 catalyst
Catalyst Binding energies of Co 2p3/2 /eV Co2+/ Co3+ ratio Co2+ Co3+ MgCo2O4 780.0 782.0 1.80 0.02K/MgCo2O4 779.8 781.9 2.10 -
[1] PACHATOURIDOU E, PAPISTA E, DELIMITIS A, VASILIADES M A, EFSTATHIOU A M, AMIRIDIS M D, ALEXEEV O S, BLOOM D, MARNELLOS G E, KONSOLAKIS M, ILIOPOULOU E. N2O decomposition over ceria-promoted Ir/Al2O3 catalysts:The role of ceria[J]. Appl Catal B:Environ, 2016, 187:259-268. doi: 10.1016/j.apcatb.2016.01.049 [2] CARABINEIRO S A C, PAPISTA E, MARNELLOS G E, TAVARES P B, MALDONADO-HÓDAR F J, KONSOLAKIS M. Catalytic decomposition of N2O on inorganic oxides:Effect of doping with Au nanoparticles[J]. Mol Catal, 2017, 436:78-89. doi: 10.1016/j.mcat.2017.04.009 [3] LIN Y, MENG T, MA Z. Catalytic decomposition of N2O over RhOx supported on metal phosphates[J]. J Ind Eng Chem, 2015, 28:138-146. doi: 10.1016/j.jiec.2015.02.009 [4] LIU Z M, HE C X, CHEN B H, LIU H Y. CuO-CeO2 mixed oxide catalyst for the catalytic decomposition of N2O in the presence of oxygen[J]. Catal Today, 2017, 297:78-83. doi: 10.1016/j.cattod.2017.05.074 [5] GRZYBEK G, WÓJCIK S, CIURA K, GRYBO Ś J, INDYKA P, OSZAJCA M, STELMACHOWSKI P, WITKOWSKI S, INGER M, WILK M, KOTARBA A, SOJKA Z. Influence of preparation method on dispersion of cobalt spinel over alumina extrudates and the catalyst deN2O activity[J]. Appl Catal B:Environ, 2017, 210:34-44. doi: 10.1016/j.apcatb.2017.03.053 [6] XIE P F, MA Z, ZHOU H B, HUANG C Y, YUE Y H, SHEN W, XU H L, HUA W M, GAO Z. Catalytic decomposition of N2O over Cu-ZSM-11 catalysts[J]. Microporous Mesoporous Mater, 2014, 191:112-117. doi: 10.1016/j.micromeso.2014.02.044 [7] WU M F, WANG H, ZHONG L S, ZHANG X Y, HAO Z P, SHEN Q, WEI W, QIAN G G, SUN Y H. Effects of acid pretreatment on Fe-ZSM-5 and Fe-beta catalysts for N2O decomposition[J]. Chin J Catal, 2016, 37(6):898-907. doi: 10.1016/S1872-2067(15)61052-X [8] ABU-ZIED B M, SOLIMAN S A, ABDELLAH S E. Pure and Ni-substituted Co3O4 spinel catalysts for direct N2O decomposition[J]. Chin J Catal, 2014, 35(7):1105-1112. doi: 10.1016/S1872-2067(14)60058-9 [9] KLYUSHINA A, PACULTOVÁ K, KARÁSKOVÁK, JIRÁTOVÁ K, RITZ M, FRIDRICHOVÁ D, VOLODARSKAJA A, OBALOVÁ L. Effect of preparation method on catalytic properties of Co-Mn-Al mixed oxides for N2O decomposition[J]. J Mol Catal A:Chem, 2016, 425:237-247. doi: 10.1016/j.molcata.2016.10.014 [10] OBALOVÁL, PACULTOVÁ K, BALABÁNOVÁ J, JIRÁTOVÁ K, BASTL Z, VALÁŠKOVÁ M, LACNY Z, KOVANDA F. Effect of Mn/Al ratio in Co-Mn-Al mixed oxide catalysts prepared from hydrotalcite-like precursors on catalytic decomposition of N2O[J]. Catal Today, 2007, 119(1/4):233-238. https://www.researchgate.net/publication/229168042_Effect_of_MnAl_ratio_in_Co-Mn-Al_mixed_oxide_catalysts_prepared_from_hydrotalcite-like_precursors_on_catalytic_decomposition_of_N2O [11] PU Z Y, LIU Y, ZHOU H, HUANG W Z, ZHENG Y F, LI X N. Catalytic combustion of lean methane at low temperature over ZrO2-modified Co3O4 catalysts[J]. Appl Surf Sci, 2017, 422:85-93. doi: 10.1016/j.apsusc.2017.05.231 [12] CHELLAM U, XU Z P, ZENG H C. Low-temperature synthesis of MgxCo1-xCo2O4 spinel catalysts for N2O decomposition[J]. Chem Mater, 2000, 12(3):650-658. doi: 10.1021/cm990355l [13] ABU-ZIED B M. Nitrous oxide decomposition over alkali-promoted magnesium cobaltite catalysts[J]. Chin J Catal, 2011, 32(2):264-272. https://www.sciencedirect.com/science/article/pii/S187220671060174X [14] ZHENG L, WU C C, XU X F. Catalytic decomposition of N2O over Mg-Co and Mg-Mn-Co composite oxides[J]. J Fuel Chem Technol, 2016, 44(12):1494-1501. doi: 10.1016/S1872-5813(17)30005-1 [15] SEVILLA M, FUERTES A B. Chemical and structural properties of carbonaceous products obtained by hydrothermal carbonization of saccharides[J]. Chem Eur J, 2009, 15(16):4195-4203. doi: 10.1002/chem.v15:16 [16] SUN X M, LI Y D. Colloidal carbon spheres and their core/shell structures with noble-metal nanoparticles[J]. Angew Chem Int Ed, 2004, 43(5):597-601. doi: 10.1002/(ISSN)1521-3773 [17] TITIRICI M M, ANTONIETTI M, THOMAS A. A generalized synthesis of metal oxide hollow spheres using a hydrothermal approach[J]. Chem Mater, 2006, 18(16):3808-3812. doi: 10.1021/cm052768u [18] YU J G, YU X X, HUANG B B, ZHANG X Y, DAI Y. Hydrothermal synthesis and visible-light photocatalytic activity of novel cage-like ferric oxide hollow spheres[J]. Cryst Growth Des, 2009, 9(3):1474-1480. doi: 10.1021/cg800941d [19] JIA G, YANG M, SONG Y H, YOU H P, ZHANG H J. General and facile method to prepare uniform Y2O3:Eu hollow microspheres[J]. Cryst Growth Des, 2009, 9(1):301-307. doi: 10.1021/cg8004823 [20] FRANKEN T, PALKOVITS R. Investigation of potassium doped mixed spinels Cux Co3-xO4 as catalysts for an efficient N2O decomposition in real reaction conditions[J]. Appl Catal B:Environ, 2015, 176-177:298-305. doi: 10.1016/j.apcatb.2015.04.002 [21] KIM M J, LEE S J, RYU I S, JEON M W, MOON S H, ROH H S, JEON S G. Catalytic decomposition of N2O over cobalt based spinel oxides:The role of additives[J]. Mol Catal, 2017, 442:202-207. doi: 10.1016/j.mcat.2017.05.029 [22] GRZYBEK G, WÓJCIK S, LEGUTKO P, GRYBOS J, INDYKA P, LESZCZYNSKI B, KOTARBA A, SOJKA Z. Thermal stability and repartition of potassium promoter between the support and active phase in the K-Co2.6Zn0.4O4|α -Al2O3 catalyst for N2O decomposition:Crucial role of activation temperature on catalytic performance[J]. Appl Catal B:Environ, 2017, 205:597-604. doi: 10.1016/j.apcatb.2017.01.005 [23] KLYUSHINA A, PACULTOVÁK, KREJCOVÁ S, SŁOWIK G, JIRÁTOVÁ K, KOVANDA F, RYCZKOWSKI J, OBALOVÁ L. Advantages of stainless steel sieves as support for catalytic N2O decomposition over K-doped Co3O4[J]. Catal Today, 2015, 257:2-10. doi: 10.1016/j.cattod.2015.05.015 [24] WANG S L, QIAN L Q, XU H, LÜ G L, DONG W J, TANG W H. Synthesis and structural characterization of cobalt hydroxide carbonate nanorods and nanosheets[J]. J Alloys Compd, 2009, 476(1/2):739-743. https://www.sciencedirect.com/science/article/pii/S0925838808015570 [25] AMROUSSE R, TSUTSUMI A, BACHAR A, LAHCENE D. N2O catalytic decomposition over nano-sized particles of Co-substituted Fe3O4substrates[J]. Appl Catal A:Gen, 2013, 450(2):253-260. http://www.doc88.com/p-9905362717867.html [26] ZABILSKIY M, DJINOVI ĆP, TCHERNYCHOVA E, PINTAR A. N2O decomposition over CuO/CeO2 catalyst:New insights intoreaction mechanism and inhibiting action of H2O and NO by operando techniques[J]. Appl Catal B:Environ, 2016, 197:146-158. doi: 10.1016/j.apcatb.2016.02.024 -





 下载:
下载: