Effect of reaction time on free radical concentration in hydrogenation liquefaction of Naomaohu coal
-
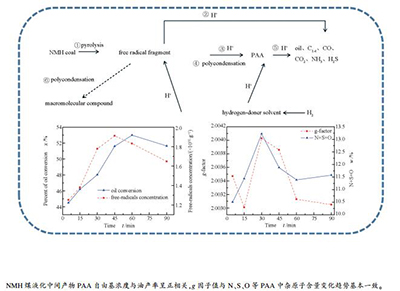
摘要: 以新疆淖毛湖煤(NMH)为原料,在间歇高压反应釜中进行加氢液化实验,通过电子顺磁共振波谱仪(EPR)分析了加氢液化过程中间产物-沥青质(PAA)自由基浓度随停留时间的变化。结果表明,在实验温度下NMH煤加氢液化总转化率先升高后降低,在60 min达到峰值96.87%,油产率为53.01%;淖毛湖原煤自由基浓度为2.6654×1018/g,PAA自由基浓度在1.2519×1018/g-1.9121×1018/g,随着反应停留时间的延长先上升后下降,中间产物PAA自由基浓度数值可以反映液化反应进行的程度,与油产率变化趋势一致;反应中间产物PAA的g值小于原煤g值(2.00434),在2.00301-2.00403,在液化加氢过程中其g值呈先上升后下降的趋势,与PAA中N、S、O等杂原子成分的变化一致,与元素分析结果相吻合。Abstract: Coal from Naomaohu (NMH) in Xinjiang as raw material, hydroliquefaction experiments were performed in a batch high-pressure liquefaction reactor. Variations of free radical concentration of PAA and the intermediate products of hydroliquefaction with reaction residence time were examined with an EPR spectrometer. The results show that conversion rate of NMH coal hydroliquefaction first rises and then decreases. The maximum conversion rate and oil yield is 96.87% and 53.01% in 60 min. The free radical concentration of NMH is 2.6654×1018/g. PAA's free radical concentration ranges between 1.2519×1018/g and 1.9121×1018/g, which first increases and then decreases with increasing reaction time. As for the intermediate products, PAA, its free radical concentration could reflect extent of hydroliquefaction reaction, which has the same variation tendency with the oil yield. PAA's g value is between 2.00301 and 2.00403, which is lower than that of NMH (2.00434). In the process of hydroliquefaction, its g value first rises and then decreases, which is closely related with the heteroatom content of N, S, O in PAA and is consistent with the element analysis result.
-
Key words:
- Naomaohu coal /
- EPR /
- free radical concentration /
- asphaltene /
- hydrogenation liquefaction
-
表 1 煤样的工业分析和元素分析
Table 1 Proximate and ultimate analyses of sample
Sample Proximate analysis w/% Ultimate analysis wdaf/% H/C
(atomic ratio)Mad Ad Vdaf FCdaf C H N St O* NMH coal 15.59 4.98 51.82 48.18 73.52 5.68 0.96 0.24 19.60 0.93 *:by difference 表 2 NMH煤的液化气体产物分析
Table 2 Liquefaction gas products analysis of NMH sample
NMH coal φ/% H2 CO CO2 CH4 C2-4 5 min 90.68 1.19 5.07 2.19 0.87 15 min 90.61 1.30 5.34 1.79 0.96 30 min 89.53 1.46 5.42 2.31 1.28 45 min 88.96 1.54 5.51 2.59 1.40 60 min 88.43 1.52 5.59 3.10 1.36 90 min 86.60 1.68 6.48 3.53 1.71 表 3 原煤和液化中间产物自由基浓度
Table 3 Free-radicals concentrations analysis of raw coal and PAAs
t/min Raw coal 5 15 30 45 60 90 Ng/(×1016·g-1) 266.54 125.19 138.22 177.80 191.21 183.24 164.78 表 4 原煤和液化中间产物的g因子值
Table 4 g-factors analysis of raw coal and PAAs
t/min Raw coal 5 15 30 45 60 90 g 2.00434 2.00347 2.00301 2.00403 2.00384 2.00313 2.00305 Heteroatom 20.80% 10.51% 11.44% 13.22% 11.88% 11.39% 11.58% 表 5 PAA元素分析
Table 5 Ultimate analysis of PAAs
Ultimate analysis
wdaf/%Time t/min 0 5 15 30 45 60 90 O 19.60 9.17 10.11 11.74 9.58 9.96 10.28 N 0.96 1.17 1.16 1.25 1.34 1.22 1.14 St 0.24 0.17 0.17 0.23 0.26 0.21 0.16 -
[1] 郭薇.新疆淖毛湖矿区煤田地质特征及可采煤层对比研究[J].环球人文地理, 2016, (24). http://d.old.wanfangdata.com.cn/Periodical/hqrwdl201624069GUO Wei. Study on the geological characteristics and the comparison of coal seams in the coal field of Naomohu in xinjiang[J]. Geol Sur, 2016, (24). http://d.old.wanfangdata.com.cn/Periodical/hqrwdl201624069 [2] 李超.淖毛湖煤及其添加铁系氧化物的热解特性研究[D].大连: 大连理工大学, 2015. http://cdmd.cnki.com.cn/Article/CDMD-10141-1015652203.htmLI Chao. Pyrolysis of naomaohu coal and the mixture of coal and iron oxide[D]. Dalian: University of Technology, 2015. http://cdmd.cnki.com.cn/Article/CDMD-10141-1015652203.htm [3] 赵鹏, 张晓静, 李军芳, 吴艳, 毛学锋, 常秋连.新疆淖毛湖煤加氢液化特性及液化产物中氢的分布规律[J].煤炭转化, 2018, 41(4):42-47. doi: 10.3969/j.issn.1004-4248.2018.04.007ZHAO Peng, ZHANG Xiao-jing, LI Jun-fang, WU Yan, MAO Xue-feng, CHANG Qiu-lian. Liquefaction characteristics and hydrogen distribution in product of Naomaohu coal from Xinjiang[J]. Coal Convers, 2018, 41(4):42-47. doi: 10.3969/j.issn.1004-4248.2018.04.007 [4] PETRAKIS L, GRANDY D W. Free radicals in coals and coal conversion. 2. Effect of liquefaction processing conditions on the formation and quenching of coal free radicals[J]. Fuel, 1980, 59(4):227-232. doi: 10.1016/0016-2361(80)90139-8 [5] PETRAKIS L, GRANDY D W. Free radicals in coals and coal conversion. 4. Investigation of the free radicals in selected macerals upon liquefaction[J]. Fuel, 1981, 60(2):120-124. doi: 10.1016/0016-2361(81)90005-3 [6] RUDNICK L R, TUETING D. Investigation of free radicals produced during coal liquefaction using ESR[J]. Fuel, 1984, 63(2):153-157. http://www.sciencedirect.com/science/article/pii/0016236184900280 [7] PILAWA B, PUSZ S, KRZESIŃSKA M, KOSZOREK A, KWIECIŃSKA B. Application of electron paramagnetic resonance spectroscopy to examination of carbonized coal blends[J]. Int J Coal Geol, 2009, 77(3):372-376. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=JJ0210368866 [8] 李刚.煤热解中间体和自由基表征及反应机理研究[D].大连: 大连理工大学, 2015. http://cdmd.cnki.com.cn/Article/CDMD-10141-1015355475.htmLI Gang. Characterization of intermediates and radicals in coal pyrolysis and investigation on reaction mechanism[D]. Dalian: University of Technology, 2015. http://cdmd.cnki.com.cn/Article/CDMD-10141-1015355475.htm [9] 万传玲, 潘铁英, 史新梅, 周丽芳, 王胜春, 张德祥.热解煤焦油随保存时间变化机理的探索[J].实验室研究与探索, 2013, (10):261-264. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=QKC20132014021800055225WAN Chuan-ling, PAN Tie-ying, SHI Xin-mei, ZHOU Li-fang, WANG Sheng-chun, ZHANG De-xiang. Mechanism investigation of pyrolysis coal tar changes with storage time[J]. Res Exp Lab, 2013, (10):261-264. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=QKC20132014021800055225 [10] 刘沐鑫.煤直接液化过程中溶剂的作用规律及煤裂解自由基的行为研究[D].北京: 中国科学院大学, 2015. http://www.wanfangdata.com.cn/details/detail.do?_type=degree&id=Y3106651LIU Mu-xin. The solvent action in liquefaction of coal and pyrolysis free radical behavior of coal[D]. Beijing: University of Chinese Academy of Sciences, 2015. http://www.wanfangdata.com.cn/details/detail.do?_type=degree&id=Y3106651 [11] HE W, LIU Q, LEI S, LIU Z, CI D, LIEVENS C, GUO X, LIU M. Understanding the stability of pyrolysis tars from biomass in a view point of free radicals[J]. Bioresour Technol, 2014, 156(3):372-375. http://europepmc.org/abstract/med/24507874 [12] LIU J, JIANG X, SHEN J, ZHANG H. Chemical properties of superfine pulverized coal particles. Part 1. Electron paramagnetic resonance analysis of free radical characteristics[J]. Adv Powder Technol, 2014, 25(3):916-925. doi: 10.1016/j.apt.2014.01.021 [13] LIU J, JIANG X, HAN X, SHEN J, ZHANG H. Chemical properties of superfine pulverized coals. Part 2. Demineralization effects on free radical characteristics[J]. Fuel, 2014, 115(12):685-696. http://www.sciencedirect.com/science/article/pii/S0016236113006984 [14] 仲晓星, 王德明, 徐永亮, 辛海会.煤氧化过程中的自由基变化特性[J].煤炭学报, 2010, 35(6):960-963. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=QK201000443295ZHONG Xiao-xing, WANG De-ming, XU Yong-liang, XIN Hai-hui. The variation characteristics of free radicals in coal oxidation[J]. J Chin Coal Soc, 2010, 35(6):960-963. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=QK201000443295 [15] 成茂, 王胜春, 张德祥.煤转化过程自由基研究进展[J].煤炭转化, 2012, 35(4):94-98. doi: 10.3969/j.issn.1004-4248.2012.04.022CHENG Mao, WANG Sheng-chun, ZHANG De-xiang. Research progress on free radicals in coal conversion progress[J]. Coal Convers, 2012, 35(4):94-98. doi: 10.3969/j.issn.1004-4248.2012.04.022 [16] 郑榕萍. EPR定量测定煤中自由基的方法及煤液化机理的研究[D].上海: 华东理工大学, 2011. http://cdmd.cnki.com.cn/Article/CDMD-10251-1011050677.htmZHENG Rong-ping. Research on quantitative determination of free radicals in coal by EPR and coal liquefaction mechanism[D]. Shanghai: East China University of Science and Technology, 2011. http://cdmd.cnki.com.cn/Article/CDMD-10251-1011050677.htm [17] 张德祥, 高晋生, 朱之培.年青煤在石油重油中加氢液化的研究[J].华东理工大学学报, 1986, (3):46-55. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=QK000001393513ZHANG De-xiang, GAO Jin-sheng, ZHU Zhi-pei. The liquefaction of some chinese low rank coals by hydrogenation in various heavy oils of petroleum[J]. J East Chin Univ Sci Technol, 1986, (3):46-55. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=QK000001393513 [18] 刘国根, 邱冠周.煤的ESR波谱研究[J].波谱学杂志, 1999, 16(2):177-180. doi: 10.3969/j.issn.1000-4556.1999.02.016LIU Guo-gen, QIU guan-zhou. A study on ESR spectrum of coal[J]. J Mag Res, 1999, 16(2):177-180. doi: 10.3969/j.issn.1000-4556.1999.02.016 [19] QIU N, LI H, JIN Z, ZHU Y. Temperature and time effect on the concentrations of free radicals in coal:Evidence from laboratory pyrolysis experiments[J]. Int J Coal Geol, 2007, 69(3):220-228. doi: 10.1016/j.coal.2006.04.002 [20] 郑榕萍, 潘铁英, 史新梅, 周丽芳, 刘瑞民, 张德祥, 高晋生.标准曲线法测定煤中自由基含量[J].波谱学杂志, 2011, 28(2):259-264. doi: 10.3969/j.issn.1000-4556.2011.02.010ZHENG Rong-ping, PAN Tie-ying, SHI Xin-mei, ZHOU Li-fang, LIU Rui-min, ZHANG De-xiang, GAO Jin-sheng. Quantitative determination of free radical content in coal by standard curve method[J]. J Mag Res, 2011, 28(2):259-264. doi: 10.3969/j.issn.1000-4556.2011.02.010 [21] WHITEHURST D D. A primer on the chemistry and constitution of coal[J]. Acs Symposium, 1978. doi: 10.1021/bk-1978-0071.ch001 [22] 周扬, 张媛媛, 陈丽诗, 潘铁英, 张德祥.两种西部煤的化学结构及加氢液化性能[J].煤炭转化, 2017, 40(6):1-6. doi: 10.3969/j.issn.1004-4248.2017.06.001ZHOU Yang, ZHANG Yuan-yuan, CHEN Li-shi, PAN Tie-ying, ZHANG De-xiang. Chemical structure and hydrogenation liquefaction performance of two kinds of western coal[J]. Coal Convers, 2017, 40(6):1-6. doi: 10.3969/j.issn.1004-4248.2017.06.001 [23] 陈丽诗.煤及加氢液化中间产物结构解析与分子模型构建[D].上海: 华东理工大学, 2018.CHEN Li-shi. Structure analysis and molecular model construction of coal and its intermediate products derived from coal hydroliquefaction[D]. Shanghai: East China University of Science and Technology, 2018. [24] ZHANG C, LEE C W, KEOGH R A, DEMIREL B, DAVIS B H. Thermal and catalytic conversion of asphaltenes[J]. Fuel, 2001, 80(8):1131-1146. doi: 10.1016/S0016-2361(00)00178-2 [25] 张茂盛.电子自旋和轨道运动的相互作用[J].时代报告, 2012, (4):79. http://d.old.wanfangdata.com.cn/Periodical/sdbg-xs201204073ZHANG Mao-sheng. The interaction of electron spin and orbital motion[J]. Time Report, 2012, (4):79. http://d.old.wanfangdata.com.cn/Periodical/sdbg-xs201204073 [26] PETRAKIS L, GRANDY D W. Electron spin resonance spectrometric study of free radicals in coals[J]. Anal Chem, 1978, 50(2):303-308. doi: 10.1021/ac50024a034 -





 下载:
下载: