Preparation and application of a novel Brönsted-Lewis acid catalyst LaPW12O40/SiO2 for the synthesis of biodiesel via esterification reaction
-
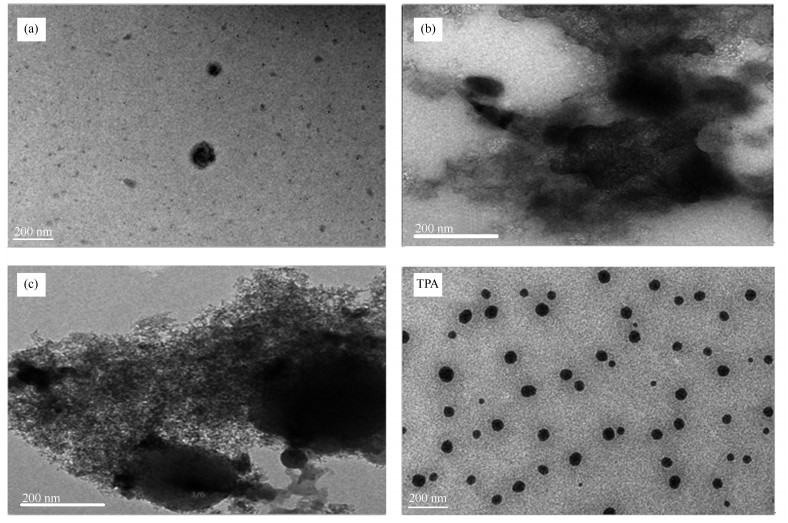
摘要: 以十二磷钨杂多酸(Tungstophosphoric acid,H3PW12O40)为基体,分别通过普通浸渍法、溶胶凝胶法和超声浸渍法进行了La3+改性作用,合成了三种固体酸催化剂A-LaPW12O40、B-LaPW12O40/SiO2和C-LaPW12O40。采用X射线荧光光谱(XRF)、孔径比表面积测定、X射线粉末衍射(XRD)、透射电镜(TEM)、红外光谱(FT-IR)、热重(TG)、N2吸附-脱附、NH3程序升温脱附(NH3-TPD)、吡啶吸附红外光谱(Py-FTIR)、X射线光电子能谱(XPS)等方法对合成的催化剂进行了表征,并比较了以上催化剂在用于催化以油酸和甲醇为反应物经酯化反应合成生物柴油时的活性和稳定性。结果表明,B-LaPW12O40/SiO2具有最高催化活性,当甲醇与油酸的物质的量比为8:1,催化剂用量为反应物总质量的2%,反应温度为65 ℃,反应1 h后,油酸的转化率即高达93%。循环使用B-LaPW12O40/SiO2催化剂六次后,油酸的转化率仍高达86.4%。B-LaPW12O40/SiO2的高催化活性和稳定性可归因于在溶胶凝胶的转化过程中,作为硅源材料的四乙氧基硅(TEOS)易在酸性条件下发生水解反应形成SiO2网络,进而SiO2网络中的硅醇键与H3PW12O40中的H+发生配位作用,生成具有强静电吸附力的(≡Si-OH2+)(H2PW12O40-)络合物。随着该络合物的形成,促进了La3+在SiO2表面的吸附而堵塞了H3PW12O40的孔道结构,抑制了H3PW12O40颗粒在焙烧过程中进一步聚集长大。SiO2将作为载体并以干凝胶状态存在于B-LaPW12O40/SiO2催化剂中,由于SiO2凝胶的高比表面积而使B-LaPW12O40/SiO2具有了较大的比表面积,从H3PW12O40的1.4 m2/g增加至31.3 m2/g。并且,通过吡啶吸附红外光谱确定B-LaPW12O40/SiO2为Brönsted-Lewis酸型固体酸,由于Brönsted酸位易与酯化反应过程中生成的水发生水合反应而失活,因而Lewis酸位的形成有助于减少催化剂的失活现象发生。Lewis酸位的出现可归因于(≡Si-OH2+)(H2PW12O40-)与吸附在其表面的具有强吸电子作用的La3+发生键合作用后生成了LaPW12O40/SiO2。
-
关键词:
- 镧改性 /
- 磷钨杂多酸 /
- 溶胶-凝胶法 /
- Brönsted-Lewis固体酸 /
- 酯化反应
Abstract: In this study, H3PW12O40 (Tungstophosphoric acid) was applied as matrix, and which was modified by La3+ through conventional impregnation method, ultrasonic impregnation method and sol-gel method, obtained three solid acid catalysts: A-LaPW12O40, B-LaPW12O40/SiO2 and C-LaPW12O40. These above catalysts were characterized by X-ray fluorescence spectrometer, specific surface area and porosity analyzer, X-ray diffraction, transmission electron microscopy, Fourier transform infrared spectoscopy, thermogravimetric analysis, N2/adsorption-desorption, NH3 temperature programmed desorption, pyridine adsorption IR spectra and X-ray photoelectron spectroscopy. The catalytic activities and stabilities of them were compared when they were used for the catalytic synthesis of biodiesel from the esterification reaction of oleic acid and methanol. Results shown that the B-LaPW12O40/SiO2 has highest catalytic activity and stability: the conversion of oleic acid can be high to 93% when the molar ratio of methanol to oleic acid was 8:1, mass ratio of catalyst to reactants was 2%, reaction temperature was 65 ℃ and reaction time was 1 h; the conversion of oleic acid maintained 86.4% after B-LaPW12O40/SiO2 had been cycled six times. The high catalytic activity and stability of B-LaPW12O40/SiO2 can be explained as follows: a SiO2 network was formed from the hydrolytic action of Si(OC2H5)4 (TEOS) under acidic conditions via Sol-Gel process. The H+ of H3PW12O40 will bond with Si-OH in SiO2 network to form a (≡Si-OH2+)(H2PW12O40-) complex with strong electrostatic adsorption force, thus promoting the adsorption of La3+ on the surface of SiO2, greatly. As a result, the pore structure of H3PW12O40 will be blockaged, the grow up of H3PW12O40 particles in the roasting process also will be inhibited. In addition, SiO2 may be existed in the form of dry gel in the B-LaPW12O40/SiO2 catalyst and acted as carrier. It will be favorable for the improvent of the surface area of B-LaPW12O40/SiO2 since SiO2 has high surface area, so the surface area of B-LaPW12O40/SiO2 has increased from the 1.4 m2/g of H3PW12O40 to the 31.3 m2/g. And more, LaPW12O40/SiO2 has been determined from the Py-FTIR spectra of pyridine adsorption analysis, which is a Brönsted-Lewis solid acid. The formation of Lewis acid sites can help to reduce the deactivation of a solid acid catalyst: some H2O will be generated from the esterification reaction, and hydration will occur between Brönsted acid site and H2O, so the deactivation will occur. The formation of Lewis acid sites can be ascribed to the strong electrophilic action of La3+ after it has been bonded with (≡Si-OH2+)(H2PW12O40-) to form LaPW12O40/SiO2. -
表 1 催化剂中各元素的含量
Table 1 Atomic percentages of different elements of four catalysts

表 2 催化剂的比表面积、孔容和孔径
Table 2 Surface area, pore volume and pore size of catalysts

表 3 不同固体酸在催化油酸和甲醇酯化反应合成生物柴油时的催化活性比较
Table 3 Comparison of activity of different solid acids for the synthesis of biodiesel from esterification of oleci acid and methanol

-
[1] HAJJARI M, TABATABAEI M, AGHBASHLO M, GHANAVATI H. A review on the prospects of sustainable biodiesel production: A global scenario with an emphasis on waste-oil biodiesel utilization[J]. Renew Sustain Energy Rev, 2017, 72: 445-464. doi: 10.1016/j.rser.2017.01.034 [2] XU Y J, LI G X, SUN Z Y. Development of biodiesel industry in China: Upon the terms of production and consumption[J]. Renew Sustain Energy Rev, 2016, 54: 318-330. doi: 10.1016/j.rser.2015.10.035 [3] VHAD M R, MARCHETTI J M. A review on recent advancement in catalytic materials for biodiesel production[J]. Sust Energ Rev, 2015, 50: 696-718. doi: 10.1016/j.rser.2015.05.038 [4] SINGH S, PATEL A. Selective green esterification and oxidation of glycerol over 12-tungstophosphoric acid anchored to MCM-48[J]. Ind Eng Chem Res, 2014, 53: 14592-14600. doi: 10.1021/ie5026858 [5] SERT E, ATALAY F S. Esterification of acrylic acid with different alcohols catalyzed by zirconia supported tungstophosphoric acid[J]. Ind Eng Chem Res, 2012, 51: 6666-6671. doi: 10.1021/ie202609f [6] LI L X, LIU B Y, WU Z W, YUAN X, LUO H A. Preparation of Keggin-type mono-lacunary phosphotungstic-ammonium salt and its catalytic performance in ammoximation of cyclohexanone[J]. Chem Eng J, 2015, 280: 670-676. doi: 10.1016/j.cej.2015.06.048 [7] CAICEDO A M E, RENGIFO-HERRERA J A, FLORIAN P, BLANCO M N, ROMANELLI G P, PIZZIO L R. Valorization of biomass derivatives: Keggin heteropolyacids supported on titania as catalysts in the suitable synthesis of 2-phenoxyethyl-2-furoate[J]. J Mol Catal A: Chem, 2016, 425: 266-274. doi: 10.1016/j.molcata.2016.10.024 [8] ZHANG X Y, ZHANG D, SUN Z, XUE L F, WANG X H, JIANG Z J. Highly efficient preparation of HMF from cellulose using temperature-responsive heteropolyacid catalysts in cascade reaction[J]. Appl Catal B: Environ, 2016, 196: 50-56. doi: 10.1016/j.apcatb.2016.05.019 [9] WU X Z, LIU Y T, LIU R, WANG L L, LU Y B, XIA X N. Hydroxyalkylation of phenol to bisphenol F over heteropolyacid catalysts: The effect of catalyst acid strength on isomer distribution and kinetics[J]. J Colloid Interf Sci, 2016, 481: 75-81. doi: 10.1016/j.jcis.2016.07.043 [10] SHI H X, ZHANG T Y, AN T C, LI B, WANG X. Enhancement of photocatalytic activity of nano-scale TiO2, particles co-doped by rare earth elements and heteropolyacids[J]. J Colloid Interf Sci, 2012, 380: 121-127. doi: 10.1016/j.jcis.2012.04.069 [11] 戈军伟, 杜治平, 袁华, 杨小俊, 吴元欣. Keggin型磷钼矾杂多化合物在氧化羰基化合成碳酸二苯酯中的应用[J].应用化工, 2009, 38(1): 19-22. http://www.cnki.com.cn/Article/CJFDTOTAL-SXHG200901007.htmGE Jun-wei, DU Zhi-ping, YUAN Hua, YANG Xiao-jun, WU Yan-xin. Application of Keggin type molybdovanadophosphoric compounds in synthesis of diphenyl carbonate by oxidative carbonylation with phenol [J]. Appl Chem Ind, 2009, 38(1): 19-22. http://www.cnki.com.cn/Article/CJFDTOTAL-SXHG200901007.htm [12] 郭晓俊, 黄崇品.反荷离子及制备方法对Keggin型杂多化合物结构和性质的影响[J].石油化工, 2008, 37(3): 216-221. http://www.cnki.com.cn/Article/CJFDTOTAL-SYHG200803005.htmGUO Xiao-jun, HUANG Chong-pin. Effects of counter-ions and preparation methods on structures and properties of Keggin-type heteropoly compounds[J]. Petrochem Technol, 2008, 37(3): 216-221. http://www.cnki.com.cn/Article/CJFDTOTAL-SYHG200803005.htm [13] MATTOS F C G D, SOUZA J A D S D, COTRIM A B D A, MACEDO J L, DIAS J A, DIAS S C L, GHESTI G F. Lewis acid/surfactant rare earth trisdodecylsulfate catalysts for biodiesel production from waste cooking oil[J]. Appl Catal A: Gel, 2012, 423/424(8): 1-6. http://www.sciencedirect.com/science/article/pii/S0926860X12001081 [14] MARCI G, GARCIA-LOPEZ E I, POMILLA F R, LIOTTA L F, PALMISANO L. Enhanced (photo) catalytic activity of Wells-Dawson (H6P2W18O62) in comparison to Keggin (H3PW12O40) heteropolyacids for 2-propanol dehydration in gas-solid regime[J]. Appl Catal A: Gen, 2016, 528: 113-122. doi: 10.1016/j.apcata.2016.10.002 [15] WANG Z, FAN Y, Li Y W, QU F R, WU D Y, KONG H N. Synthesis of zeolite/hydrous lanthanum oxide composite from coal fly ash for efficient phosphate removal from lake water[J]. Micropor Mesopor Mat, 2016, 222: 226-234. doi: 10.1016/j.micromeso.2015.10.028 [16] ZHANG Y, WONG W T, YUNG K F. Biodiesel production via, esterification of oleic acid catalyzed by chlorosulfonic acid modified zirconia[J]. Appl Energy, 2014, 116(1): 191-198. http://www.cabdirect.org/abstracts/20143319972.html [17] SANTOS J S, DIAS J A, DIAS S C L, DE MACEDO J L, GARCIA F A C, ALMEIDA L S, D E CAVALHO E N C B. Acidic characterization and activity of (NH4)xCs2.5-xH0.5PW12O40 catalysts in the esterification reaction of oleic acid with ethanol[J]. Appl Catal A: Gen, 2012, 443/444: 33-39. doi: 10.1016/j.apcata.2012.07.013 [18] MORENO J I, JAIMES R, GOMEZ R, GOMEZ M E N. Evaluation of sulfated tin oxides in the esterification reaction of free fatty acids[J]. Catal Today, 2011, 172(1): 34-40. doi: 10.1016/j.cattod.2011.03.052 [19] JUNIOR C A R M, ALBURQUERQUE C E R, CARNEIRO J S A, DARIVA C, FORTUNY M, SANTOS A F, EGUES S M S, RAMOS A L. Solid-acid-catalyzed esterification of oleic acid assisted by microwave heating[J]. Ind Eng Chem Res, 2010, 49(23): 12135-12139. doi: 10.1021/ie100501d [20] CAMPOSECO R, CASTILLO S, MEJIA-CENTENO I, NAVARRETE J, RODRIGUEZ-GONZALEZ V. Behavior of lewis and brönsted surface acidity featured by Ag, Au, Ce, La, Fe, Mn, Pd, Pt, V and W decorated on protonated titanate nanotubes[J]. Micropor Mesopor Mat, 2016, 236: 235-243. doi: 10.1016/j.micromeso.2016.08.033 [21] JALIL P A, FAIZ M, TABET N, HAMDAN N M, HUSSAIN Z. A study of the stability of tungstophosphoric acid, H3PW12O40, using synchrotron XPS, XANES, hexane cracking, XRD, and IR spectroscopy[J]. J Catal, 2002, 217: 292-297. http://www.sciencedirect.com/science/article/pii/S0021951703000666 [22] ZHANG J Q, WONG H, KAKUSHIMA K, IWAI H. XPS study on the effects of thermal annealing on CeO2/La2O3 stacked gate dielectrics[J]. Thin Solid Films, 2016, 600: 30-35. doi: 10.1016/j.tsf.2016.01.001 -





 下载:
下载: