Study on the activity and mechanism of selective catalytic reduction of NO with NH3 over MnαTi1-α catalyst at medium-low temperatures
-
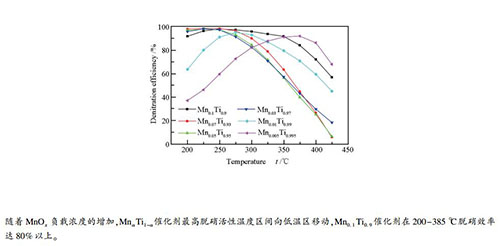
摘要: 采用浸渍法制备了不同MnOx负载量的SCR催化剂,检测其在中低温下的脱硝活性和抗SO2中毒性能,并分析影响MnαTi1-α催化剂中低温活性的机理。采用BET、XRD、XPS、NH3-TPD和H2-TPR对催化剂表征。研究表明,随着MnOx负载量的增加,MnαTi1-α催化剂最高脱硝活性温度区间向低温区移动,Mn0.1Ti0.9催化剂在200-385 ℃脱硝效率达80%以上。SO2会造成MnαTi1-α催化剂脱硝活性显著下降,且不可逆。当MnOx负载量增加时,催化剂比表面积先增大后略微减小、H2-TPR中Mn4+峰面积增大、表面化学吸附氧增加,有利于NH3-SCR反应在低温下的进行。MnαTi1-α催化剂的酸性位点随MnOx含量增加而增多,H2还原峰出现温度较低,表明MnαTi1-α催化剂具有良好的中低温氧化还原性。Abstract: MnαTi1-α catalysts for selective catalytic reduction (SCR) of NO were prepared with impregnation method and their denitration activity and SO2 resistance at medium-low temperature were evaluated. The catalysts were characterized using BET, XRD, XPS, NH3-TPD and H2-TPR. The results showed that the temperature range of the highest denitration activity of MnαTi1-α catalyst shifted to the lower temperature zone along with the increase of MnOx loading. The denitration efficiency of Mn0.1Ti0.9 catalyst reached over 80% at 200-385 ℃. SO2 could bring down denitration activity of MnαTi1-α catalyst greatly and resulted in irreversible deactivation. The specific surface area of the catalyst first increased then slightly decreased with the increase of Mnx loading. Both Mn4+ peak area in H2-TPR and surface chemical adsorbed oxygen increased along with the increase of MnOx loading. All these factors were beneficial to the proceeding of NH3-SCR reaction at low temperature. With the increase of MnOx loading, the acid sites of MnαTi1-α catalyst increased and the reduction peak at low temperature appeared, indicating that MnαTi1-α catalyst had good redox performance at medium and low temperature.
-
Key words:
- selective catalytic reduction /
- MnOx /
- medium-low temperature SCR /
- NH3 reduction /
- SO2 resistance
-
表 1 MnαTi1-α催化剂孔隙结构特征参数
Table 1 Pore structure parameters of the MnαTi1-α catalyst
Catalyst Specific surface area A/(m2·g-1) Pore volume v/(cm3·g-1) Average pore diameter d/nm Mn0.1Ti0.9 55.4 0.4482 33.6 Mn0.07Ti0.93 57.0 0.4975 34.9 Mn0.05Ti0.95 51.5 0.4648 35.5 Mn0.03Ti0.97 50.9 0.4023 31.6 Mn0.01Ti0.99 52.5 0.3934 30.0 Mn0.005Ti0.995 49.8 0.3533 28.4 表 2 MnαTi1-α催化剂的表面元素含量
Table 2 Surface element contents of the MnαTi1-α catalysts
Catalyst Content w/% Mn3+ Mn4+ Oα Oβ Mn0.1Ti0.9 31.2 68.8 35.0 65.0 Mn0.05Ti0.95 35.6 64.4 29.4 70.6 Mn0.01Ti0.99 40.8 59.2 28.2 71.8 表 3 MnαTi1-α催化剂NH3-TPD分峰数据
Table 3 NH3-TPD peak parameters of the MnαTi1-α catalysts
Catalyst Peak position t/℃ peak1 peak2 peak3 peak4 peak5 peak6 Mn0.1Ti0.9 224.13 285.46 351.64 448.91 553.32 - Mn0.05Ti0.95 192.05 244.62 296.31 346.37 425.92 495.47 Mn0.01Ti0.99 196.43 244.04 305.02 362.75 443.37 - peak area peak1 peak2 peak3 peak4 peak5 peak6 Mn0.1Ti0.9 259.43 143.73 245.12 366.88 13.58 - Mn0.05Ti0.95 131.73 289.42 84.86 244.68 242.42 41.88 Mn0.01Ti0.99 86.67 156.50 158.38 106.03 124.68 - -
[1] YANG Z Q, LI H L, LIU X, LI P, YANG J P, LEE P H, SHIH K. Promotional effect of CuO loading on the catalytic activity and SO2 resistance of MnOx/TiO2 catalyst for simultaneous NO reduction and Hg0 oxidation[J]. Fuel, 227: 79-88. doi: 10.1016/j.fuel.2018.04.074 [2] QIU K Z, SONG J, SONG H, GAO X, LUO Z Y, CEN K F. A novel method of microwave heating mixed liquid-assisted regeneration of V2O5-WO3/TiO2 commercial SCR catalysts[J]. Environ Geochem Health, 2015, 37(5): 905-914. doi: 10.1007/s10653-014-9663-y [3] 马子然, 林德海, 马少丹, 马静, 孙琦, 李永龙, 徐文强, 王宝冬. 600 MW机组脱硝催化剂失活机理及中试再生[J].环境工程学报, 2018, 12(6): 1702-1712. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=hjwrzljsysb201806015MA Zi-ran, LIN De-hai, MA Shao-dan, MA Jing, SUN Qi, LI Yong-long, XU Wen-qiang, WANG Bao-dong. Deactivation mechanism and regeneration of SCR catalyst used in 600MW unit of coal fired power plant[J]. Chin J Environ Eng, 2018, 12(6): 1702-1712. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=hjwrzljsysb201806015 [4] 李燕, 刘毅, 王鹏, 韩涛, 余学海, 赵瑞, 廖海燕. Zn-W/TiO2宽温度窗口SCR催化剂在燃煤烟气中的应用[J].环境工程, 2018, 36(11): 89-93. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=hjgc201811018LI Yan, LIU Yi, WANG Peng, HAN Tao, YU Xue-hai, ZHAO Rui, LIAO Hai-yan. Application research on Zn-W/TiO2 SCR catalyst with wide operation temperature window to the coal-fired flue gas[J]. Environ Energy, 2018, 36(11): 89-93. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=hjgc201811018 [5] YANG Y R, WANG M H, TAO Z L, LIU Q, FEI Z Y, CHEN X, ZHANG Z X, TANG J H, CUI M F, QIAO X. Mesoporous Mn-Ti amorphous oxides: A robust low-temperature NH3-SCR catalyst[J]. Catal Sci Technol, 2018, 8(24): 6396-6406. doi: 10.1039/C8CY01313F [6] 闫东杰, 李亚静, 玉亚, 黄学敏, 周卫可, 刘颖慧.碱金属沉积对Mn-Ce/TiO2低温SCR催化剂性能影响[J].燃料化学学报, 2018, 46(12): 1513-1519. http://manu60.magtech.com.cn/rlhxxb/CN/abstract/abstract29318.shtmlYAN Dong-jie, LI Ya-jing, YU Ya, HUANG Xue-min, ZHOU Wei-ke, LIU Ying-hui. Effect of alkali metal deposition on Mn-Ce/TiO2 catalyst for NO reduction by NH3 at low temperature[J]. J Fuel Chem Technol, 2018, 46(12): 1513-1519. http://manu60.magtech.com.cn/rlhxxb/CN/abstract/abstract29318.shtml [7] MU W T, ZHU J, ZHANG S, GUO Y Y, SU L Q, LI X Y, LI Z. Novel proposition on mechanism aspects over Fe-Mn/ZSM-5 catalyst for NH3-SCR of NOx at low temperature: rate and direction of multifunctional electron-transfer-bridge and in situ DRIFTs analysis[J]. Catal Sci Technol, 2016, 6(20): 7532-7548. doi: 10.1039/C6CY01510G [8] 杜学森.钛基SCR脱硝催化剂中毒失活及抗中毒机理的实验和分子模拟研究[D].杭州: 浙江大学, 2014. http://kns.cnki.net/KCMS/detail/detail.aspx?dbcode=CDFD&dbname=CDFD&filename=1015539632.nhDU Xue-sen. An experimental and theoretical study on the anti-poisoning of the titania-based SCR catalyst[D]. Hangzhou: Zhejiang University, 2014. http://kns.cnki.net/KCMS/detail/detail.aspx?dbcode=CDFD&dbname=CDFD&filename=1015539632.nh [9] WEI L, CUI S P, GUO H X, ZHANG L J. The effect of alkali metal over Mn/TiO2 for low-temperature SCR catalyst of NO with NH3 through DRIFT and DFT[J]. Comput Mater Sci, 2018, 144: 216-222. doi: 10.1016/j.commatsci.2017.12.013 [10] PARK E, KIM M, JUNG H, CHIN S, JURNG J. Effect of sulfur on Mn/Ti catalysts prepared using chemical vapor condensation (CVC) for low-temperature NO reduction[J]. ACS Catal, 2013, 3(7): 1518-1525. doi: 10.1021/cs3007846 [11] PAPPAS D K, BONINGARI T, BOOLCHAND P, SMIRNIOTIS P G. Novel manganese oxide confined interweaved titania nanotubes for the low-temperature selective catalytic reduction (SCR) of NOx by NH3[J]. J Catal, 2016, 334: 1-13. doi: 10.1016/j.jcat.2015.11.013 [12] CHEN J Y, ZHU B Z, SUN Y L, YIN S L, ZHU Z C, LI J X. Investigation of low-temperature selective catalytic reduction of NOx with ammonia over Mn-modified Fe2O3/AC catalysts[J]. J Braz Chem Soc, 2018, 29(1): 79-87. [13] GAO F Y, TANG X L, YI H H, ZHAO S Z, WANG J E, GU T. Improvement of activity, selectivity and H2O & SO2-tolerance of micro-mesoporous CrMn2O4 spinel catalyst for low-temperature NH3-SCR of NOx[J]. Appl Surf Sci, 2019, 466: 411-424. doi: 10.1016/j.apsusc.2018.09.227 [14] QU L, LI C T, ZENG G M, ZHNAG M Y, FU M F, MA J F, ZHAN F M, LUO D Q. Support modification for improving the performance of MnOx-CeOy/gamma-Al2O3 in selective catalytic reduction of NO by NH3[J]. Chem Eng J, 2014, 242: 76-85. doi: 10.1016/j.cej.2013.12.076 [15] LIU C, SHI J W, GAO C, NIU C M. Manganese oxide-based catalysts for low-temperature selective catalytic reduction of NOx with NH3: A review[J]. Appl Catal A: Gen, 2016, 522: 54-69. doi: 10.1016/j.apcata.2016.04.023 [16] 周佳丽, 王宝冬, 马静, 李歌, 孙琦, 徐文强, 李永龙.锰基低温SCR脱硝催化剂抗硫抗水性能研究进展[J].环境化学, 2018, 37(4): 782-791. http://d.old.wanfangdata.com.cn/Periodical/hjhx201804018ZHOU Jia-li, WANG Bao-dong, MA Jing, LI Ge, SUN Qi, XU Wen-qiang, LI Yong-long. SO2 and H2O poisoning resistance of manganese oxide-based catalysts for low-temperature selective catalytic reduction of NOx[J]. Environ Chem, 2018, 37(4): 782-791. http://d.old.wanfangdata.com.cn/Periodical/hjhx201804018 [17] QU L, LI C T, ZENG G M, ZHANG M Y, FU M F, MA J F, ZHAN F M, LUO D Q. Support modification for improving the performance of MnOx-CeOy/gamma-Al2O3 in selective catalytic reduction of NO by NH3[J]. Chem Eng J, 2014, 242: 76-85. doi: 10.1016/j.cej.2013.12.076 [18] 杨超, 程华, 黄碧纯.抗SO2和H2O中毒的低温NH3-SCR脱硝催化剂研究进展[J].化工进展, 2014, 33(4): 907-913. http://d.old.wanfangdata.com.cn/Periodical/hgjz201404020YANG Chao, CHENG Hua, HUANG Bi-chun. Review of deNOx catalysts with SO2 and H2O poisoning resistance for low-temperature NH3-SCR[J]. Chem Ind Eng Prog, 2014, 33(4): 907-913. http://d.old.wanfangdata.com.cn/Periodical/hgjz201404020 [19] LIU Z, YANG Y, MI J H, TAN X L, SONG Y. Synthesis of copper-containing ordered mesoporous carbons for selective hydrogenation of cinnamaldehyde[J]. Catal Commun, 2012, 21: 58-62. doi: 10.1016/j.catcom.2012.01.024 [20] CHEN X, XU X, FEI Z Y, XIE X X, LOU J W, TANG J H, CUI M F, QIAO X. CeO2 nanodots embedded in a porous silica matrix as an active yet durable catalyst for HCl ocidation[J]. Catal Sci Technol, 2016, 6(13): 5116-5123. doi: 10.1039/C5CY02300A [21] 黄金, 仲兆平, 朱林, 薛建明, 许月阳, 吴培亭.锰铈改性钒钨钛中低温SCR催化剂脱硝及抗水抗硫性能[J].化工进展, 2018, 37(6): 2242-2248. http://d.old.wanfangdata.com.cn/Periodical/hgjz201806026HUANG Jin, ZHONG Zhao-ping, ZHU Lin, XUE Jian-ming, XU Yue-yang, WU Pei-ting. DeNOx performance and resistance to H2O and SO2 of Mn-Ce doped V-W/Ti catalyst at middle-low temperature[J]. Chem Ind Eng Prog, 2018, 37(6): 2242-2248. http://d.old.wanfangdata.com.cn/Periodical/hgjz201806026 [22] 李伟, 张成, 李鑫, 谭鹏, 方庆艳, 陈刚. Ho掺杂对Mn-Ce/TiO2低温SCR催化剂的脱硝性能影响[J].燃料化学学报, 2017, 45(12): 1508-1513. http://manu60.magtech.com.cn/rlhxxb/CN/abstract/abstract19141.shtmlLI Wei, ZHANG Cheng, LI Xin, TAN Peng, FANG Qing-yan, CHEN Gang. Influence of Ho doping on the deNOx performance of Mn-Ce/TiO2 low temperature SCR catalyst[J]. J Fuel Chem Technol, 2017, 45(12): 1508-1513. http://manu60.magtech.com.cn/rlhxxb/CN/abstract/abstract19141.shtml [23] 胡宇峰, 薛建明, 王小明, 盛重义, 廖伟平. Mn-Ce/TiO2低温选择性催化还原催化剂二氧化硫中毒及再生特性[J].工业催化, 2013, 21(4): 27-33. doi: 10.3969/j.issn.1008-1143.2013.04.006HU Yu-feng, XUE Jian-ming, WANG Xiao-ming, SHENG Zhong-yi, LIAO Wei-ping. Research on characteristics of SO2-poison and regeneration of Mn-Ce/TiO2 catalyst for low temperature selective catalytic reduction[J]. Ind Catal, 2013, 21(4): 27-33. doi: 10.3969/j.issn.1008-1143.2013.04.006 [24] DELIMARIS D, LOANNIDES T. VOC oxidation over MnOx-CeO2 catalysts prepared by a combustion method[J]. Appl Catal B: Environ, 2008, 84(12): 303-312. http://www.sciencedirect.com/science/article/pii/S0926337308001355 [25] KANG M, PARK E D, KIM J M, YIE J E. Manganese oxide catalysts for NOx reduction with NH3 at low temperatures[J]. Appl Catal A: Gen, 2007, 327(2): 261-269. doi: 10.1016/j.apcata.2007.05.024 [26] LIU F D, HE H, DING Y, ZHANG C B. Effect of manganese substitution on the structure and activity of iron titanate catalyst for the selective catalytic reduction of NO with NH3[J]. Appl Catal B: Environ, 2009, 93(12): 194-204. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=fd24003bd391131bd3be8817ed7852be [27] VENKATASWAMY P, RAO K N, JAMPAIAH D, REDDY B M. Nanostructured manganese doped ceria solid solutions for CO oxidation at lower temperatures[J]. Appl Catal B: Environ, 2015, 162: 122-132. doi: 10.1016/j.apcatb.2014.06.038 [28] SANTOS V P, PEREIRA M F R, ORFAO J J M, FIGUEIREDO J L. The role of lattice oxygen on activity of mangnese oxides towards the oxidation of volatile organic compounds[J], Appl Catal B: Environ, 2010, 99(1/2): 353-363. http://www.sciencedirect.com/science/article/pii/S0926337310003139 [29] WU Z B, JIN R B, LIU Y, WANG H Q. Ceria modified MnOx/TiO2 as a superior catalyst for NO reduction with NH3 at low-temperature[J]. Catal Commun, 2008, 9(13): 2217-2220. doi: 10.1016/j.catcom.2008.05.001 [30] ETTIREDDY P R, ETTIREDDY N, MAMEDOV S, BOOLCHAND P, SMIRNIOTIS P G. Surface characterization studies of TiO2 supported manganese oxide catalysts for low temperature SCR of NO with NH3[J]. Appl Cataly B: Environ, 2007, 76(1/2): 123-134. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=35cb995e5856d8b4b81e52a4390273ea -





 下载:
下载: