Effect of supports on the catalytic performance of Pt/WO3-ZrO2 catalysts for hydroisomerization
-
摘要: 以平衡吸附过氧钨酸的水合氧化锆为前驱体,经焙烧得到WO3-ZrO2固体酸,并采用XRD、UV-vis、NH3-TPD等手段考察了过氧钨酸吸附液浓度及焙烧温度对WO3-ZrO2固体酸组成、结构及酸性的影响。通过BET、H2-TPR、H2-TPD等表征手段和正戊烷临氢异构反应,考察了负载铂后相应催化剂的结构、还原与氢吸附性质及其催化正戊烷临氢异构反应的性能。结果表明,焙烧温度为700℃时,随着吸附液浓度的增加,所得载体酸度及相应催化剂比表面积均先增加后减小,且在吸附液浓度为82 mmol W/L时达到最大值。吸附液浓度为59 mmol W/L时,随着焙烧温度的升高,所得载体四方相氧化锆含量、酸度及相应催化剂比表面积均降低。吸附液浓度为82 mmol W/L、焙烧温度为700℃所得载体负载0.5%(质量分数)铂后催化活性最高。该催化剂在250℃常压临氢操作、n(H2)/n(n-C5H12)为3、WHSV为1.0 h-1的条件下,催化正戊烷异构反应中异戊烷收率可达57.7%。
-
关键词:
- Pt/WO3-ZrO2 /
- 平衡吸附 /
- 正戊烷 /
- 临氢异构
Abstract: A series of WO3-ZrO2 solid acids were synthesized by calcining the equilibrium adsorbed peroxotungstic acid/hydrated zirconia precursors. The influences of peroxotungstic acid concentration and calcination temperature on the composition, structure and acidity of the obtained solid acids were evaluated by using XRD, UV-vis and NH3-TPD. Pt/WO3-ZrO2 catalysts were prepared by impregnation method and characterized by BET, H2-TPR and H2-TPD. The catalytic performance in the hydroisomerization of n-pentane was investigated. It was found that under the same calcination temperature, both the support acidity and the catalyst surface area first increase and then decrease with the increase of peroxotungstic acid concentration, and are maximized when the peroxotungstic acid concentration reaches 82 mmol W/L. When the peroxotungstic acid possesses the same concentration of 59 mmol W/L, the tetragonal zirconia fraction, support acidity and the catalyst surface area decrease with the increase of calcination temperature. When the peroxotungstic acid concentration and the calcination temperature of the support are 82 mmol W/L and 700℃ respectively, the obtained catalyst shows the best catalytic performance. The yield of isopentane reaches 57.7% under the reaction condition of ambient pressure, 250℃, n(H2)/n(n-C5H12)=3 and WHSV=1.0 h-1.-
Key words:
- Pt/WO3-ZrO2 /
- equilibrium adsorption /
- n-pentane /
- hydroisomerization
-
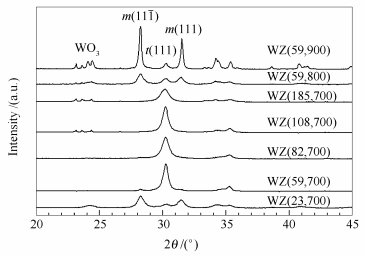
表 1 WO3-ZrO2载体中四方相氧化锆的体积分数
Table 1 Volume fractions of tetragonal zirconia in WO3-ZrO2 supports
Support Vt /% WZ (23, 700) 17 WZ (59, 700) 97 WZ (82, 700) 100 WZ (108, 700) 100 WZ (185, 700) 100 WZ (59, 800) 22 WZ (59, 900) 6.5 表 2 WO3-ZrO2载体的酸位分布
Table 2 Acid distribution data of WO3-ZrO2 supports
Support Acid amount w/(μmol·g-1)/Temperature t/℃ Total acid amount w/(μmol·g-1) peak 1 peak 2 peak 3 WZ (23, 700) 36/175 69/240 178/363 283 WZ (59, 700) 55/174 89/231 166/318 310 WZ (82, 700) 74/176 162/243 244/329 480 WZ (108, 700) 71/177 134/239 212/326 417 WZ (185, 700) 69/175 135/234 258/326 461 WZ (59, 800) 54/170 87/228 160/323 301 WZ (59, 900) 34/168 53/223 85/317 171 表 3 Pt/WO3-ZrO2催化剂的比表面积与孔结构
Table 3 Surface area and porosity data of Pt/WO3-ZrO2 catalysts
Catalyst Surface area A/(m2·g-1) Pore size d/nm Pore volume v/(cm3·g-1) Pt/WZ (23, 700) 44.1 12.3 0.17 Pt/WZ (59, 700) 67.4 7.9 0.19 Pt/WZ (82, 700) 79.6 6.6 0.19 Pt/WZ (108, 700) 72.6 5.6 0.13 Pt/WZ (185, 700) 70.5 7.9 0.18 Pt/WZ (59, 800) 50.7 6.9 0.21 Pt/WZ (59, 900) 23.0 17.1 0.11 -
[1] 徐铁钢, 吴显军, 王刚, 李瑞峰.轻质烷烃异构化催化剂研究进展[J].化工进展, 2015, 34(2):397-401. http://www.cnki.com.cn/Article/CJFDTOTAL-HGJZ201502017.htmXU Tie-gang, WU Xian-jun, WANG Gang, LI Rui-feng. Light paraffin isomerization catalyst and its development[J]. Chem Ind Eng Prog, 2015, 34(2):397-401. http://www.cnki.com.cn/Article/CJFDTOTAL-HGJZ201502017.htm [2] WEYDA H, KÖHLER E. Modern refining concepts-an update on naphtha-isomerization to modern gasoline manufacture[J]. Catal Today, 2003, 81(1):51-55. doi: 10.1016/S0920-5861(03)00101-9 [3] 孔晓翠, 濮仲英, 于中伟.固体超强酸催化正戊烷异构化反应失活因素的考察[J].石油学报 (石油加工), 1999, 15(4):33-38. http://www.cnki.com.cn/Article/CJFDTOTAL-SXJG904.006.htmKONG Xiao-cui, PU Zhong-ying, YU Zhong-wei. Study on deactivation of solid super-acid catalyst for n-pentane isomerization[J]. Acta Pet Sin (Pet Process Sect), 1999, 15(4):33-38. http://www.cnki.com.cn/Article/CJFDTOTAL-SXJG904.006.htm [4] RESOFSZKI G, MUHLER M, SPRENGER S, WILD U, PAÁL Z. Electron spectroscopy of sulfated zirconia, its activity in n-hexane conversion and possible reasons of its deactivation[J]. Appl Catal A:Gen, 2003, 240(1/2):71-81. https://www.researchgate.net/publication/223640368_Electron_spectroscopy_of_sulfated_zirconia_its_activity_in_n-hexane_conversion_and_possible_reasons_of_its_deactivation [5] 汪颖军, 张海菊, 孙博, 田性刚. WO3/ZrO2催化烷烃异构化反应研究进展[J].石油学报 (石油加工), 2009, 25(2):283-290. http://www.cnki.com.cn/Article/CJFDTotal-SXJG200902031.htmWANG Ying-jun, ZHANG Hai-ju, SUN Bo, TIAN Xing-gang. Research progress of WO3/ZrO2 in alkane isomerization[J]. Acta Pet Sin (Pet Process Sect), 2009, 25(2):283-290. http://www.cnki.com.cn/Article/CJFDTotal-SXJG200902031.htm [6] 宋华, 宋华林, 崔雪涵, 张旭. Pd含量对SO42-/ZrO2-WO3固体超强酸催化剂其异构化性能的影响[J].燃料化学学报, 2012, 40(11):1346-1352. http://rlhxxb.sxicc.ac.cn/CN/abstract/abstract18066.shtmlSONG Hua, SONG Hua-lin, CUI Xue-han, ZHANG Xu. Effect of Pd content on the catalytic performance of SO42-/ZrO2-WO3 solid superacid in pentane isomerization[J]. J Fuel Chem Technol, 2012, 40(11):1346-1352. http://rlhxxb.sxicc.ac.cn/CN/abstract/abstract18066.shtml [7] BARTON D G, SOLED S L, MEITZNER G D, FUENTES G A, IGLESIA E. Structural and catalytic characterization of solid acids based on zirconia modified by tungsten oxide[J]. J Catal, 1999, 181(1):57-72. doi: 10.1006/jcat.1998.2269 [8] SANTIESTEBAN J G, VARTULI J C, HAN S, BASTIAN R D, CHANG C D. Influence of the preparative method on the activity of highly acidic WOx/ZrO2 and the relative acid activity compared with zeolites[J]. J Catal, 1997, 168(2):431-441. doi: 10.1006/jcat.1997.1658 [9] SIGNORETTO M, SCARPA M, PINNA F, STRUKUL G, CANTON P, BENEDETTI A. WO3/ZrO2 catalysts by sol-gel processing[J]. J Non-Cryst Solids, 1998, 225(1):178-183. [10] VALIGI M, GAZZOLI D, PETTITI I, MATTEI G, COLONNA S, DE ROSSI S, FERRARIS G. WOx/ZrO2 catalysts. Part 1. Preparation, bulk and surface characterization[J]. Appl Catal A:Gen, 2002, 231(1/2):159-172. [11] HERNÁNDEZ-PICHARDO M L, MONTOYA J A, DEL ANGEL P, VARGAS A, NAVARRETE J. A comparative study of the WOx dispersion on Mn-promoted tungstated zirconia catalysts prepared by conventional and high-throughput experimentation[J]. Appl Catal A:Gen, 2008, 345(2):233-240. doi: 10.1016/j.apcata.2008.05.005 [12] VALIGI M, GAZZOLI D, CIMINO A, PROVERBIO E. Ionic size and metal uptake of chromium (Ⅵ), molybdenum (Ⅵ), and tungsten (Ⅵ) species on ZrO2-based catalyst precursors[J]. J Phys Chem B, 1999, 103(51):11318-11326. doi: 10.1021/jp9922716 [13] LORIDANT S, FECHE C, ESSAYEM N, FIGUERAS F. WOx/ZrO2 catalysts prepared by anionic exchange:In situ Raman investigation from the precursor solutions to the calcined catalysts[J]. J Phys Chem B, 2005, 109(12):5631-5637. doi: 10.1021/jp044494o [14] SHUPYK I, PIQUEMAL J Y, BRIOT E, VAULAY M J, CONNAN C, TRUONG S, ZAITSEV V, BOZON-VERDURAZ F. The use of low-nuclearity oxoperoxo molybdenum species to achieve high dispersions on zirconia materials[J]. Appl Catal A:Gen, 2007, 325(1):140-153. doi: 10.1016/j.apcata.2007.03.033 [15] 田戈, 徐云鹏, 徐竹生, 田志坚, 林励吾. Al助剂对超强酸WOx/ZrO2机械应力稳定性的改善[J].催化学报, 2008, 29(5):415-417. doi: 10.1016/S1872-2067(08)60041-8TIAN Ge, XU Yun-peng, XU Zhu-sheng, TIAN Zhi-jian, LIN Li-wu. Effect of aluminum on the mechanical stress stability of WOx/ZrO2 superacid[J]. Chin J Catal, 2008, 29(5):415-417. doi: 10.1016/S1872-2067(08)60041-8 [16] TORAYA H, YOSHIMURA M, SOMIYA S. Calibration curve for quantitative-analysis of the monoclinic-tetragonal ZrO2 system by X-ray diffraction[J]. J Am Ceram Soc, 1984, 67(6):C119-C121. [17] BARTON D G, SHTEIN M, WILSON R D, SOLED S L, IGLESIA E. Structure and electronic properties of solid acids based on tungsten oxide nanostructures[J]. J Phys Chem B, 1999, 103(4):630-640. doi: 10.1021/jp983555d [18] ZHAO B Y, XU X P, MA H R, SUN D H, GAO J M. Monolayer dispersion of oxides and salts on surface of ZrO2 and its application in preparation of ZrO2-supported catalysts with high surface areas[J]. Catal Lett, 1997, 45(3/4):237-244. doi: 10.1023/A:1019048503124 [19] 王春明, 赵璧英, 谢有畅.盐类和氧化物在载体上自发单层分散研究新进展[J].催化学报, 2003, 24(6):475-482. http://www.cnki.com.cn/Article/CJFDTOTAL-CHUA200306016.htmWANG Chun-ming, ZHAO Bi-ying, XIE You-chang. Advances in the studies of spontaneous monolayer dispersion of oxides and salts on supports[J]. Chin J Catal, 2003, 24(6):475-482. http://www.cnki.com.cn/Article/CJFDTOTAL-CHUA200306016.htm [20] SCHEITHAUER M, GRASSELLI R K, KNÖZINGER H. Genesis and Structure of WOx/ZrO2 Solid Acid Catalysts[J]. Langmuir, 1998, 14(11):3019-3029. doi: 10.1021/la971399g [21] JIN T, YAMAGUCHI T, TANABE K. Mechanism of acidity generation on sulfur-promoted metal-oxides[J]. J Phys Chem, 1986, 90(20):4794-4796. doi: 10.1021/j100411a017 [22] PANAGIOTOPOULOU P, KONDARIDES D I. Effects of alkali additives on the physicochemical characteristics and chemisorptive properties of Pt/TiO2 catalysts[J]. J Catal, 2008, 260(1):141-149. doi: 10.1016/j.jcat.2008.09.014 [23] TSUCHIYA S, AMENOMIYA Y, CVETANOVI-R J. Study of metal catalysts by temperature programmed desorption. Ⅱ. Chemisorption of hydrogen on platinum[J]. J Catal, 1970, 19(3):245-255. [24] KHOOBIAR S. Particle to particle migration of hydrogen atoms on platinum-alumina catalysts from particle to neighboring particles[J]. J Phys Chem, 1964, 68(2):411-412. doi: 10.1021/j100784a503 [25] IGLESIA E, BARTON D G, SOLED S L, MISEO S, BAUMGARTNER J E, GATES W E, FUENTES G A, MEITZNER G D. Selective isomerization of alkanes on supported tungsten oxide acids[J]. Stud Surf Sci Catal, 1996, 101:533-542. doi: 10.1016/S0167-2991(96)80264-3 [26] SHISHIDO T, HATTORI H. Spillover of hydrogen over zirconium oxide promoted by sulfate ion and platinum[J]. Appl Catal A:Gen, 1996, 146(1):157-164. doi: 10.1016/0926-860X(96)00161-5 [27] TRIWAHYONO S, YAMADA T, HATTORI H. IR study of acid sites on WO3-ZrO2[J]. Appl Catal A:Gen, 2003, 250(1):75-81. doi: 10.1016/S0926-860X(03)00303-X [28] CHEN K, BELL A T, IGLESIA E. The relationship between the electronic and redox properties of dispersed metal oxides and their turnover rates in oxidative dehydrogenation reactions[J]. J Catal, 2002, 209(1):35-42. doi: 10.1006/jcat.2002.3620 [29] BAERTSCH C D, SOLED S L, IGLESIA E. Isotopic and chemical titration of acid sites in tungsten oxide domains supported on zirconia[J]. J Phys Chem B, 2001, 105(7):1320-1330. doi: 10.1021/jp003073d [30] PRINS R. Hydrogen Spillover. Facts and fiction[J]. Chem Rev, 2012, 112(5):2714-2738. doi: 10.1021/cr200346z -





 下载:
下载: