Performance of the modified Cu-Mn/SAPO-34 catalysts in the selective catalytic reduction of NOx by NH3
-
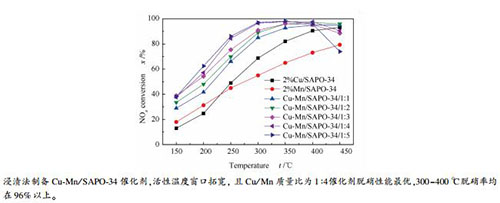
摘要: 采用浸渍法制备了系列铜锰复合氧化物分子筛催化剂(Cu-Mn/SAPO-34),在固定床反应器上考察不同Cu/Mn质量比对分子筛催化剂选择催化还原NO的影响,利用XRD、NH3-TPD、H2-TPR、XPS等手段对催化剂进行了表征分析。结果表明,双金属改性的Cu-Mn/SAPO-34催化剂在NH3-SCR反应中表现出较为优异的催化活性,具有较宽的活性温度窗口。当Cu/Mn质量比为1:4时,催化剂具有最宽的活性温度窗口,NOx转化率在250℃已达到85.39%,在300-400℃转化率均达到96%以上,450℃时仍能达到90%。铜和锰物种高度分散于催化剂表面,未改变SAPO-34的晶体结构,且构成协同作用。Cu-Mn共同负载促进了Cu2+向Cu+的转变,增加了高价态Mn4+和Mn3+的比例,有利于提高低温活性,促进催化反应的进行。Cu-Mn/SAPO-34/1:4具备丰富的酸性位、良好的氧化还原性能和抗SO2/H2O性能,该配比有助于催化剂的催化活性和稳定性的提高。
-
关键词:
- 脱硝 /
- Cu-Mn/SAPO-34 /
- NH3-SCR /
- 氮氧化物 /
- 催化剂
Abstract: A series of Cu-Mn/SAPO-34 catalysts with different mass ratios of Cu to Mn were prepared by impregnation method. The influence of Cu and Mn loading on the denitrification performance was investigated in a fixed-bed reactor. XRD, NH3-TPD, H2-TPR, XPS were used to characterize and analyze the catalysts. The results show that the bimetallic modified Cu-Mn/SAPO-34 have excellent catalytic activity and broad active temperature window. Especially, the Cu-Mn/SAPO-34/1:4 catalyst with a Cu/Mn mass ratio of 1:4 has the widest active temperature window, its denitrification rates could reach 85.39% at 250℃, 96% at 300-400℃, and up to 90% at 450℃. Cu and Mn species are highly dispersed on the surface of the catalyst and do not change the crystal structure of SAPO-34. Co-doping of Cu and Mn promotes the transformation of Cu2+ to Cu+, increases the ratio of Mn4+ to Mn3+, improves the activity at low temperature and promotes the catalytic reaction. Cu-Mn/SAPO-34/1:4 catalyst has rich acid sites, good redox performance and resistance to SO2 and H2O, which can improve the activity and stability of the catalysts.-
Key words:
- denitration /
- Cu-Mn/SAPO-34 /
- NH3-SCR /
- NOx /
- catalyst
-
表 1 3种不同催化剂的XPS表征
Table 1 XPS results of the catalysts
Catalyst Binding energy E/eV Relative content w/% Mn2+ Mn3+ Mn4+ Cu+ Cu2+ Mn2+ Mn3+ Mn4+ Cu+ Cu2+ Cu/SAPO-34 - - - 932.89 935.33 - - - 31.16 68.84 Mn/SAPO-34 641.4 643.02 645.39 - - 48.13 26.84 25.02 - - Cu-Mn/SAPO-34 641.44 642.86 645.9 932.99 935.06 31.27 40.97 27.76 50.74 49.26 -
[1] 郭凤, 余剑, 牟洋, 初茉, 许光文.宽工作温度烟气脱硝催化剂制备及反应机理研究[J].燃料化学学报, 2014, 42(1):101-109. http://www.ccspublishing.org.cn/article/id/100033059GUO Feng, YU Jian, MU Yang, CHU Mo, XU Guang-wen. Preparation of catalyst with wide working-temperature and the reaction mechanism of flue gas denitration[J]. J Fuel Chem Technol, 2014, 42(1):101-109. http://www.ccspublishing.org.cn/article/id/100033059 [2] 胡海鹏, 王学涛, 张兴宇, 苏晓昕, 杨晓东, 史瑞华. Fe-Cu/ZSM-5催化剂的NH3-SCR脱硝性能[J].燃料化学学报, 2018, 46(2):225-232. doi: 10.3969/j.issn.0253-2409.2018.02.013HU Hai-peng, WANG Xue-tao, ZHANG Xing-yu, SU Xiao-xin, YANG Xiao-dong, SHI Rui-hua. Performance of Fe-Cu/ZSM-5 catalyst in the DeNOx process via NH3-SCR[J]. J Fuel Chem Technol, 2018, 46(2):225-232. doi: 10.3969/j.issn.0253-2409.2018.02.013 [3] 熊志波, 路春美.铁铈复合氧化物催化剂SCR脱硝的改性研究[J].燃料化学学报, 2013, 41(3):361-367. doi: 10.3969/j.issn.0253-2409.2013.03.016XIONG Zhi-bo, LU Chun-mei. Study on the modification of iron-cerium mixed oxide catalyst for selective catalytic reduction of NO[J]. J Fuel Chem Technol, 2013, 41(3):361-367. doi: 10.3969/j.issn.0253-2409.2013.03.016 [4] PANAHI P N, SALARI D, TSENG H H, NIAEI A, MOUSAVI S M. Effect of the preparation method on activity of Cu-ZSM-5 nanocatalyst for the selective reduction of NO by NH3[J]. Environ Technol, 2017, 38(15):1852-1861. doi: 10.1080/09593330.2016.1238964 [5] VENNESTRØM P N R, JANSSENS T V W, KUSTOV A, GRILL M, PUIG-MOLINA A, LUNDEGAARD L F, TIRUVALAM R R, CONCEPCIÓN P, CORMA A. Influence of lattice stability on hydrothermal deactivation of Cu-ZSM-5 and Cu-IM-5 zeolites for selective catalytic reduction of NOx by NH3[J]. J Catal, 2014, 309:477-490. doi: 10.1016/j.jcat.2013.10.017 [6] PARK J H, PARK H J, BAIK J H, NAM I S, SHIN C H, LEE J H, CHO B K, OH S H. Hydrothermal stability of CuZSM5 catalyst in reducing NO by NH3 for the urea selective catalytic reduction process[J]. J Catal, 2006, 240(1):47-57. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=4f72f19d59c5af4fddcbfc8b430c6b33 [7] YAN C D, CHENG H, YUAN Z S, WANG S D. The role of isolated Cu2+ location in structural stability of Cu-modified SAPO-34 in NH3-SCR of NO[J]. Environ Technol, 2015, 36(2):169-177. https://www.researchgate.net/publication/268790882_The_role_of_isolated_Cu2_location_in_structural_stability_of_Cu-modified_SAPO-34_in_NH3-SCR_of_NO [8] HUANG L M, WANG X M, YAO S L, JIANG B Q, CHEN X Y, WANG X. Cu-Mn bimetal ion-exchanged SAPO-34 as an active SCR catalyst for removal of NOx from diesel engine exhausts[J]. Catal Commun, 2016, 81:54-57. doi: 10.1016/j.catcom.2016.03.010 [9] 乔南利, 杨忆新, 刘清龙, 宋焕巧, 禹耕之, 罗明生.载体物化性质对锰铈催化剂NH3-SCR脱硝性能的影响[J].燃料化学学报, 2018, 46(6):733-742. doi: 10.3969/j.issn.0253-2409.2018.06.012QIAO Nan-li, YANG Yi-xin, LIU Qing-long, SONG Huan-qiao, YU Geng-zhi, LUO Ming-sheng. Influence of different supports on the physicochemical properties and denitration performance of the supported MnCe-based catalysts for NH3-SCR[J]. J Fuel Chem Technol, 2018, 46(6):733-742.). doi: 10.3969/j.issn.0253-2409.2018.06.012 [10] TAN J, LIU Z M, BAO X H, LIU X C, HAN X W, HE C Q, ZHAI R S. Crystallization and Si incorporation mechanisms of SAPO-34[J]. Microporous Mesoporous Mater, 2002, 53(1):97-108. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=57911f4417e0a542e0ca45557e54feff [11] LIU G Y, TIAN P, LI J Z, ZHANG D Z, FAN Z, LIU Z M. Synthesis, characterization and catalytic properties of SAPO-34 synthesized using diethylamine as a template[J]. Microporous Mesoporous Mater, 2008, 111(1):143-149. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=269eb2a13b14fd0a1fbde2934100ed3e [12] 朱彦涛, 吕刚, 宋崇林, 李博, 陈科.一价铜改性ZSM-5催化剂及其催化碳烟氧化反应性能研究[J].燃料化学学报, 2017, 45(1):106-112. doi: 10.3969/j.issn.0253-2409.2017.01.015ZHU Yan-tao, LÜ Gang, SONG Chong-lin, LI Bo, CHEN Ke. Catalytic oxidation of soot over monovalent copper modified ZSM-5[J]. J Fuel Chem Technol, 2017, 45(1):106-112. doi: 10.3969/j.issn.0253-2409.2017.01.015 [13] CAO Y, LAN L, FENG X, YANG Z Z, ZOU S, XU H D, LI Z Q, GONG M C, CHEN Y Q. Cerium promotion on the hydrocarbon resistance of a Cu-SAPO-34 NH3-SCR monolith catalyst[J]. Catal Sci Technol, 2015, 5(9):4511-4521. doi: 10.1039/C5CY00704F [14] 张强, 刘璐, 于梦云, 周洲.氧化铝载体硫酸化对锰铈催化剂SCR脱硝性能的影响[J].燃料化学学报, 2019, 47(9):1137-1145. doi: 10.3969/j.issn.0253-2409.2019.09.014ZHANG Qiang, LIU Lu, YU Meng-yun, ZHOU Zhou. Effect of sulfuric acid modification of Al2O3 support on the SCR performance of MnCe/Al2O3 catalysts[J]. J Fuel Chem Technol, 2019, 47(9):1137-1145. doi: 10.3969/j.issn.0253-2409.2019.09.014 [15] 方祺隆, 朱宝忠, 孙运兰, 尹寿来, 訾朝辉, 史金星, 李国波. Mn-Fe/Al2O3催化剂的低温脱硝性能研究[J].分子催化, 2018, 32(4):18-27. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=fzch201804002FANG Qi-long, ZHU Bao-zhong, SUN Yun-lan, YIN Shou-lai, ZI Zhao-hui, SHI Jin-xing, LI Guo-bo. Study on the performance of low temperature De-NOx based on Mn-Fe/Al2O3 catalysts[J]. J Mol Catal, 2018, 32(4):18-27. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=fzch201804002 [16] 黄增斌, 李翠清, 王振, 徐胜美, 冯凌波, 王虹, 宋永吉, 张伟.不同分子筛负载锰铈催化剂的低温NH3-SCR脱硝性能[J].燃料化学学报, 2016, 44(11):1388-1393. doi: 10.3969/j.issn.0253-2409.2016.11.016HUANG Zeng-bin, LI Cui-qing, WANG Zhen, XU Sheng-mei, FENG Ling-bo, WANG Hong, SONG Yong-ji, ZHANG Wei. Performance of Mn-Ce catalysts supported on different zeolites in the NH3-SCR of NOx[J]. J Fuel Chem Technol, 2016, 44(11):1388-1393. doi: 10.3969/j.issn.0253-2409.2016.11.016 [17] LONG R Q, YANG R T. Temperature-programmed desorption/surface reaction (TPD/TPSR) study of Fe-exchanged ZSM-5 for selective catalytic reduction of nitric oxide by ammonia[J]. J Catal, 2001, 198(1):20-28. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=b73eb8429a4151890db2c92acdb621bc [18] 杨颖欣, 马杰文, 喻成龙, 孙梦婷, 黄碧纯, 吴友明.不同SAPO分子筛负载MnOx催化剂的低温NH3-SCR性能研究[J].环境科学学报, 2016, 36(9):3400-3408. http://d.old.wanfangdata.com.cn/Periodical/hjkxxb201609037YANG Ying-xin, MA Jie-wen, YU Cheng-long, SUN Meng-ting, HUNAG Bi-chun, WU You-ming. Low temperature NH3-SCR activity of manganese oxides supported on different SAPO molecular sieves catalysts[J]. Acta Sci Circumstantiae, 2016, 36(9):3400-3408. http://d.old.wanfangdata.com.cn/Periodical/hjkxxb201609037 [19] WANG C, ZHAO Y, ZHANG C, YAN X, CAO P. Effect of iron doping on SO2 and H2O resistance of honeycomb cordierite-based Mn-Ce/Al2O3 catalyst for NO removal at low temperature[J]. Res Chem Intermed, 2018, 44(5):3135-3150. doi: 10.1007/s11164-018-3297-0 [20] KAPTELJN F, SMGOREDJO L, ANDREML A, MOULIJN J A. Activity and selectivity of pure manganese oxides in the selective catalytic reduction of nitric oxide with ammonia[J]. Appl Catal B:Environ, 1994, 3(3):173-189. doi: 10.1016-0926-3373(93)E0034-9/ [21] LIU Q, FU Z, MA L, NIU H, LIU C, LI J, ZHANG Z. MnOx-CeO2 supported on Cu-SSZ-13:A novel SCR catalyst in a wide temperature range[J]. Appl Catal A:Gen, 2017, 547:146-154. doi: 10.1016/j.apcata.2017.08.024 [22] 张川, 郭芳, 许俊强, 秦娅华, 谢家庆. Ce、Zr改性Mn/ZSM-5分子筛催化剂对C3H6-SCR反应性能的影响[J].硅酸盐学报, 2019, 47(4):486-493. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=gsyxb201904009ZAHNG Chuan, GUO Fang, XU Jun-qaing, QIN Ya-hua, XIE Jia-qing. Influence of Ce and Zr Modified Mn/ZSM-5 Catalyst on C3H6-SCR Reaction Performance[J]. J Chin Ceram Soc, 2019, 47(4):486-493. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=gsyxb201904009 [23] PANAHI P N. MnOx catalysts supported on γ-Al2O3, ZSM-5, and SAPO-34:Effect of support on the activity of Mn supported catalysts in NO abatement by NH3[J]. Russ J Appl Chem, 2016, 89(8):1365-1371. doi: 10.1134/S1070427216080243 [24] 骆航. Mn/SAPO-34分子筛催化剂的制备及低温NH3-SCR性能研究[D].重庆: 重庆大学, 2018.LUO Hang. Preparation of Mn/SAPO-34 molecular sieve catalyst and NH3-SCR performance at low temperature[D]. Chongqing: Chongqing University, 2018. [25] 唐南, 黄妍, 李元元, 赵永谦, 周婷, 张俊丰, 杨柳春.水热法制备铁锰催化剂脱硝性能及抗水抗硫性能研究[J].分子催化, 2018, 32(3):240-248. http://d.old.wanfangdata.com.cn/Periodical/fzch201803006TANG Nan, HUANG Yan, LI Yuan-yuan, ZHAO Yong-qian, ZHOU Ting, ZHANG Jun-feng, YANG Liu-chun. Low temperature selective catalytic reduction of NO with NH3 over Fe-Mn catalysts prepared by hydrothermal method[J]. J Mol Catal, 2018, 32(3):240-248. http://d.old.wanfangdata.com.cn/Periodical/fzch201803006 [26] JIN Q, SHEN Y, ZHU S, LI X, HU M. Promotional effects of erincorporation in CeO2 (ZrO2)/TiO2 for selective catalytic reduction of NO by NH3[J]. Chin J Catal, 2016, 37(9):1521-1528. doi: 10.1016/S1872-2067(16)62450-6 [27] 万义玲, 张传辉, 郭杨龙, 郭耘, 卢冠忠. CeO2-MnOx催化剂上氯乙烯有机废气的催化燃烧[J].催化学报, 2012, 33(3):557-562.WAN Yi-ling, ZHANG Chuan-hui, GUO Yang-long, GUO Yun, LU Guan-zhong. Catalytic combustion of vinyl chloride emission over CeO2-MnOx catalyst[J]. Chin J Catal, 2012, 33(3):557-562. [28] 何鹏飞, 沈德魁, 刘国富.改性SAPO-34分子筛催化剂的SCR脱硝性能[J].东南大学学报:自然科学版, 2017, 47(3):513-520. http://d.old.wanfangdata.com.cn/Periodical/dndxxb201703017HE Peng-fei, SHEN De-kui, LIU Guo-fu. NH3-SCR performance of modified SAPO-34 molecular sieve[J]. J Southeast Univ:Nat Sci Ed, 2017, 47(3):513-520. http://d.old.wanfangdata.com.cn/Periodical/dndxxb201703017 [29] SHAN J H, LIU X Q, SUN L B, RONG C. Cu-Ce bimetal ion-exchanged Y zeolites for selective adsorption of thiophenic sulfur[J]. Energy Fuels, 2008, 22(6):3955-3959. doi: 10.1021/ef800296n [30] CHOI E Y, NAM I S, KIM Y G. TPD study of mordenite-type zeolites for selective catalytic reduction of NO by NH3[J]. J Catal, 1996, 161(2):597-604. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=2219130e6206c1cd4f14a92bea8b9cbd [31] WANG C, WANG J, WANG J Q, YU T, SHEN M Q, WANG W L, LI W. The effect of sulfate species on the activity of NH3-SCR over Cu/SAPO-34[J]. Appl Catal B:Environ, 2017, 204:239-249. doi: 10.1016/j.apcatb.2016.11.033 [32] 陈潇雪, 宋敏, 孟凡跃, 卫月星. FexMnCe1-AC低温SCR催化剂SO2中毒机理研究[J].化工学报, 2019, 70(8):3000-3010. http://d.old.wanfangdata.com.cn/Periodical/hgxb201908020CHEN Xiao-xue, SONG Min, MENG Fan-yue, WEI Yue-xing. Mechanism study on SO2 poisoning of FexMnCe1-AC catalyst for low-temperature SCR[J]. CIESC J, 2019, 70(8):3000-3010. http://d.old.wanfangdata.com.cn/Periodical/hgxb201908020 [33] 王晓波, 归柯庭.铁基催化剂低温脱硝性能研究[J].工程热物理学报, 2013, 34(9):1671-1674. http://d.old.wanfangdata.com.cn/Thesis/D685897WANG Xiao-bo, GUI Ke-ting. Low-temperature selective catalytic reduction of NO with NH3 over Iron based catalysts[J]. J Eng Thermophys, 2013, 34(9):1671-1674. http://d.old.wanfangdata.com.cn/Thesis/D685897 [34] QIAO J S, WANG N, WANG Z H, SUN W, SUN K N. Porous bimetallic Mn2Co1Ox catalysts prepared by a one-step combustion method for the low temperature selective catalytic reduction of NOx with NH3[J]. Catal Commun, 2015, 72:111-115. doi: 10.1016/j.catcom.2015.09.023 [35] LIU G F, ZHANG W J, HE P F, GUAN S P, YUAN B, LI R, SUN Y, SHEN D K. H2O and/or SO2 tolerance of Cu-Mn/SAPO-34 catalyst for NO reduction with NH3 at low temperature[J]. Catal, 2019, 9(3):289. doi: 10.3390/catal9030289 [36] 束韫, 张凡, 王昌, 石应杰, 朱金伟.稻壳基活性炭催化剂的低温NH3-SCR抗硫性能[J].中国环境科学, 2019, 39(11):4620-4627. doi: 10.3969/j.issn.1000-6923.2019.11.017SHU Yun, ZHANG Fan, WANG Chang, SHI Ying-jie, ZHU Jin-wei. Sulfur resistance of rice husk based activated carbon catalyst for the low-temperature selective catalytic reduction of NO by NH3[J]. Chin Environ Sci, 2019, 39(11):4620-4627. doi: 10.3969/j.issn.1000-6923.2019.11.017 -





 下载:
下载: