Effect of preparation method of nanosized zeolite HY-Al2O3 composite as NiMo catalyst support on diesel HDS
-
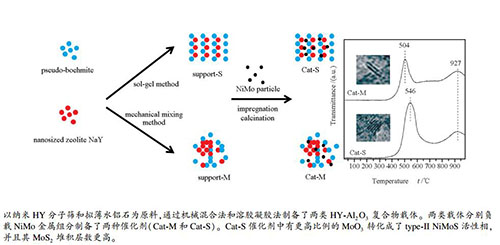
摘要: 以纳米HY分子筛-氧化铝混合物为载体,根据两者混合方式的不同(溶胶凝胶法和机械混合法)制备了两种NiMo加氢脱硫催化剂,并对其进行了XRD、BET、TPD、H2-TPR、HRTEM和FT-IR等表征。与溶胶凝胶法催化剂相比,机械混合法催化剂表现出了较好的纹理结构和更高酸量,其金属相更易还原,边角位Mo原子的分散度更高,表现出了更高的加氢脱硫性能。但溶胶凝胶法催化剂的type-Ⅱ Ni-Mo-S活性相前驱物比例更高,MoS2晶片长度更大,堆垛程度更高,活性组分分散度较差。虽然溶胶凝胶法有利于提高type-Ⅱ Ni-Mo-S活性相前驱物比例,但是该方法导致的较差孔结构抑制了这种优势,并且降低了活性组分分散度,减弱了催化活性。Abstract: Two NiMo catalysts using the nanosized zeolite HY-Al2O3 composite (labeled as NYA) prepared by mechanical mixing method and sol-gel method as the support were prepared and characterized by XRD, BET, TPD, H2-TPR, HRTEM and FT-IR spectroscopy. The former catalyst possessed larger pore volume and specific surface area, more acid amount, superior reducibility of metal phase and higher dispersion of edge and corner Mo atoms, and showed higher hydrodesulfurization (HDS) performance. Compared with the former catalyst, the latter catalyst had more MoO3 to be converted to active type-Ⅱ NiMoS phase, possessed higher stacking degree and bigger length of MoS2 slabs, and showed lower active phase dispersion. Although the sol-gel method was beneficial to increase the precursor ratio of type-Ⅱ NiMoS phase, the poor pore structure caused by this method inhibited this advantage and reduced the catalytic activity of the catalyst.
-
Key words:
- nanosized zeolite /
- hydrodesulfurization /
- hybrid mode
-
Table 1 Textural properties of two catalysts
Sample SBET/
(m2·g-1)Smi/
(m2·g-1)Pore volume
v/(cm3·g-1)Pore size
d/nmCat-S 124.9 15.3 0.27 9.1 Cat-M 207.6 27.6 0.41 9.2 Table 2 Amounts of the acid sites of two catalysts
Sample Amount of acid sites/(mmol·g-1 catalyst) B/L (ratio) L B Cat-S 0.32 0.27 0.84 Cat-M 0.53 0.45 0.85 Table 3 Average stacking degree (NA), average slab length (LA) and fraction available Mo (fMo) of two catalysts
Catalyst NA LA /nm fMo Cat-M 3.1 3.52 0.32 Cat-S 3.6 4.32 0.27 Table 4 Hydrotreating activity results of FCC diesel over two catalysts
Feedstock Cat-M Cat-S d20 ℃/(g·cm-3) 0.970 0.930 0.935 S concentration, w/(μg·g-1) 7216 652 981 HDS rate/% - 91.7 86.4 Distillation range t/℃ IBP 222 198 201 5% 234 214 219 10% 245 221 226 50% 288 249 255 90% 357 319 306 FBP 380 326 327 Table 5 S removal rate of DBT, 4-MDBT and 4, 6-DMDBT over different catalysts
Removal rate Cat-M Cat-S DBT/% 99.93 95.47 4-MDBT/% 81.11 76.15 4, 6-DMDBT/% 63.42 61.22 -
[1] ZHOU W W, LIU M F, ZHANG Q, WEI Q, DING S J, ZHOU Y S. Synthesis of NiMo catalysts supported on gallium-containing mesoporous Y zeolites with different gallium contents and their high activities in the hydrodesulfurization of 4, 6-dimethyldibenzothiophene[J]. ACS Catal, 2017, 7(11):7665-7679. doi: 10.1021/acscatal.7b02705 [2] DING L, ZHENG Y, ZHANG Z, RING Z, CHEN J. HDS, HDN, HDA and hydrocracking of model compounds over Mo-Ni catalysts with various acidities[J]. Appl Catal A:Gen, 2007, 319:25-37. doi: 10.1016/j.apcata.2006.11.016 [3] JORGE R, AÍDA G A, FELIPE S M, VÍCTOR M A, PERLA C V, LAETITIA O, FRANÇOISE M. HDS of 4, 6-DMDBT over NiMoP/(x)Ti-SBA-15 catalysts prepared with H3PMo12O40[J]. Energy Fuels, 2012, 26(2):773-782. doi: 10.1021/ef201590g [4] GUTIERREZ O Y, KLIMOVA T J. Effect of the support on the high activity of the (Ni)Mo/ZrO2-SBA-15 catalyst in the simultaneous hydrodesulfurization of DBT and 4, 6-DMDBT[J]. J Catal, 2011, 281(1):50-62. doi: 10.1016/j.jcat.2011.04.001 [5] NAVA R, INFANTES M A, CASTANO P, LOPEZ R G, PAWELEC B. Inhibition of CoMo/HMS catalyst deactivation in the HDS of 4, 6-DMDBT by support modification with phosphate[J]. Fuel, 2011, 90(8):2726-2737. doi: 10.1016/j.fuel.2011.03.049 [6] FU W Q, ZHANG L, TANG T D, KE Q P, WANG S, HU J B, FANG G Y, LI J X, XIAO F S. Extraordinarily high activity in the hydrodesulfurization of 4, 6-Dimethyldibenzothiophene over Pd supported on mesoporous zeolite Y[J]. J Am Chem Soc, 2011, 133(39):15346-15349. doi: 10.1021/ja2072719 [7] ZHANG L, FU W Q, KE Q P, ZHANG S, JIN H L, HU J B, WANG S, TANG T D. Study of hydrodesulfurization of 4, 6-DM-DBT over Pd supported on mesoporous USY zeolite[J]. Appl Catal A:Gen, 2012, 433/434:251-257. doi: 10.1016/j.apcata.2012.05.028 [8] RICHARD F, BOITA T, PÉROT G. Reaction mechanism of 4, 6-dimethyldibenzothiophene desulfurization over sulfided NiMoP/Al2O3-zeolite catalysts[J]. Appl Catal A:Gen, 2007, 320:69-79. [9] YIN H L, ZHOU T N, LIU Y Q. NiMo/Al2O3 catalyst containing nano-sized zeolite Y for deep hydrodesulfurization and hydrodenitrogenation of diesel[J]. J Nat Gas Chem, 2011, 20(4):441-448. doi: 10.1016/S1003-9953(10)60204-6 [10] TANG T, ZHANG L, FU W. Design and synthesis of metal sulfide catalysts supported on zeolite nanofiber dundles with unprecedented hydrodesulfurization activities[J]. J Am Chem Soc, 2013, 135(31):11437-11440. doi: 10.1021/ja4043388 [11] SRINIVAS B N, MAITY S K, PRASAD V V D N, RANA M S, KUMAR M, DHAR G M, RAO P T S R. Support effect studies on TiO2-Al2O3 mixed oxide hydroprocessing catalysts[J]. Stud Surf Sci Catal, 1998, 113:497-506. http://www.osti.gov/scitech/biblio/20050862-solid-state-vanadium-nmr-studies-supported-sub-sub-wo-sub-tio-sub-catalysts [12] MAITY S K, ANCHEYTA J, RANA M S, RAYO P. Alumina-titania mixed oxide used as support for hydrotreating catalysts of maya heavy crude-effect of support preparation methods[J]. Energy Fuels, 2016, 20(2):427-431. [13] BARRERA M C, VINIEGRA M, ESCOBAR J, VRINAT M, DE LOS REYES J A, MURRIETA F, GARCÍA J. Highly active MoS2 on wide-pore ZrO2-TiO2 mixed oxides[J]. Catal Today, 2004, 98(1/2):131-139. [14] BARRERA M C, ESCOBAR J, DE LOS REYES J A, CORTÉS M A, VINIEGRA M, HERNÁNDEZ A. Effect of solvo-thermal treatment temperature on the properties of sol-gel ZrO2-TiO2 mixed oxides as HDS catalyst supports[J]. Catal Today, 2006, 116(4):498-504. doi: 10.1016/j.cattod.2006.06.030 [15] YIN H L, LIU X L, YUAN Y Y, ZHOU T N. Nanosized HY zeolite-alumina composite support for hydrodesulfurization of FCC diesel[J]. J Porous Mater, 2015, 22(1):29-36. doi: 10.1007/s10934-014-9869-5 [16] LIU B J, ZHA X J, MENG Q M, HOU H J, GAO S S, ZHANG J X, SHENG S S, YANG W S. Preparation of NiW/TiO2-Al2O3 hydrodesulfurization catalyst with microwave technique[J]. Chin J Catal, 2005, 26(6):458-462. [17] PONCELET G, DUBRU M L. An infrared study of the surface acidity of germanic near-faujasite zeolite by pyridine adsorption[J]. J Catal, 1978, 52(2):321-331. [18] EMEIS C A. Determination of integrated molar extinction coefficients for infrared absorption bands of pyridine adsorbed on solid acid catalysts[J], J Catal, 1993, 141(2):347-354. doi: 10.1006/jcat.1993.1145 [19] TOPSØE H, CLAUSEN B S, CANDIA R, WIVEL C, MØRUP S. In situ mössbauer emission spectroscopy studies of unsupported and supported sulfided CoMo hydrodesulfurization catalysts:Evidence for and nature of a CoMoS phase[J]. J Catal, 1981, 68(2):433-452. doi: 10.1016/0021-9517(81)90114-7 [20] TOPSØE H, CLAUSEN B S, TOPSØE N Y, ZEUTHEN P. Progress in the design of hydrotreating catalysts based on fundamental molecular insight[J]. Stud Surf Sci Catal, 1989, 53:77-102. doi: 10.1016/S0167-2991(08)61061-7 [21] MARZARI J A, RAJAGOPAL S, MIRANDA R. Bifunctional mechanism of pyridine hydrodenitrogenation[J]. J Catal, 1995, 156(2):255-264. doi: 10.1006/jcat.1995.1252 [22] HENSEN E, KOOYMAN P, VAN M Y, VAN K A M. The relation between morphology and hydrotreating activity for supported MoS2 particles[J]. J Catal, 2001, 199(2):224-235. https://www.researchgate.net/publication/241951970_Morphology_study_of_MoSsub_2-_and_WSsub_2-based_hydrotreating_catalysts_by_high-resolution_electron_microscopy [23] HENSEN E J M, DE BEER V H J, VAN VEEN J A R, VAN SANTEN R A. A refinement on the notion of type Ⅰ and Ⅱ (Co)MoS phases in hydrotreating catalysts[J]. Catal Lett, 2002, 84(1/2):59-67. [24] EIJSBOUTS S, HEINERMAN J J L, ELZERMAN H J W. MoS2 structures in high-activity hydrotreating catalysts:Ⅰ. Semi-quantitative method for evaluation of transmission electron microscopy results. Correlations between hydrodesulfurization and hydrodenitrogenation activities and MoS2 dispersion[J]. Appl Catal A:Gen, 1993, 105(1):53-68. doi: 10.1016/0926-860X(93)85133-A -





 下载:
下载: