Effect of calcination temperature of LaNiO3 on CuO/LaNiO3 catalyst for hydrogen production via methanol steam reforming
-
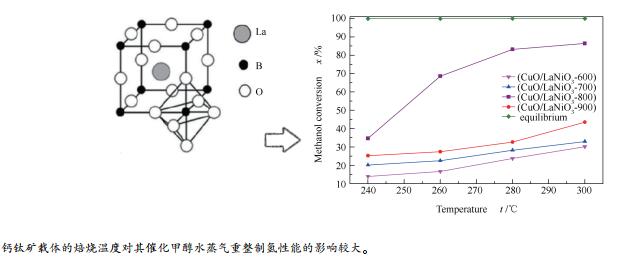
摘要: 采用溶胶凝胶法合成了LaNiO3钙钛矿型氧化物载体,再采用浸渍法制备了CuO/LaNiO3催化剂,并通过XRF、XRD、BET、H2-TPR和XPS等手段对催化剂进行了表征,考察了LaNiO3钙钛矿的焙烧温度对CuO/LaNiO3催化剂结构及其催化甲醇水蒸气重整制氢性能的影响。结果表明,载体焙烧温度主要影响了催化剂的表面晶格氧缺位,活性组分和载体间的相互作用。当载体焙烧温度为800℃时,催化剂表面氧空穴较多,活性组分与载体间相互作用较强,因此,催化甲醇水蒸气重整制氢活性较好。Abstract: The LaNiO3 perovskite support was synthesized by sol-gel method. The CuO/LaNiO3 catalyst was prepared by impregnation method. The catalyst was characterized by XRF, XRD, BET, H2-TPR and XPS. The effect of calcination temperature of LaNiO3 perovskite on the structure of CuO/LaNiO3 catalyst and its catalytic performance for methanol steam reforming were investigated. The results show that the calcination temperature of the support mainly affects the surface oxygen vacancy of the catalyst, the interaction between the active component and the support. When the calcination temperature of the support is 800℃, the surface of the catalyst has more oxygen vacancy, and the interaction between the active component and the support is stronger. Therefore, the hydrogenation activity of methanol steam reforming is higher.
-
Key words:
- perovskite /
- methanol steam reforming /
- hydrogen /
- calcination temperature
-
表 1 不同焙烧温度下制备的钙钛矿催化材料的元素含量
Table 1 Elemental content of perovskite catalytic materials prepared at different calcination temperatures
Catalyst Content of element w/% Cu La Ni O CuO/LaNiO3-600 8.1 53.2 21.7 17.0 CuO/LaNiO3-700 8.2 50.0 24.4 17.4 CuO/LaNiO3-800 8.5 51.2 23.0 17.3 CuO/LaNiO3-900 8.3 50.1 24.3 17.3 Normal value 9.0 51.4 21.7 17.9 表 2 催化剂的物化性质及其用于催化甲醇水蒸气重整反应中氢气产率
Table 2 Physicochemical properties and catalytic performance of the catalysts
Catalyst ABET /(m2·g-1) Pore volume v/(cm3·g-1) Bore diameter d/nm dCuO /nm Cu surface areaa A/(m2·gcat-1) H2 production rateb /(cm3·kg-1·s-1) LaNiO3-600 11.5 0.03 3.86 - - - LaNiO3-700 7.9 0.03 3.36 - - - LaNiO3-800 7.4 0.02 3.06 - - - LaNiO3-900 5.3 0.01 3.37 - - - CuO/LaNiO3-600 12.4 0.04 3.79 26.1 0.8 218.0 CuO/LaNiO3-700 10.8 0.02 3.41 30.1 1.0 372.9 CuO/LaNiO3-800 7.5 0.02 3.03 20.2 1.2 1007.2 CuO/LaNiO3-900 7.6 0.02 3.85 23.6 1.1 491.6 a: determined by N2O experiments; b: reaction conditions: atmospheric pressure, 240 ℃, W/M=1.2:1, GHSV=800 h-1, no carrier gas 表 3 不同焙烧温度下催化剂和O 1s XPS曲线拟合
Table 3 O 1s XPS curve-fitting results of the catalysts calcined at various temperatures
Catalyst Oads/(Oads+O-OH+Olatt) CuO/LaNiO3-600 0.20 CuO/LaNiO3-700 0.24 CuO/LaNiO3-800 0.31 CuO/LaNiO3-900 0.21 -
[1] LIN L, ZHOU W, GAO R, YAO S, ZHANG X, XU W. Low-temperature hydrogen production from water and methanol using Pt/α-MoC catalysts[J]. Nature, 2017, 544(7648):80-83. doi: 10.1038/nature21672 [2] LIU X, MEN Y, WANG J, HE R, WANG Y. Remarkable support effect on the reactivity of Pt/In2O3/MOx catalysts for methanol steam reforming[J]. J Power Sources, 2017, 364:341-350. doi: 10.1016/j.jpowsour.2017.08.043 [3] BUTTNER W, RIVKIN C, BURGESS R, HARTMANN K, BLOOMFIELD I, BUBAR M. Hydrogen monitoring requirements in the global technical regulation on hydrogen and fuel cell vehicles[J]. Int J Hydrogen Energy, 2017, 42(11):7664-7671. doi: 10.1016/j.ijhydene.2016.06.053 [4] HAN H, MU Z, LIU Z, ZHAO F. Abating transport GHG emissions by hydrogen fuel cell vehicles:Chances for the developing world[J]. Front Energy, 2018, 12(3):1-15. http://d.old.wanfangdata.com.cn/Periodical/zggdxxxswz-nyydlgc201803013 [5] ROSES L, MANZOLINI G, CAMPANARI S, WIT E, WALTER M. Techno-economic assessment of membrane reactor technologies for pure hydrogen production for fuel cell vehicle fleets[J]. Energy Fuels, 2013, 27(8):4423-4431. doi: 10.1021/ef301960e [6] CHOI H J, KANG M. Hydrogen production from methanol/water decomposition in a liquid photosystem using the anatase structure of Cu loaded[J]. Int J Hydrogen Energy, 2007, 32(16):3841-3848. doi: 10.1016/j.ijhydene.2007.05.011 [7] 苏石龙, 张磊, 张艳, 雷俊腾, 桂建舟, 刘丹, 刘道胜, 潘立卫.千瓦级PEMFC甲醇水蒸气重整制氢过程热力学模拟[J].石油化工高等学校学报, 2015, 28(2):19-25. doi: 10.3969/j.issn.1006-396X.2015.02.004SU Shi-long, ZHANG Lei, ZHANG Yan, LEI Jun-teng, GUI Jian-zhou, LIU Dan, LIU Dan-sheng, PAN Li-wei. Thermodynamic simulation for hydrogen production in the methanol steam reforming system of kilowatt PEMFC[J]. J Petrochem Univ, 2015, 28(2):19-25. doi: 10.3969/j.issn.1006-396X.2015.02.004 [8] YANG H, CHEN Y, CUI X, WANG G, CEN Y, DENG T. A highly stable copper-based catalyst for clarifying the catalytic roles of Cu0 and Cu+ species in methanol dehydrogenation[J]. Angew Chem Int Ed, 2018, 130(7):1836-1840. doi: 10.1002/anie.201710605 [9] JEON N J, NOH J H, YANG W S, KIM Y C, RYU S, SEO J. Compositional engineering of perovskite materials for high-performance solar cells[J]. Nature, 2015, 517(7535):476-480. doi: 10.1038/nature14133 [10] KHALESI A, ARANDIYAN H R, PARVARI M. effects of lanthanum substitution by strontium and calcium in la-ni-al perovskite oxides in dry reforming of methane[J]. J Catal, 2008, 29(10):18-26. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=cuihuaxb200810004 [11] YANG S Q, ZHOU F, LIU Y J, ZHANG L, YU C, WANG H H, TIAN Y, ZHANG C S, LIU D S. Morphology effect of ceria on the performance of CuO/CeO2 catalysts for hydrogen production by methanol steam reforming[J]. Int J Hydrogen Energy, 2019, 44(14):7252-7261. doi: 10.1016/j.ijhydene.2019.01.254 [12] 杨淑倩, 贺建平, 张娜, 隋晓伟, 张磊, 杨占旭.稀土掺杂改性对Cu/ZnAl水滑石衍生催化剂甲醇水蒸气重整制氢性能的影响[J].燃料化学学报, 2018, 46(2):179-188. doi: 10.3969/j.issn.0253-2409.2018.02.007YANG Shu-qian, HE Jian-ping, ZHANG Na, SUI Xiao-wei, ZHANG Lei, YANG Zhan-xu. Effect of rare-earth element modification on the performance of Cu/ZnAl catalysts derived from hydrotalcite precursor in methanol steam reforming[J]. J Fuel Chem Technol, 2018, 46(2):179-188. doi: 10.3969/j.issn.0253-2409.2018.02.007 [13] HE J P, YANG Z X, ZHANG L, LI Y, PAN L W. Cu supported on ZnAl-LDHs precursor prepared by in-situ synthesis method on γ-Al2O3 as catalytic material with high catalytic activity for methanol steam reforming[J]. Int J Hydrogen Energy, 2017, 42(15):9930-9937. doi: 10.1016/j.ijhydene.2017.01.229 [14] 杨淑倩, 张娜, 贺建平, 张磊, 王宏浩, 白金, 张健, 刘道胜, 杨占旭. Ce的浸渍顺序对Cu/Zn-Al水滑石衍生催化剂用于甲醇水蒸气重整制氢性能的影响[J].燃料化学学报, 2018, 46(4):479-488. doi: 10.3969/j.issn.0253-2409.2018.04.014YANG Shua-qian, ZHANG Na, HE Jian-ping, ZHANG Lei, WANG Hong-hao, BAI Jin, ZHANG Jian, LIU Dao-sheng, YANG Zhan-xu. Effect of impregnation sequence of Ce on the performance of Cu/Zn-Al catalysts derived from hydrotalcite precursor in methanol steam reforming[J]. J Fuel Chem Technol, 2018, 46(4):479-488. doi: 10.3969/j.issn.0253-2409.2018.04.014 [15] 贺建平, 张磊, 陈琳, 杨占旭, 佟宇飞. CeO2改性Cu/Zn-Al水滑石衍生催化剂对甲醇水蒸气重整制氢性能的影响[J].高等学校化学学报, 2017, 38:1822-1828. doi: 10.7503/cjcu20170158HE Jian-ping, ZHANG Lei, CHEN Lin, YANG Zhan-xu, TONG Yu-fei. Effect of CeO2 on Cu/Zn-Al catalysts derived from hydrotalcite precursor for methanol steam reforming[J]. Chem J Chin Univ, 2017, 38:1822-1828. doi: 10.7503/cjcu20170158 [16] ZHANG L, PAN L W, NI C J, SUN T J, ZHAO S S, WANG S D, WANG A J, HU Y K. CeO2-ZrO2-promoted CuO/ZnO catalyst for methanol steam reforming[J]. Int J Hydrogen Energy, 2013, 38(11):4397-4406. doi: 10.1016/j.ijhydene.2013.01.053 [17] TANG P S, SUN H, CAO F, YANG J T, NI S L, CHEN H F. Visible-light driven LaNiO3 nanosized photocatalysts prepared by a sol-gel process[J]. Adv Mater Res, 2011, 279(11):83-87. https://www.scientific.net/AMR.279.83 [18] LI Y Y, YAO S S, WEN W, XUE L H, YAN Y W. Sol-gel combustion synthesis and visible-light-driven photocatalytic property of perovskite LaNiO3[J]. J Alloys Compd, 491(1/2):560-564. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=7b9be17f1150e380fb4e3fe9d5db2f0b [19] MORADI G R, KHOSRAVIAN F, RAHMANZADEH M. Effects of partial substitution of Ni by Cu in LaNiO3 perovskite catalyst for dry methane reforming[J]. Chin J Catal, 2012, 33(4/6):797-801. https://www.sciencedirect.com/science/article/pii/S1872206711603781 [20] 牛春艳, 徐占林.不同焙烧温度制备的类钙钛矿型复合氧化物La2NiO4的性能研究[J].硅酸盐通报, 2010, 29(5):1231-1234. http://d.old.wanfangdata.com.cn/Periodical/gsytb201005048NIU Chun-yan, XU Zhan-lin. Study on properties of perovskite-like composite oxides La2NiO4 prepared at different roasting temperatures[J]. Bull Chin Ceram Soc, 2010, 29(5):1231-1234. http://d.old.wanfangdata.com.cn/Periodical/gsytb201005048 [21] 杨彩虹, 韩怡卓, 李文彬. Ni-La2O3/C催化剂上甲醇羰基化反应性能的研究[J].燃料化学学报, 2000, 28(5):392-395. doi: 10.3969/j.issn.0253-2409.2000.05.003YANG Cai-hong, HAN Yi-zhuo, LI Wen-bin. Study on the carbonylation of methanol over Ni-La2O3/C catalyst[J]. J Fuel Chem Techno, 2000, 28(5):392-395. doi: 10.3969/j.issn.0253-2409.2000.05.003 [22] MICKEVIČIUS S, GREBINSKIJ S, BONDARENKA V, VENGALIS B, ORLOWSKI A. Investigation of epitaxial LaNiO3-x thin films by high-energy XPS[J]. J Alloys Compd, 2006, 423(1):107-111. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=aea5667a7ce453924861fe0cf50cc6ca [23] HONMA T, BENINO Y, FUJIWARA T, KOMATSU T, DIMITROV V. Electronic polarizability, optical basicity, and interaction parameter of La2O3 and related glasses[J]. J Appl Phys, 2002, 91(5):2942-2950. doi: 10.1063/1.1436292 [24] FU Z, HU J, HU W, YANG S, LUO Y. Quantitative analysis of Ni2+/Ni3+ in Li[NixMnyCoz]O2 cathode materials:Non-linear least-squares fitting of XPS spectra[J]. Appl Surf Sci, 2018, 441:1048-1056. doi: 10.1016/j.apsusc.2018.02.114 [25] GONZALEZ-DELACRUZ V M, TERNERO F, PEREIGUEZ R, CABALLERO A, HOLGADO J P. Study of nanostructured Ni/CeO2 catalysts prepared by combustion synthesis in dry reforming of methane[J]. Appl Catal A:Gen, 2010, 384(1/2):1-9. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=83b73d40dbfbd7a7dae7a097a8cd353e [26] SEIM H, MÖLSÄ H, NIEMINEN M, FJELLVG H, NIINIST L. Deposition of LaNiO3 thin films in an atomic layer epitaxy reactor[J]. J Mater Chem, 1997, 7(3):449-454. doi: 10.1039/a606316k [27] PENG X, OMASTA T J, ROLLER J M, MUSTAIN W E. Highly active and durable Pd-Cu catalysts for oxygen reduction in alkaline exchange membrane fuel cells[J]. Front Energy, 2017, 11(3):299-309. [28] WANG C, CHENG Q P, WANG X L, MA K, BAI X Q, TAN S R, TIAN Y, TONG D, ZHENG L R, ZHANG J, LI X G. Enhanced catalytic performance for CO preferential oxidation over CuO catalysts supported on highly defective CeO2 nanocrystals[J]. Appl Surf Sci, 2017, 422(2):932-943. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=391accdd48df1b637266f8ce42ac61b4 [29] BENNICI S, GERVASINI A, RAVASIO N, ZACCHERIA F. Optimization of tailoring of CuOx species of silica alumina supported catalysts for the selective catalytic reduction of NOx[J]. J Phys Chem B, 2003, 107(22):5168-5176. doi: 10.1021/jp022064x [30] AFONASENKO T N, TSYRULNIKOV P G, GULYAEVA T I, LEONTEVA N N, SMIRNOVA N S, KOCHUBEI D I, SUPRUN E A, SALANOV A N. (CuO-CeO2)/glass cloth catalysts for selective CO oxidation in the presence of H2:The effect of the nature of the fuel component used in their surface self-propagating high-temperature synthesis on their properties[J]. Kinet Catal, 2013, 54(1):59-68. [31] KULKARNI G U, RAO C N R. EXAFS and XPS investigations of Cu/ZnO catalysts and their interaction with CO and methanol[J]. Top Catal, 2003, 22(3):183-189. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=c18db8e5a8333691cda5a303df6a1fb8 [32] PEREÑÍGUEZ R, GONZÁLEZ-DELACRUZ V M, HOLGADO J P, CABALLERO A. Synthesis and characterization of a LaNiO3 perovskite as precursor for methane reforming reactions catalysts[J]. Appl Catal B:Environ, 2010, 93(3):346-353. [33] 王东哲, 田志强, 张宣娇, 冯旭, 张磊, 白金.制备方法对甲醇水蒸气重整制氢CuO/CeO2催化剂的影响[J].石油化工, 2019, 48(4):335-341. doi: 10.3969/j.issn.1000-8144.2019.04.002WANG Dong-zhe, TIAN Zhi-qiang, ZHANG Xuan-jiao, FENG Xu, ZHANG Lei, BAI Jin. Effect of preparation methods on CuO/CeO2 catalysts for hydrogen production from methanol steam reforming[J]. Petro Technol, 2019, 48(4):335-341. doi: 10.3969/j.issn.1000-8144.2019.04.002 [34] 庆绍军, 侯晓宁, 刘雅杰, 王磊, 李林东, 高志贤. Cu-Ni-Al尖晶石催化甲醇水蒸气重整制氢性能的研究[J].燃料化学学报, 2018, 46(10):69-76. http://www.ccspublishing.org.cn/article/id/b14d7a58-9df6-4e70-aff6-8011a66c65eaQING Shao-jun, HOU Xiao-ning, LIU Ya-jie, WANG Lei, LI Lin-dong, GAO Zhi-xian. Study on Cu-Ni-Al spinel catalytic performance for hydrogen production from methanol steam reforming[J]. J Fuel Chem Technol, 2018, 46(10):69-76. http://www.ccspublishing.org.cn/article/id/b14d7a58-9df6-4e70-aff6-8011a66c65ea -





 下载:
下载: