Preparation of Ag-Mn/γ-Al2O3-TiO2 catalysts by complexation-impregnation process with citric acid and its application in propane catalytic combustion
-
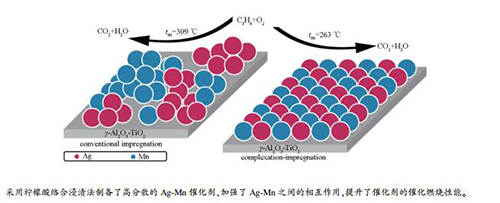
摘要: 采用不同浸渍方法制备了系列Ag-Mn/γ-Al2O3-TiO2催化剂,利用BET、XRD、TEM、XPS和H2-TPR等技术对催化剂进行了表征,通过丙烷催化燃烧反应考察了催化性能。结果表明,与常规浸渍法相比,柠檬酸络合浸渍法促进了催化剂表面Ag与Mn颗粒的分散及加强了Ag与Mn之间的相互作用,从而提高了活性氧物种的相对含量和催化剂的低温还原性能,进而促进丙烷催化燃烧活性的提升。其中,络合浸渍法制备的Ag1Mn3/γ-Al2O3-TiO2催化剂在263℃时丙烷转化率即可达90%。Abstract: A series of Ag-Mn/γ-Al2O3-TiO2 catalysts were prepared by different impregnation procedures. The catalysts were characterized by BET, XRD, TEM, XPS and H2-TPR, and the catalytic properties were investigated by propane catalytic combustion. Results show that compared with the conventional impregnation method, complexation-impregnation procedure with citric acid promotes the dispersion of Ag and Mn particles on the surface of catalyst and strengthens the interaction between Ag and Mn, so as to increase the relative content of reactive oxygen species and improve the reducibility of catalysts, which further improves the catalytic activity of propane combustion reaction. Especially, the Ag1Mn3/γ-Al2O3-TiO2 catalyst prepared by complexation-impregnation process with citric acid exhibits the best activity for propane catalytic combustion with 90% conversion at 263℃.
-
Key words:
- complexation-impregnation /
- Ag-Mn catalysts /
- propane /
- catalytic combustion /
- interaction
-
表 1 不同催化剂的织构性质
Table 1 Textural properties of different catalysts
Sample Surface
area A/
(m2·g-1)Pore
volume v/
(cm3·g-1)Average
pore diameter
d/nmAlTi 163 0.41 9.9 Ag4/AlTi 105 0.31 11.4 Ag4/AlTi(CA) 112 0.36 10.6 Mn4/AlTi 94 0.30 10.5 Mn4/AlTi(CA) 122 0.34 10.4 Ag1Mn3/AlTi 93 0.23 9.9 Ag1Mn3/AlTi(CA) 126 0.40 11.1 表 2 催化剂的XPS数据
Table 2 XPS results of different catalysts
Sample Surface element contents wmol/% Ag0/
(Ag++Ag0)Mnδ+/Mntotal O species contents Ag Mn O Al Mn2+ Mn3+ Mn4+ OⅠ OⅡ OⅢ Ag4/AlTi 1.80 - 64.80 33.40 42.52 - - - 32.55 47.65 19.80 Ag4/AlTi(CA) 4.00 - 64.61 31.39 43.34 - - - 28.73 51.76 19.50 Mn4/AlTi - 1.08 75.41 23.51 - 22.12 45.18 32.70 34.89 49.23 15.88 Mn4/AlTi(CA) - 8.52 61.6 29.88 - 12.28 31.89 55.83 38.97 51.22 9.80 Ag1Mn3/AlTi 0.9 1.51 72.7 24.89 33.54 22.56 39.42 38.02 32.41 52.73 14.86 Ag1Mn3/AlTi(CA) 1.09 4.72 70.81 23.39 38.33 12.08 28.02 59.90 36.32 54.57 9.11 -
[1] HUANG H, XU Y, FENG Q, DENNIS Y, LEUNG D Y C. Low temperature catalytic oxidation of volatile organic compounds:A review[J]. Catal Sci Technol, 2015, 5(5):2649-2669. doi: 10.1039/C4CY01733A [2] LI J, LIU H, DENG Y, LIU G, CHEN Y, YANG J. Emerging nanostructured materials for the catalytic removal of volatile organic compounds[J]. Nanotechnol Rev, 2016, 5(1):147-181. http://cn.bing.com/academic/profile?id=bcfd327dbf0299ce5bf6bf0ba5483161&encoded=0&v=paper_preview&mkt=zh-cn [3] BARANOWAKA K, OKAL J. Bimetallic Ru-Re/gamma-Al2O3 catalysts for the catalytic combustion of propane:Effect of the Re addition[J]. Appl Catal A:Gen, 2015, 499:158-167. doi: 10.1016/j.apcata.2015.04.023 [4] HU Z, QIU S, YOU Y, GUO Y, GUO Y, WANG L, ZHAN W, LU G. Hydrothermal synthesis of NiCeOx nanosheets and its application to the total oxidation of propane[J]. Appl Catal B:Environ, 2018, 225:110-120. doi: 10.1016/j.apcatb.2017.08.068 [5] CHEN S, LI Y, MA F, CHEN F, LU W. The relationship between the surface oxygen species and the acidic properties of mesoporous metal oxides and their effects on propane oxidation[J]. Catal Sci Technol, 2015, 5(2):1213-1221. doi: 10.1039/C4CY01231C [6] HE C, CHENG J, ZHANG X, DOUTHWAITE M, PATTISSON S, HAO Z. Recent advances in the catalytic oxidation of volatile organic compounds:A review based on pollutant sorts and sources[J]. Chem Rev, 2019, 119(7):4471-4568. doi: 10.1021/acs.chemrev.8b00408 [7] QU Z, SHEN S, CHEN D, WANG Y. Highly active Ag/SBA-15 catalyst using post-grafting method for formaldehyde oxidation[J]. J Mol Catal A:Chem, 2012, 356:171-177. doi: 10.1016/j.molcata.2012.01.013 [8] QIN Y, QU Z, DONG C, HUANG N. Effect of pretreatment conditions on catalytic activity of Ag/SBA-15 catalyst for toluene oxidation[J]. Chin J Catal, 2017, 38(9):1603-1612. doi: 10.1016/S1872-2067(17)62842-0 [9] 赖潇潇, 冯洁, 周晓英, 侯忠燕, 林涛, 陈耀强.钾改性Mn/Ce0.65Zr0.35O2催化剂催化氧化甲苯[J].物理化学学报, 2020, 36(x):1-10.LAI Xiao-xiao, FENG Jie, ZHOU Xiao-ying, HOU Zhong-yan, LIN Tao, CHE Yao-qiang. Catalytic oxidation of toluene over potassium modified Mn/Ce0.65Zr0.35O2 catalyst[J]. Acta Phys-Chim Sin, 2020, 36(x):1-10. [10] QIN Y, QU Z, DONG C, WANG Y, HUANG N. Highly catalytic activity of Mn/SBA-15 catalysts for toluene combustion improved by adjusting the morphology of supports[J]. J Environ Sci, 2019, 76:208-216. doi: 10.1016/j.jes.2018.04.027 [11] WANG J, LI J, JIANG C, ZHOU P, ZHANG P, YU J. The effect of manganese vacancy in birnessite-type MnO2 on room-temperature oxidation of formaldehyde in air[J]. Appl Catal B:Environ, 2017, 204:147-155. doi: 10.1016/j.apcatb.2016.11.036 [12] WANG J, ZHANG P, LI J, JIANG C, YUNUS R, KIM J. Room-temperature oxidation of formaldehyde by layered manganese oxide:Effect of water[J]. Environ Sci Technol, 2015, 49(20):12372-12379. doi: 10.1021/acs.est.5b02085 [13] LIN R, LIU W, ZHONG Y, LUO M. Catalyst characterization and activity of Ag-Mn complex oxides[J]. Appl Catal A:Gen, 2001, 220(1/2):165-171. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=d3404ec1c8f4ea3477a7ecf54ad540cf [14] DENG J, HE S, XIE S, YANG H, LIU Y, GUO G, DAI H. Ultralow loading of silver nanoparticles on Mn2O3 nanowires derived with molten salts:A high-efficiency catalyst for the oxidative removal of toluene[J]. Environ Sci Technol, 2015, 49(18):11089. doi: 10.1021/acs.est.5b02350 [15] KHARLAMOVA T, MAMONTOV G, SALAEV M, ZAIKOVSKII V, POPOVA G, SOBOLEV V, KNYAZEV A, VODYANKINA O. Silica-supported silver catalysts modified by cerium/manganese oxides for total oxidation of formaldehyde[J]. Appl Catal A:Gen, 2013, 467(10):519-529. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=d5ee243aaa1c4a94d7a762c97c265745 [16] QU Z, BU Y, QIN Y, WANGY, FU Q. The improved reactivity of manganese catalysts by Ag in catalytic oxidation of toluene[J]. Appl Catal B:Environ, 2013, 132/133:353-362. doi: 10.1016/j.apcatb.2012.12.008 [17] PAN H, SU Q, CHEN J, YE Q, LIU Y, SHI Y. Promotion of Ag/H-BEA by Mn for lean NO reduction with propane at low temperature[J]. Environ Sci Technol, 2009, 43(24):9348-9353. doi: 10.1021/es901504b [18] LUO M, YUANX, ZHENG X. Catalyst characterization and activity of Ag-Mn, Ag-Co and Ag-Ce composite oxides for oxidation of volatile organic compounds[J]. Appl Catal A:Gen, 1998, 175(1/2):121-129. doi: 10.1016/S0926-860X(98)00210-5 [19] 袁善良, 兰海, 薄其飞, 张彪, 肖熙, 蒋毅. TiO2掺杂CuMnCe/Al2O3催化剂对甲烷催化燃烧脱氧反应的影响[J].燃料化学学报, 2017, 45(2):243-248. doi: 10.3969/j.issn.0253-2409.2017.02.015YUAN Shan-liang, LAN Hai, BO Qi-fei, ZHANG Biao, XIAO Xi, JIANG Yi. Effect of TiO2 doping on methane catalytic combustion deoxidation of CuMnCe/Al2O3 catalyst[J]. J Fuel Chem Technol, 2017, 45(2):243-248. doi: 10.3969/j.issn.0253-2409.2017.02.015 [20] PEREZ H, NAVARRO P, DELGADO J, MONTES M. Mn-SBA15 catalysts prepared by impregnation:Influence of the manganese precursor[J]. Appl Catal A:Gen, 2011, 400(1/2):238-248. doi: 10.1016/j.apcata.2011.05.002 [21] ZHANG Y, QIN Z, WANG G, ZHU H, DONG M, LI S, WU Z, LI Z, WU Z, ZHANG J, HU T, FAN W, WANG J. Catalytic performance of MnOx-NiO composite oxide in lean methane combustion at low temperature[J]. Appl Catal B:Environ, 2013, 129:172-181. doi: 10.1016/j.apcatb.2012.09.021 [22] XIE Y, GUO Y, GUO Y, WANG L, ZHAN W, WANG Y, GONG X, LU G. A highly effective Ni-modified MnOx catalyst for total oxidation of propane:the promotional role of nickel oxide[J]. RSC Adv, 2016, 6(55):50228-50237. doi: 10.1039/C6RA09039G [23] LI G, HU W, HUANG F, CHEN J, GONG M, YUAN S, CHEN Y, ZHONG L. Pd catalyst supported on ZrO2-Al2O3 by double-solvent method for methane oxidation under lean conditions[J]. Can J Chem Eng, 2017, 95(6):1117-1123. doi: 10.1002/cjce.22750 [24] HU W, LI G, CHEN J, HUANG F, GONG M, ZHONG L, CHEN Y. Enhancement of activity and hydrothermal stability of Pd/ZrO2-Al2O3 doped by Mg for methane combustion under lean conditions[J]. Fuel, 2017, 194:368-374. doi: 10.1016/j.fuel.2016.11.028 [25] QU Z, HUANG W, CHENG M, BAO X. Restructuring and redispersion of silver on SiO2 under oxidizing/reducing atmospheres and its activity toward CO oxidation[J]. J Phys Chem B, 2005, 109(33):15842-15848. doi: 10.1021/jp050152m [26] TANG X, CHEN J, LI Y, LI Y, XU Y, SHEN W. Complete oxidation of formaldehyde over Ag/MnOx-CeO2 catalysts[J]. Chem EngJ, 2006, 118(1):119-125. doi: 10.1016/j.cej.2006.02.002 [27] BAI B, QIAO Q, ARANDIYAN H, LI J, HAO J. Three-dimensional ordered mesoporous MnO2-supported Ag nanoparticles for catalytic removal of formaldehyde[J]. Environ Sci Technol, 2016, 50(5):2635-2640. doi: 10.1021/acs.est.5b03342 -





 下载:
下载: