Catalytic performance of NiMo/Al2O3-USY in the hydrocracking of low-temperature coal tar
-
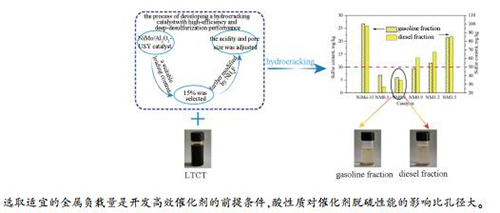
摘要: 采用等体积浸渍法制得一系列NiMo/Al2O3-USY催化剂,在200 mL固定床上考察了不同金属负载量对其中低煤焦油加氢裂化催化性能的影响,进一步用NH4F溶液改性USY以提高催化剂的脱硫性能,并结合XRD、氮气吸附-脱附、XPS、HR-TEM、H2-TPR和NH3-TPD等手段对催化剂进行了表征分析。结果表明,NiMo/Al2O3-USY催化剂适宜的MoO3负载量为15%(质量分数);当MoO3含量超过15%后,MoS2活性相在载体上团聚,硫化程度趋于稳定,强酸酸量和孔径减少,增加金属负载量对煤焦油加氢裂化转化率影响较小。NH4F改性USY可增大NiMo/Al2O3-USY催化剂的孔径,有利于提高煤焦油加氢裂化转化率。表面强酸酸量减少后,产品中的硫含量明显增加,说明强酸酸量是影响产物硫含量的关键因素。当NH4F浓度为0.6 mol/L时,NH4F改性USY制得的NM0.6催化剂上煤焦油加氢裂化的转化率为87.65%,产品汽油馏分(≤ 180 ℃)硫含量为5.96 mg/kg,柴油馏分(180-320 ℃)硫含量为34.98 mg/kg。
-
关键词:
- 中低温煤焦油 /
- 加氢裂化 /
- 改性USY /
- 硫含量 /
- NiMo/Al2O3-USY
Abstract: A series of NiMo/Al2O3-USY catalysts with different MoO3 contents were prepared through incipient wetness method and further modified with NH4F. The NiMo/Al2O3-USY catalysts were characterized by XRD, XPS, HR-TEM, NH3-TPD, H2-TPR and N2 adsorption and their catalytic performance in the hydrocracking of low-temperature coal tar (LTCT) was investigated in a 200 mL fixed-bed reactor. The results indicate that the appropriate MoO3 content is 15% (mass ratio); higher MoO3 content may lead to the agglomeration of active metals on the support, although it has little influence on the sulfidation degree of Mo species and the conversion of coal tar upon hydrocracking. In addition, the amount of strong acid sites and pore diameter decrease gradually with a further increase in the MoO3 content, which is disadvantageous for deep hydrocracking. The modification of USY zeolite with NH4F solution can enlarge the average pore diameter of resultant NiMo/Al2O3-USY catalysts and then improve the residue conversion of coal tar. However, the amount of strong acid sites decreases obviously when the concentration of NH4F solution exceeds 0.6 mol/L, which may lead to an increase of the sulfur content in the hydrocracking product. Over the NiMo/Al2O3-USY catalyst modified with 0.6 mol/L NH4F solution, the residue conversion of coal tar reaches 87.65%; the sulfur contents in the gasoline fraction (< 180 ℃) and diesel fraction (180-320 ℃) are 5.96 and 34.98 mg/kg, respectively.-
Key words:
- low-temperature coal tar /
- hydrocracking /
- modified USY /
- sulfur content /
- NiMo/Al2O3-USY
-
表 1 原材料的基本性质
Table 1 Properties of the LTCT feedstock
Item Value Density ρ/(g·cm-3) 0.9548 Elemental analysis w/% C 84.97 H 11.53 S 0.15 Distillation range t/℃ IBP/10% 199/265 30%/50% 293/351 90%/FBP 467/504 表 2 不同金属含量催化剂的织构参数
Table 2 Textural properties of NiMo/USY-Al2O3 catalysts with different MoO3 contents
Catalyst ABET /(m2·g-1) Pore volume v/(cm3·g-1) Pore diameter d/nm vtotal vmicro vmacro CAY 359 0.30 0.007 0.297 4.41 NiMo-9 346 0.32 0.021 0.307 3.80 NiMo-12 280 0.20 0.005 0.195 4.30 NiMo-15 257 0.19 0.005 0.188 4.25 NiMo-18 230 0.21 0.009 0.205 3.98 NiMo-21 191 0.18 0.006 0.1756 3.98 表 3 不同金属含量催化剂的酸性分布
Table 3 Acid site distribution of NiMo/USY-Al2O3 catalysts with different MoO3 contents
Catalyst 20-200℃ 200-350℃ 350-600℃ Overall relative value area (a.u.) relative area (a.u.) relative area (a.u.) relative CAY70 26.71 1 44.32 1 28.97 1 1 NiMo-9 31.06 1.43 46.79 1.3 22.15 0.94 1.23 NiMo-12 28.85 1.49 45.82 1.42 25.32 1.2 1.38 NiMo-15 27.89 1.35 45.63 1.33 26.48 1.18 1.3 NiMo-18 30.75 1.38 48.49 1.28 20.76 0.84 1.17 NiMo-21 29.52 1.38 53.74 1.52 16.74 0.72 1.25 表 4 不同金属含量催化剂硫化后的价态分布
Table 4 Valence distribution of various NiMo/USY-Al2O3 catalysts after sulfidation
Catalyst Ratio of different Mo species /% Mo4+ Mo5+ Mo6+ total NiMo-9 57 19 24 100.00 NiMo-12 65 17 18 100.00 NiMo-15 78 15 7 100.00 NiMo-18 82 14 5 100.00 NiMo-21 86 11 3 100.00 表 5 不同金属含量催化剂的平均MoS2片层长度和层数
Table 5 Average length and stack layer number of various NiMo/USY-Al2O3 catalysts after sulfidation
Catalyst Average length of slabs d/nm Average stack layer number of slabs NiMo-9 3.63 3.56 NiMo-12 3.06 2.2 NiMo-15 3.88 3.01 NiMo-18 3.59 2.63 NiMo-21 3.94 2.92 表 6 不同NMy催化剂的织构参数
Table 6 Textural properties of the NMy catalysts
Catalyst Surface area
A/(m2·g-1)Pore volume v/(cm3·g-1) Average pore diameter d/nm vtotal vmicro vmacro NiMo-15 257 0.19 0.005 0.188 4.14 NiMo-15(0.3) 254 0.22 0.008 0.212 3.93 NiMo-15(0.6) 248 0.22 0.006 0.216 4.36 NiMo-15(0.9) 254 0.24 0.007 0.238 4.38 NiMo-15(1.2) 268 0.22 0.004 0.2189 4.45 NiMo-15(1.5) 253 0.25 0.010 0.239 4.40 表 7 不同NMy催化剂的酸性分布
Table 7 Acid sites distribution of the NMy catalysts
Catalyst 20-200℃ 200-350℃ 350-600℃ Overall relative value area (a.u.) relative area (a.u.) relative area (a.u.) relative NiMo-15 926.7 1 1039 1 582.4 1 1 NM0.3 1001 1.08 933 0.9 388 0.67 0.91 NM0.6 930.5 1 1071 1.03 305.8 0.53 0.91 NM0.9 840 0.91 1108 1.07 248 0.43 0.86 NM1.2 746.6 0.81 1068 1.03 218.6 0.38 0.8 NM1.5 682.9 0.74 820.2 0.79 99.4 0.17 0.63 -
[1] 闫伦靖.煤焦油气相催化裂解生成轻质芳烃的研究[D].太原: 太原理工大学, 2016. http://cdmd.cnki.com.cn/Article/CDMD-10112-1016714272.htmYAN Lun-jing. Analysis on converting gaseous tar to light arenes by catalytic cracking[D]. Taiyuan: Taiyuan University of Technology, 2016. http://cdmd.cnki.com.cn/Article/CDMD-10112-1016714272.htm [2] SUN J M, LI D, YAO R Q, SUN Z H, LI X K, LI W H. Modeling the hydrotreatment of full range medium temperature coal tar by using a lumping kinetic approach[J]. React Kinet Mech Catal, 2015, 114(2):451-471. doi: 10.1007/s11144-014-0791-2 [3] 马宝岐.煤焦油制燃料油品[M].北京:化学工业出版社, 2011.MA Bao-qi. Preparation of Fuel Oil from Coal Tar[M]. Beijing:Chemical Industry Press, 2011. [4] CUI W G, ZHENG H A, NIU M L, ZHANG S J, LI D, QIAO J, LI W H. Product compositions from catalytic hydroprocessing of low temperature coal tar distillate over three commercial catalysts[J]. React Kinet Mech Catal, 2016, 119(2):491-509. doi: 10.1007/s11144-016-1068-8 [5] 夏良燕.多联产中低温煤焦油加氢工艺及催化剂研究[D].杭州: 浙江大学, 2015. http://cdmd.cnki.com.cn/Article/CDMD-10335-1015539673.htmXIA Liang-yan. Study on hydroprocessing technology and catalysts of LTCT from polygeneration[D]. Hangzhou: Zhejiang University, 2015. http://cdmd.cnki.com.cn/Article/CDMD-10335-1015539673.htm [6] FENG X, LI D, CHEN J H, NIU M L, LIU X, LESTER L T, LI W H. Kinetic parameter estimation and simulation of trickle-bed reactor for hydrodesulfurization of whole fraction low-temperature coal tar[J]. Fuel, 2018, 230:113-125. doi: 10.1016/j.fuel.2018.05.023 [7] 张世万.煤焦油催化加氢轻质化及催化剂的研究[D].上海: 华东理工大学, 2012. http://cdmd.cnki.com.cn/Article/CDMD-10251-1012309953.htmZHANG Shi-wan. Study on light-end products of coal tar with catalytic hydrogenation and catalyst[D]. Shanghai: East China University of Science and Technology, 2012. http://cdmd.cnki.com.cn/Article/CDMD-10251-1012309953.htm [8] ZHANG H Y, CHEN G W, BAI L, CHANG N, WANG Y G. Selective hydrogenation of aromatics in coal-derived liquids over novel NiW and NiMo carbide catalysts[J]. Fuel, 2019, 244:359-365. doi: 10.1016/j.fuel.2019.02.015 [9] VAN N B, DOROTHEE L, PAVEL A, CHRISTOPHE G. Hydrodeoxygenation of guaiacol with CoMo catalysts. Part Ⅰ:Promoting effect of cobalt on HDO selectivity and activity[J]. Appl Catal B:Environ, 2011, 101(3/4):239-245. [10] ZHANG D Q, DUAN A J, ZHAO Z, WANG X Q, JIANG G Y, LIU J, WANG C Y. Synthesis, characterization and catalytic performance of meso-microporous material Beta-SBA-15-supported NiMo catalysts for hydrodesulfurization of dibenzothiophene[J]. Catal Today, 2011, 175(1):477-484. doi: 10.1016/j.cattod.2011.03.060 [11] 许楠, 梁乃森, 张舜光, 段艳, 侯凯湖.β-MCM-41的制备及在汽油异构/脱硫中的应用[J].石油学报(石油加工), 2012, 28(6):913-919. doi: 10.3969/j.issn.1001-8719.2012.06.005XU Nan, LIANG Nai-sen, ZHANG Shun-guang, DUAN Yan, HOU Kai-hu. Synthesis of β-MCM-41 and its application in gasoline isomerization/hydrodesulfurization[J]. Acta Pet Sin(Pet Process Sect), 2012, 28(6):913-919. doi: 10.3969/j.issn.1001-8719.2012.06.005 [12] MENG J P, WANG Z Y, MA Y H, LU J Y. Hydrocracking of low-temperature coal tar over NiMo/Beta-KIT-6 catalyst to produce gasoline oil[J]. Fuel Process Technol, 2017, 165:62-71. doi: 10.1016/j.fuproc.2017.05.009 [13] KAZAKOV M O, NADEINA K A, DANILOVA I G, DIK P P, KLIMOV O V, PEREYMA V Y, PAUKSHTIS E A, GOLUBEV I S, PROSVIRIN I P, GERASIMOV E Y, DOBRYAKOVA I V, KNYAZEVA E E, IVANOVA I I, NOSKOV A S. Influence of USY zeolite recrystallization on physicochemical properties and catalytic performance of NiMo/USY-Al2O3 hydrocracking catalysts[J]. Catal Today, 2019, 329:108-115. doi: 10.1016/j.cattod.2019.01.003 [14] RAYO P, TORRES M P, CENTENTO G, FERNANDO A, JOSE A D, ANCHEYTA J. Effect of silicon incorporation method in the supports of NiMo catalysts for hydrotreating reactions[J]. Fuel, 2019, 239:1293-1303. doi: 10.1016/j.fuel.2018.10.102 [15] FERRAZ S, ZOTIN F, ARAUJO L. Influence of support acidity of NiMoS catalysts in the activity for hydrogenation and hydrocracking of tetralin[J]. Appl Catal A:Gen, 2010, 384(1):51-57. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=66b6232b9d2820ec4fff73894a76e421 [16] 陈松, 许杰, 阳永荣, 王靖岱, 张晓萍, 张奎喜.以介孔分子筛为硅源合成的超微β沸石特性及其加氢裂化性能[J].催化学报, 2006, 27(3):255-258. doi: 10.3321/j.issn:0253-9837.2006.03.013CHEN Song, XU Jie, YANG Yong-rong, WANG Jing-dai, ZHANG Xiao-ping, ZHANG Kui-xi. Nanometer β zeolite made from mesoporous zeolite and its hydrocracking performance[J]. Chin J Catal, 2006, 27(3):255-258. doi: 10.3321/j.issn:0253-9837.2006.03.013 [17] 张学军, 王宗贤, 郭爱军, 袁宗胜, 王甫村.高中油型加氢裂化催化剂用Y型沸石的改性研究[J].燃料化学学报, 2008, 36(5):606-609. doi: 10.3969/j.issn.0253-2409.2008.05.017ZHANG Xue-jun, WANG Zong-xian, GUO Ai-jun, YUAN Zong-sheng, WANG Fu-cun. Modification of zeolite Y for preparation of the maxinizing middle distillates hydrocracking catalyst[J]. J Fuel Chem Technol, 2008, 36(5):606-609. doi: 10.3969/j.issn.0253-2409.2008.05.017 [18] SHALI N B, SUGUNAN S. Influence of transition metals on the surface acidic properties of titania prepared by sol-gel route[J]. Mater Res Bull, 2007, 42(9):1777-1783. doi: 10.1016/j.materresbull.2006.11.016 [19] RAJAGOPAL S, MARZARI J A, MIRANDA R. Silica-alumina-supported Mo oxide catalysts:Genesis and demise of Brønsted-Lewis acidity[J]. J Catal, 1995, 151(1):192-203. doi: 10.1006/jcat.1995.1021 [20] JOSE E, MARIA C B, ANA W G, MARIA A. CORTES J, CARLOS A C, JOSE A T. Highly active P-doped sulfided NiMo/alumina HDS catalysts from Mo-blue by using saccharose as reducing agents precursor[J]. Appl Catal B:Environ, 2018, 237:708-720. doi: 10.1016/j.apcatb.2018.06.034 [21] KLIMOV O V, NADEINA K A, DIK P P, KORYAKINA G I, PEREYMA V Y, KAZAKOV M O, BUDUKVA S V, GERASIMOV E Y, PROSVIRIN I P, KOCHUBEY D I, NOSKOV A S. CoNiMo/Al2O3 catalysts for deep hydrotreatment of vacuum gasoil[J]. Catal Today, 2016, 271:56-63. doi: 10.1016/j.cattod.2015.11.004 [22] 孟欣欣, 邱泽刚, 郭兴梅, 李振荣, 胡乃方, 宋毛宁, 赵亮富.不同金属含量Ni-W催化剂的煤焦油加氢脱硫脱氮性能研究[J].燃料化学学报, 2016, 44(5):570-578. doi: 10.3969/j.issn.0253-2409.2016.05.009MENG Xin-xin, QIU Ze-gang, GUO Xing-mei, LI Zhen-rong, HU Nai-fang, SONG Mao-ning, ZHAO Liang-fu. Hydrodenitrogenation and hydrodesulfurization of coal tar on Ni-W catalysts with different metal loadings[J]. J Fuel Chem Technol, 2016, 44(5):570-578. doi: 10.3969/j.issn.0253-2409.2016.05.009 [23] BERIT H, N R J K, HENRSK T E. A density functional study of the chemical differences between Type Ⅰ and Type Ⅱ MoS2-based structures in hydrotreating catalysts[J]. J Phys Chem B, 2005, 109(6):2245-2253. doi: 10.1021/jp048842y [24] YIN H L, ZHOU T N, LLU Y Q, CHAI Y M, LIU C G. NiMo/Al2O3 catalyst containing nano-sized zeolite Y for deep hydrodesulfurization and hydrodenitrogenation of diesel[J]. J Nat Gas Chem, 2011, 20(4):441-448. doi: 10.1016/S1003-9953(10)60204-6 [25] YU F, HAN X, GANG S, LIU H Y, QIAN Y, WANG T H, GONG G B, BAO X J. Citrc acid-assisted hydrothermal method for preparing NiW/USY-Al2O3 ultradeep hydrodesulfurization catalysts[J]. J Catal, 2011, 279(5):27-35. [26] 方向晨.加氢裂化[M].北京:中国石化出版社, 2008.FANG Xiang-chen. Hydrocracking[M]. Beijing:China Petrochemical Press, 2008. [27] 张登前.介微孔材料的合成及其在柴油HDS催化剂中的应用研究[D].北京: 中国石油大学, 2010. http://d.wanfangdata.com.cn/Thesis/Y2169840ZHANG Deng-qian. Synthesis of meso-microporous composite materials and their applications in the catalysts for the hydrodesulfurization of diesel[D]. Beijing: China University of Petroleum, 2010. http://d.wanfangdata.com.cn/Thesis/Y2169840 [28] 任亮, 毛以朝, 刘坤红, 聂红.正癸烷在不同酸性Y型分子筛催化剂上的加氢裂化反应[J].石油学报(石油加工), 2009, 25(1):31-35. doi: 10.3969/j.issn.1001-8719.2009.01.006REN Liang, MAO Yi-chao, LIU Kun-hong, NIE Hong. Hydrocracking of decane on different acidity Y zeolite catalysts[J]. Acta Pet Sin(Pet Process Sect), 2009, 25(1):31-35. doi: 10.3969/j.issn.1001-8719.2009.01.006 [29] AROLDY P, JONGE J C M D, MOULIJNN J A. Temperature-programed reduction of molybdenum(Ⅵ) oxide and molybdenum(Ⅳ) oxide[J]. J Phys Chem, 1985, 89(21):4517-4526. doi: 10.1021/j100267a021 [30] HENKER M, WENDLANDT K P, VALYON J, BORNMANN P. Structure of MoO3/Al2O3-SiO2 catalysts[J]. Appl Catal, 1991, 69(1):205-220. doi: 10.1016/S0166-9834(00)83303-5 [31] SHEILA G A, FATIMA M, LUCIA R, RADDI A, JOSE L Z. Influence of support acidity of NiMoS catalysts in the activity for hydrogenation and hydrocracking of tetralin[J]. Appl Catal A:Gen, 2010, 384(1/2):51-57. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=66b6232b9d2820ec4fff73894a76e421 -





 下载:
下载: