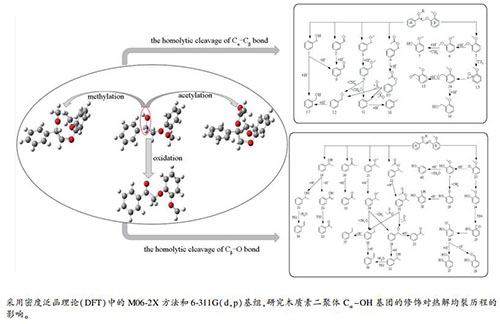
Density functional theory study on the effect of Cα-OH functional group modification on the homolytic cracking reaction routes during the pyrolysis of lignin dimer
-
摘要: 采用密度泛函理论方法,对四种β-O-4型二聚体木质素模型化合物2-(2-甲氧基苯氧基)-1-苯基乙烷-1-醇、2-(2-甲氧基苯氧基)-1-苯基乙烷-1-酮、1-甲氧基-2-(2-甲氧基-2-苯基乙氧基)苯、2-(2-甲氧基苯氧基)-1-苯乙基乙酸酯的Caromatic-O、Caromatic-Cα、Cα-Cβ、Cβ-O键均裂解离能进行了理论计算,并对所述二聚体的热解均裂历程进行了理论计算研究,分析了不同二聚体的热解产物形成途径。结果表明,Cβ-O键均裂是二聚体初次热解的主要反应,Cα-Cβ键均裂是竞争反应。Cα-OH官能团被氧化、乙酰化修饰后,Cβ-O键均裂解离能降低,而Cα-Cβ键的键解离能升高,Cβ-O键裂解概率增大,Cα-Cβ键均裂竞争性降低。基于上述四种模型化合物热解的主要芳香族产物有苯甲醇、甲苯、苯甲醛和愈创木酚等,Cα-OH官能团的选择性修饰可调控热解产物种类,其中,氧化修饰后的二聚体的热解产物种类变少,产物选择性增强;甲基化、乙酰化修饰后的二聚体热解可产生苯乙烷和甲苯。Abstract: Density functional theory method was used to calculate the bond dissociation energies of the Caromatic-Cα, Cα-Cβ, Cβ-O bond, and Caromatic-O bonds in four lignin dimer model compounds, viz., (2-(2-methoxyphenoxy)-1-phenylethan-1-ol, 2-(2-methoxyphenoxy)-1-phenylethan-1-one, 1-methoxy-2-(2-methoxy-2-phenylethoxy)benzene, and 2-(2-methoxyphenoxy)-1-phenylethyl acetate; the homolytic cracking reaction during pyrolysis of these dimers was then invetigated and the formation pathways of pyrolysis products of different dimers were analyzed. The results show that the homogenization of Cβ-O bond is the main reaction in the initial pyrolysis of dimer, whereas the homolysis of Cα-Cβ bond is a competitive reaction. After the oxidation and acetylation of Cα-OH, the bond dissociation energy of Cβ-O bond decreases, whereas the dissociation energy of Cα-Cβ bond increases, ccompanied with an increase in the probability of the Cβ-O bond dissociation and a decrease in the competitive ability of Cα-Cβ bond homolysis. For the pyrolysis of four model compounds, the main aromatic products include benzyl alcohol, toluene, benzaldehyde, guaiacol, etc. The selective modification of the Cα-OH functional groups can regulate the types of pyrolysis products. In particular, the product types for the pyrolysis of model compounds modified by oxidation become less, accompanied with an increase in the selectivity to ceratin products. Ethylbenzene and toluene can be produced from the hydrolysis of dimers modified by methylation and acetylation.
-
Key words:
- lignin /
- pyrolysis /
- homolysis /
- Cα-OH functional group /
- β-O-4 bonding /
- density functional theory
-
表 1 四种β-O-4型木质素二聚体各主要键的解离能和键长
Table 1 Bond dissociation energies and bond lengths of four β-O-4 linkage lignin model compounds
Bond type Dissociation energy E/(kJ·mol-1) Bond length d/nm BG1 BG2 BG3 BG4 BG1 BG2 BG3 BG4 Caromatic-Cα 411.4 401.5 402.6 402.1 0.15141 0.15004 0.15167 0.15135 Cα-Cβ 302.7 333.2 302.6 323.6 0.15213 0.15253 0.15263 0.15304 Cβ-O 270.7 234.6 271.2 254.2 0.14214 0.14054 0.14294 0.14274 Caromatic-O 417.5 426.7 416.3 403.3 0.13668 0.13740 0.13673 0.13689 -
[1] LIU X, WEI W, WU S, LEI M, LIU Y. A promptly approach from monosaccharides of biomass to oligosaccharides via sharp-quenching thermo conversion (SQTC)[J]. Carbohydr Polym, 2018, 189:204-209. doi: 10.1016/j.carbpol.2018.01.107 [2] LEI M, WU S, LIANG J, LIU C. Comprehensive understanding the chemical structure evolution and crucial intermediate radical in situ observation in enzymatic hydrolysis/mild acidolysis lignin pyrolysis[J]. J Anal Appl Pyrolysis, 2019, 138:249-260. doi: 10.1016/j.jaap.2019.01.004 [3] ZOU R, WANG Y, JIANG L, YU Z, ZHAO Y, WU Q, DAI L, KE L, LIU Y, RUAN R. Microwave-assisted co-pyrolysis of lignin and waste oil catalyzed by hierarchical ZSM-5/MCM-41 catalyst to produce aromatic hydrocarbons[J]. Bioresour Technol, 2019, 289:121609. doi: 10.1016/j.biortech.2019.121609 [4] SHUAI L, AMIRI M T, QUESTELL-SANTIAGO Y M, HEROGUEL F, LI Y, KIM H, MEILAN R, CHAPPLE C, RALPH J, LUTERBACHER J S. Formaldehyde stabilization facilitates lignin monomer production during biomass depolymerization[J]. Science, 2016, 354(6310):329-333. doi: 10.1126/science.aaf7810 [5] RAGAUSKAS A J, BECKHAM G T, BIDDY M J, CHANDRA R, CHEN F, DAVIS M F, DAVISON B H, DIXON R A, GILNA P, KELLER M, LANGAN P, NASKAR A K, SADDLER J N, TSCHAPLINSKI T J, TUSKAN G A, WYMAN C E. Lignin valorization:Improving lignin processing in the biorefinery[J]. Science, 2014, 344(6185):1246843. doi: 10.1126/science.1246843 [6] DAI G, ZHU Y, YANG J, PAN Y, WANG G, WANG S, REUBROYCHAROEN P. Mechanism study on the pyrolysis of the typical ether linkages in biomass[J]. Fuel, 2019, 249:146-153. doi: 10.1016/j.fuel.2019.03.099 [7] HA J, HWANG K, KIM Y, JAE J, KIM K H, LEE H W, KIM J, PARK Y. Recent progress in the thermal and catalytic conversion of lignin[J]. Renewable Sustainable Energy Rev, 2019, 111:422-441. doi: 10.1016/j.rser.2019.05.034 [8] WANG S, DAI G, YANG H, LUO Z. Lignocellulosic biomass pyrolysis mechanism:A state-of-the-art review[J]. Prog Energy Combust, 2017, 62:33-86. doi: 10.1016/j.pecs.2017.05.004 [9] ARO T, FATEHI P. Production and application of lignosulfonates and sulfonated lignin[J]. ChemSusChem, 2017, 10(9):1861-1877. doi: 10.1002/cssc.201700082 [10] LORA J H, GLASSER W G. Recent industrial applications of lignin:A sustainable alternative to nonrenewable materials[J]. J Polym Environ, 2002, 10(1):39-48. http://cn.bing.com/academic/profile?id=cea4d37e14c1dce746b84bbd574ad545&encoded=0&v=paper_preview&mkt=zh-cn [11] KAWAMOTO H, RYORITANI M, SAKA S. Different pyrolytic cleavage mechanisms of β-ether bond depending on the side-chain structure of lignin dimers[J]. J Anal Appl Pyrolysis, 2008, 81(1):88-94. doi: 10.1016/j.jaap.2007.09.006 [12] CHEN Y, FANG Y, YANG H, XIN S, ZHANG X, WANG X, CHEN H. Effect of volatiles interaction during pyrolysis of cellulose, hemicellulose, and lignin at different temperatures[J]. Fuel, 2019, 248:1-7. doi: 10.1016/j.fuel.2019.03.070 [13] KAWAMOTO H, NAKAMURA T, SAKA S. Pyrolytic cleavage mechanisms of lignin-ether linkages:A study on p-substituted dimers and trimers[J]. Holzforschung, 2008, 62(1):50-56. doi: 10.1515/HF.2008.007 [14] YERRAYYA A, SURIAPPARAO D V, NATARAJAN U, VINU R. Selective production of phenols from lignin via microwave pyrolysis using different carbonaceous susceptors[J]. Bioresour Technol, 2018, 270:519-528. doi: 10.1016/j.biortech.2018.09.051 [15] JIANG W, WU S, LUCIA L A, CHU J. A comparison of the pyrolysis behavior of selected β-O-4 type lignin model compounds[J]. J Anal Appl Pyrolysis, 2017, 125:185-192. doi: 10.1016/j.jaap.2017.04.003 [16] JIANG W, CHU J, WU S, LUCIA L A. Modeling pyrolytic behavior of pre-oxidized lignin using four representative β-ether-type lignin-like model polymers[J]. Fuel Process Technol, 2018, 176:221-229. doi: 10.1016/j.fuproc.2018.03.041 [17] KIM J, HAFEZI-SEFAT P, CADY S, SMITH R G, BROWN R C. Premethylation of lignin hydroxyl functionality for improving storage stability of oil from solvent liquefaction[J]. Energy Fuels, 2019, 33(2):1248-1255. doi: 10.1021/acs.energyfuels.8b03894 [18] ZHU G, QIU X, ZHAO Y, QIAN Y, PANG Y, OUYANG X. Depolymerization of lignin by microwave-assisted methylation of benzylic alcohols[J]. Bioresour Technol, 2016, 218:718-722. doi: 10.1016/j.biortech.2016.07.021 [19] LOHR T L, LI Z, MARKS T J. Selective ether/ester C-O cleavage of an acetylated lignin model via tandem catalysis[J]. ACS Catal, 2015, 5(11):7004-7007. doi: 10.1021/acscatal.5b01972 [20] HUANG J, LIU C, WU D, TONG H, REN L. Density functional theory studies on pyrolysis mechanism of β-O-4 type lignin dimer model compound[J]. J Anal Appl Pyrolysis, 2014, 109:98-108. doi: 10.1016/j.jaap.2014.07.007 [21] BRITT P F, BUCHANAN A C, COONEY M J, MARTINEAU D R. Flash vacuum pyrolysis of methoxy-substituted lignin model compounds[J]. J Org Chem, 2000, 65(5):1376-1389. doi: 10.1021/jo991479k [22] BESTE A, BUCHANAN A C, BRITT P F, HATHORN B C, HARRISON R J. kinetic analysis of the pyrolysis of phenethyl phenyl ether:Computational prediction of α/β-selectivities[J]. J Phys Chem A, 2007, 111:12118-12126 doi: 10.1021/jp075861+ [23] BRITT P F, KIDDER M K, BUCHANAN A C. Oxygen substituent effects in the pyrolysis of phenethyl phenyl ethers[J]. Energy Fuels, 2007, 21(6):3102-3108. doi: 10.1021/ef700354y [24] HUANG X, LIU C, HUANG J, LI H. Theory studies on pyrolysis mechanism of phenethyl phenyl ether[J]. Comput Theor Chem, 2011, 976(1/3):51-59. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=2dbfa3d97330434573be123ae336e556 [25] CHEN L, YE X, LUO F, SHAO J, LU Q, FANG Y, WANG X, CHEN H. Pyrolysis mechanism of β-O-4 type lignin model dimer[J]. J Anal Appl Pyrolysis, 2015, 115:103-111. doi: 10.1016/j.jaap.2015.07.009 [26] ASARE S O, HUANG F, LYNN B C. Characterization and sequencing of lithium cationized β-O-4 lignin oligomers using higher-energy collisional dissociation mass spectrometry[J]. Anal Chim Acta, 2019, 1047:104-114. doi: 10.1016/j.aca.2018.09.068 [27] JARVIS M W, DAILY J W, CARSTENSEN H, DEAN A M, SHARMA S, DAYTON D C, ROBICHAUD D J, NIMLOS M R. Direct detection of products from the pyrolysis of 2-phenethyl phenyl ether[J]. J Phys Chem A, 2011, 115(4):428-438. doi: 10.1021/jp1076356 [28] FRISCH M J, TRUCKS G W, SCHLEGEL H B, et al. Gaussian09[CP]. Gaussian, Inc.Pittsburgh PA, 2009. [29] ELDER T. A computational study of pyrolysis reactions of lignin model compounds[J]. Holzforschung, 2010, 64(4). http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=7cce4c80ce24895d747a7bc532b60c7c [30] JIANG X, LU Q, HU B, LIU J, DONG C, YANG Y. Intermolecular interaction mechanism of lignin pyrolysis:A joint theoretical and experimental study[J]. Fuel, 2018, 215:386-394. doi: 10.1016/j.fuel.2017.11.084 [31] BESTE A, BUCHANAN A C. Substituent effects on the reaction rates of hydrogen abstraction in the pyrolysis of phenethyl phenyl ethers[J]. Energy Fuels, 2010, 24(5):2857-2867. doi: 10.1021/ef1001953 [32] PARTHASARATHI R, ROMERO R A, REDONDO A, GNANAKARAN S. Theoretical study of the remarkably diverse linkages in lignin[J]. J Phys Chem Lett, 2011, 2(20):2660-2666. doi: 10.1021/jz201201q [33] BESTE A, BUCHANAN A C. Kinetic simulation of the thermal degradation of phenethyl phenyl ether, a model compound for the β-O-4 linkage in lignin[J]. Chem Phys Lett, 2012, 550:19-24. doi: 10.1016/j.cplett.2012.08.040 -





 下载:
下载: