Synthesis and deep oxidative desulfurization of vanadium-substituted polyoxotungstate phase transfer catalyst
-
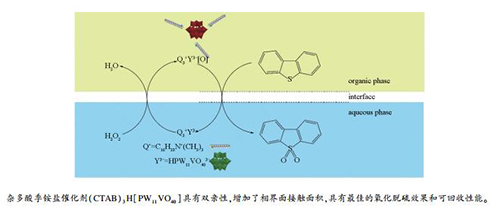
摘要: 以钒原子取代的Keggin型磷钨杂多酸与不同的季铵类阳离子表面活性剂反应合成了一系列磷钨钒杂多酸相转移催化剂,采用红外和X射线衍射对催化剂进行了表征。以H2O2为氧化剂,对模型柴油的氧化脱硫反应进行了研究,考察了季胺类表面活性剂种类、不同季胺盐含量、催化剂用量、氧硫比、反应温度等参数对反应的影响。结果表明,所制备的杂多酸相转移催化剂保留有杂多酸阴离子和季铵盐阳离子的结构特征。[(C16H33(CH3)3)N]3H[PW11VO40]催化剂具有最佳的氧化脱硫性能和重复使用性能,在n(催化剂)/n(模型柴油)=1:80,n(H2O2)/n(模型柴油)=8:1,反应温度50℃,反应时间3 h的反应条件下,二苯并噻吩的转化率可达到100%;催化剂重复使用五次后,转化率为99.7%。反应过程中,该催化剂与反应物形成微乳体系,如同一个均相混合物,而反应结束体系静置一段时间后,催化剂和产物又形成两相,通过离心法就可以快速分离和回收催化剂。Abstract: A series of phase transfer catalysts, composed of vanadium-substituted phosphotungstic acid(H4[PW11VO40]) and different quaternary cationics were synthesized through ion exchange method. The characterization of FT-IR spectroscopy and X-ray diffraction confirmed that the integrity of polyoxometalate anions and quaternary ammonium cations immobilized in the phase transfer catalyst. The as-prepared catalysts were applied to the catalytic oxidative desulfurization of model diesel oil using H2O2 as oxidant. The influencing factors such as quaternary cationics species, catalyst composition, catalyst amount, oxygen-sulfur ratio and reaction temperature were investigated.[(C16H33(CH3)3)N]3H[PW11VO40] is found to be efficient and reusable catalyst for oxidative desulfurization reaction. Under the optimized reaction conditions of n(catalyst)/n(model diesel)=1:80, n(H2O2)/n(model diesel)=8:1, 50℃, 3 h, the catalyst exhibits the dibenzothiophene conversion of 100% and excellent reusability with 99.7% conversion after five times reaction. The catalyst and reactants form a microemulsion system and behave like homogeneous mixture during reaction, but precipitates with biphase separation when the reaction ends. The catalyst could be quickly separated and recycled by centrifugation.
-
表 1 不同催化剂对DBT氧化脱硫性能的比较
Table 1 Comparison of catalytic efficiency for oxidative desulfurization of DBT on different catalysts
Entry Catalyst t /℃ t /h n(catalyst)/n(s) n(H2O2)/n(S) DBT conversion x/% 1 without catalyst 50 3 0 4:1 3.37 2 H3[PW12O40] 50 3 1:20 4:1 24.38 3 H4[PW11VO40] 50 3 1:20 4:1 36.15 4 (CTAB)3[PW12O40] 50 3 1:20 4:1 72.26 5 (CTAB)3H[PW11VO40] 50 3 1:20 4:1 86.41 6 (TTAB)3H [PW11VO40] 50 3 1:20 4:1 65.35 7 (TOAB)3 H [PW11VO40] 50 3 1:20 4:1 53.78 8 (TBAB) 3H [PW11VO40] 50 3 1:20 4:1 44.62 9 (TMAB) 3H [PW11VO40] 50 3 1:20 4:1 38.93 10 (STAB) 3H [PW11VO40] 50 3 1:20 16:1 99.44 11 (CTAB)3H[PW11VO40] 50 3 1:20 16:1 100 -
[1] BANISHARIF F, DEHGHANI M R, CAMPOS-MARTIN J M. Oxidative desulfurization of diesel using vanadium-substituted dawson-type emulsion catalysts[J]. Energy Fuels, 2017, 31(5):5419-5427. doi: 10.1021/acs.energyfuels.6b02791 [2] SONG C. An overview of new approaches to deep desulfurization for ultra-clean gasoline, diesel fuel and jet fuel[J]. Catal Today, 2003, 86(1):211-263. doi: 10.1016-S0920-5861(03)00412-7/ [3] ABRO R, GAO S R, CHEN X C. Oxidative desulfurization of gasoline by ionic liquids coupled with extraction by organic solvents[J]. J Brazil Chem Soc, 2016, 27(6):998-1006. http://cn.bing.com/academic/profile?id=52d127ff7d767e46e3f15106a82ca792&encoded=0&v=paper_preview&mkt=zh-cn [4] WANG D, EIKA W Q, AMANO H, OKATA K, LSHIHARA A, KABE T. Oxidative desulfurization of fuel oil Part Ⅰ. Oxidation of dibenzothiophenes using tert-butyl hydroperoxide[J]. Appl Catal A:Gen, 2003, 253(1):91-99. doi: 10.1016/S0926-860X(03)00528-3 [5] OTSUKI S, NONAKA T, TAKASHIMA N, QIAN W, LSHIHARA A, LMAI T, KABE T. Oxidative desulfurization of light gas oil and vacuum gas oil by oxidation and solvent extraction[J]. Energy Fuels, 2000, 14(6):1234-1239. doi: 10.1021-ef000096i/ [6] LIANG W D, ZHANG S, LI H F, ZHANG G. Oxidative desulfurization of simulated gasoline catalyzed acetic acid-based ionice liquids at room temperature[J]. Fuel Process Technol, 2013, 109(2):27-31. http://cn.bing.com/academic/profile?id=497c1033a3387b637c122e0beb96ee55&encoded=0&v=paper_preview&mkt=zh-cn [7] SHIRAISHI Y, NAITO T, HIRAI T. Vanadosilicate molecular sieve as a catalyst for oxidative desulfurization of light oil[J]. Ind Eng Chem Res, 2003, 42(24):6034-6039. doi: 10.1021/ie030328b [8] WANG R, ZHANG G, ZHAO H. Polyoxometalate as effective catalyst for the deep desulfurization of diesel oil[J]. Catal Today, 2010, 149(1/2):15358-15359. http://cn.bing.com/academic/profile?id=2f38ebec2ba159a297465917f88840fc&encoded=0&v=paper_preview&mkt=zh-cn [9] BANISHARIF F, DEHGHANI M R, CAPEL-SANCHEZ M C, CAMPOS-MARTIN J M. Desulfurization of fuel by extraction and catalytic oxidation using a vanadium substituted dawson-type emulsion catalyst[J]. Ind Eng Chem Res, 2017, 56(14):3839-3852. doi: 10.1021/acs.iecr.7b00089 [10] MAHDIEH S, BABAK M, HAMID R M, KUROSH T H, ALI S, MOJTABA M. Oxidative desulfurization of diesel fuel using a bronsted acidic ionic liquid supported on silica gel[J]. Energy Fuels, 2017, 31(9):10196-10205. doi: 10.1021/acs.energyfuels.6b03505 [11] LI S W, GAO R M, ZHAO J S. Deep oxidative desulfurization of fuel catalyzed by modified heteropolyacid:The comparison performance of three kinds of ionic liquids[J]. ACS Sustainable Chem Eng, 2018, 6(11):15858-15866 doi: 10.1021/acssuschemeng.8b04524 [12] ZHANG J, WANG A J, LI X, MA X H. Oxidative desulfurization of dibenzothiophene and diesel over[Bmim]3PMo12O40[J]. J Catal, 2011, 279(2):269-275. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=85306610c42e2e43818aae01b2f5cdd9 [13] HUA Z, BAKER G A. Oxidative desulfurization of fuels using ionic liquids[J]. Front Chem Sci Eng, 2015, 9(3):262-279. doi: 10.1007/s11705-015-1528-0 [14] 王恩波.多酸化学导论[M].北京:化工出版社, 1998.WANG En-bo. Guidance to Polyoxometalates[M]. Beijing:Chemical Industry Press, 1998. [15] 张海燕, 代跃利, 蔡蕾.杂多酸催化剂催化氧化脱硫研究进展[J].化工进展, 2013, 32(4):809-815. http://d.old.wanfangdata.com.cn/Periodical/hgjz201304014ZHANG Hai-yan, DAI Yue-li, CAI Lei. Research progress of heteropoly acid catalyzed oxidative desulfurization[J]. Chem Ind Eng Prog, 2013, 32(4):809-815. http://d.old.wanfangdata.com.cn/Periodical/hgjz201304014 [16] TE M, FAIRBRIDGE C, RING Z. Oxidation reactivities of dibenzothiophenes in polyoxometalate-H2O2 and formic acid-H2O2 systems[J]. Appl Catal A:Gen, 2001, 219:267-280. doi: 10.1016/S0926-860X(01)00699-8 [17] LI B, LIU Z, HAN C. In situ synthesis, characterization, and catalytic performance of tungtophosphoric acid encapsulated into the framework of mesoporous silica pillared clay[J]. J Colloid Interf Sci, 2012, 377(1):334-341. doi: 10.1016/j.jcis.2012.03.067 [18] ZHANG Y N, WANG L, ZHANG Y L, JIANG Z X, LI C. Ultra-deep oxidative desulfurization of fuel oil catalyzed by dawson-type polyoxotungstate emulsion catalysts[J]. Chin J Catal, 2011, 32(1):235-239. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=cuihuaxb201102005 [19] QI W, WANG Y Z, LI W, WU L X. Surfactant-encapsulated polyoxometalates as immobilized supramolecular catalysts for highly efficient and selective oxidation reactions[J]. Chem Eur J, 2010, 16(3):1068-1078. doi: 10.1002/chem.200902261 [20] HUANG D, ZHAI Z, LU Y C, YANG L M, LUO G S. Optimization of composition of a directly combined catalyst in dibenzothiophene oxidation for deep desulfurization[J]. Ind Eng Chem Res, 2007, 46(5):1447-1451. doi: 10.1021/ie0611857 [21] LI C, JIANG Z X, GAO J B, YANG Y X, WANG S J, TIAN F P, SUN F X, SUN X P, YING P L, HAN C R. Ultra-deep desulfurization of diesel:Oxidation with a recoverable catalyst assembled in emulsion[J]. Chem Eur J, 2004, 10(9):2277-2280. doi: 10.1002/chem.200305679 [22] LI C, GAO J B, JIANG Z G, WANG S G, LU H Y, YANG Y X, JING F. Selective oxidations on recoverable catalysts assembled in emulsions[J]. Top Catal, 2005, 35(1/2):169-175. http://cn.bing.com/academic/profile?id=19fa54de202ecdfc20b45eae5f333971&encoded=0&v=paper_preview&mkt=zh-cn [23] ZHANG Y J, GU Y F, DONG X B, WU P F, LI Y L, HU H M, XUE G L. Deep oxidative desulfurization of refractory sulfur compounds with cesium salts of mono-substituted phosphomolybdate as efficient catalyst[J]. Catal Lett, 2017, 147(7):1811-1819. doi: 10.1007/s10562-017-2078-5 [24] LU H Y, GAO J B, JIANG Z X, JING F, YANG Y X, WANG G, LI C. Ultra-deep desulfurization of diesel by selective oxidation with[C18H37N(CH3)3]4[H2NaPW10O36]catalyst assembled in emulsion droplets[J]. J Catal, 2006, 239(2):369-375. doi: 10.1016/j.jcat.2006.01.025 [25] XU J H, ZHAO S, JI Y C, SONG Y F. Deep desulfurization by amphiphilic lanthanide-containing polyoxometalates in ionic-liquid emulsion systems under mild conditions[J]. Chem Eur J, 2013, 19(2):709-715. doi: 10.1002/chem.201202595 [26] 张烨红. Keggin型杂多酸相转移催化剂的合成、表征及催化性能研究[D].兰州: 兰州理工大学, 2009.ZHANG Ye-hong. Synthesis, characterization and catalytic performance of keggin type heteropolyacid phase-transfer catalysts[D]. Lanzhou: Lanzhou University of Technology, 2009. [27] 鄢景森, 艾丽梅, 王强, 王泽青, 鄂永胜, 刘海彬.酸功能化-温控型三元杂多酸离子液体的合成、表征及其酯催化性能[J].无机化学学报, 2018, 34(12):2179-2187. doi: 10.11862/CJIC.2018.272YAN Jing-sen, AI Li-mei, WANG Qiang, WANG Ze-qing, E Yong-sheng, LIU Hai-bin. Synthesis, characterization and esterification application of acid-functionalized ternary heteropolyanion-based ionic liquids with temperature-responsive behaviour[J]. Chin J Inorg Chem, 2018, 34(12):2179-2187. doi: 10.11862/CJIC.2018.272 [28] WU X F, HUANG T P, TONG X, XIE Z R, CHEN W X, WU Q Y, YAN W F. Thermoregulated polyoxometalate-based ionic-liquid gel electrolytes[J]. RSC Adv, 2015, 5(28):21973-21977. doi: 10.1039/C5RA02209F [29] WU X F, TONG X, WU Q Y, DING H, YAN W F. Reversible phase transformation-type electrolyte based on layered shape polyoxometalate[J]. J Mater Chem A, 2014, 2(16):5780-5784. doi: 10.1039/c3ta15237e [30] 齐月红, 刘倩, 柳云骐, 刘晨光.锌钨酸季铵盐的制备及其催化氧化脱硫性能研究[J].石油炼制与化工, 2015, 46(6):52-56. doi: 10.3969/j.issn.1005-2399.2015.06.016QI Yue-hong, LIU Qian, LIU Yun-qi, LIU Chen-guang. Synthesis of polytungstozincic quaternary ammonium salts and application in catalytic oxidation desulfurization[J]. Pet Process Pet Chem, 2015, 46(6):52-56. doi: 10.3969/j.issn.1005-2399.2015.06.016 [31] YAMAURA T, KAMATA K, YAMAGUCHI K. Efficient sulfoxidation with hydrogen peroxide catalyzed by a divanadium-subtituted phosphotungstate[J]. Catal Today, 2013, 203:76-81. doi: 10.1016/j.cattod.2012.01.026 [32] 王亮.杂多酸微乳液催化氧化油品脱硫的研究[D].青岛: 中国海洋大学, 2011. http://cdmd.cnki.com.cn/Article/CDMD-10423-1011231223.htmWANG Liang. Study on desulfurization of oil catalyzed oxidation by supported heteropoly acid in microemulsion[D]. Qingdao: Ocean University of China, 2011. http://cdmd.cnki.com.cn/Article/CDMD-10423-1011231223.htm [33] 刘日嘉, 王睿, KORCHAK V. Keggin结构杂多酸盐的合成、表征及催化燃油深度脱硫[J].无机化学学报, 2014, 30(3):563-572. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=wjhxxb201403015LIU Ri-jia, WANG Rui, KORCHAK V. Synthesis, charaterization and catalytic fuel ultra-deep desulfurization of keggin-type polyoxometalates[J]. Chin J Inorg Chem, 2014, 30(3):563-572. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=wjhxxb201403015 -





 下载:
下载: