Influence of acid treatment on the structure and extraction performance of Xinjiang Hefeng low-rank coal
-
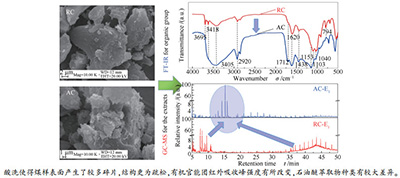
摘要: 对新疆和丰低阶煤样进行酸洗脱灰处理,通过相关表征,分析了脱灰处理对煤样主体结构、石油醚和CS2萃取性能的影响。FT-IR表征表明,煤样经酸洗脱灰处理,结构仅发生了微弱改变,酸洗煤样(AC)仅在1712 cm-1处出现了较弱的原煤样(RC)所没有的羧酸类C=O吸收峰。由TG-DTG表征可知,酸洗使得煤中小分子键断裂,但并未破坏煤样的大分子网络主体结构。以石油醚(PE)和CS2为溶剂对RC和AC两煤样进行常温两级超声萃取的研究表明,AC煤样的PE和CS2萃取率均高于RC煤样,分别从0.16%和0.53%(RC煤样)增加到0.17%和0.64%,且萃取速率也更大,显著降低了煤样的溶剂萃取次数。萃取物的FT-IR和GC-MS分析表明,酸洗处理不仅能有效脱除煤样中的杂原子,且使得煤样CS2萃取物的种类增加。另外,由萃余物TG-DTG结果可知,超声萃取主要是一个物理溶胀过程,并没有破坏煤样的大分子主体结构。Abstract: The acid treatment experiment to remove the inorganic minerals in Xinjiang Hefeng low-rank coal was conducted, and the effects of deashing treatment on the main structure of coal sample and its extraction performance by petroleum ether and CS2 were analyzed by instrumental characterizations. The FT-IR results show that the main structure of the treated coal changes slightly compared with the untreated one. And the acid-washed coal sample (AC) presents a very weak absorption peak at 1712 cm-1 attributed to carboxylic acid (C=O), which could not be observed in the raw coal sample (RC). It can also be seen from TG-DTG characterization that the process of acid treatment results in a cleavage of small molecular bonds in the coal without destroying the macromolecular network structure. The performance of two-stage ultrasonic extraction of RC and AC samples with petroleum ether (PE) and CS2 as solvents proposes that the extraction proportions of PE and CS2 of AC sample are higher than that of RC sample, from 0.16% and 0.53% (RC) to 0.17% and 0.64%, respectively, and the extraction rate of AC sample is larger than that of RC sample, reducing the number of solvent extraction operation significantly.FT-IR and GC-MS analysis of the extracts shows that the acid treatment not only effectively removes the heteroatoms in the coal sample, but also increases the type of CS2 extracts. In addition, from the results of TG-DTG for the residues, it can be noted that the ultrasonic extraction is a physical swelling process, and does not destroy the macromolecular structure of the coal sample.
-
Key words:
- Hefeng coal /
- acid treatment /
- coal structure /
- extraction performance
-
表 1 煤样的工业和元素分析
Table 1 Proximate and ultimate analyses of the samples
Sample Proximate analysis w/% Ultimate analysis wdaf/% H/C
(atomic ratio)Mad Ad Vdaf FCdaf C H N S O* RC 5.88 21.18 42.81 57.19 74.91 5.65 1.50 0.37 17.57 0.91 AC 1.52 1.84 44.37 55.63 73.05 5.33 1.50 0.38 19.74 0.88 *:by difference 表 2 两种煤样各官能团的相对含量及其变化
Table 2 Contents and changes of groups in two coal samples
Band position σ/cm-1 Functional group Area percentage/% △=(AC-RC)/
RC*100%RC AC 3600-3500 OH-π 17.02 14.70 -13.63 3500-3350 self-associated OH 23.76 22.09 -7.03 3350-3260 OH-ether O 35.61 35.31 -0.84 3260-3170 cyclic OH 23.60 27.91 18.26 2950 aliphatic -CH3 26.04 19.06 -26.80 2920 asymmetric aliphatic -CH2 20.56 35.83 74.27 2890 aliphatic -CH 38.92 24.95 -35.89 2850 symmetric aliphatic -CH2 14.47 20.16 39.32 1710 carboxylic acids C=O 0 12.83 - 1600 conjugated C=O 12.51 14.70 17.51 1500 aromatic C=C 7.46 8.42 12.87 1436 asymmetric CH3-, CH2- 9.13 10.46 14.57 1371 CH3-Ar, R 8.27 9.22 11.49 1269 symmetric deformation -CH3 9.48 10.16 7.17 1165 carboxyl C-O 15.85 10.64 -32.87 1110 grease C-O 19.57 14.05 -28.21 1035 alkyl ethers 16.72 9.53 -43.00 900-860 five adjacent H deformation 22.35 4.71 -78.93 860-810 four adjacent H deformations 26.13 12.85 -50.82 810-750 three adjacent H deformations 29.41 16.09 -45.29 750-720 two adjacent H deformations 22.11 66.35 200.1 表 3 两种煤样石油醚、CS2两级萃取率
Table 3 Extraction rate of two coals with petroleum ether and CS2 as agents
Extraction times Extraction rate/% △E1/% Extraction rate/% △E2/% RC-E1 AC-E1 RC-E2 AC-E2 10 0.09 0.12 33.3 0.36 0.59 63.89 15 0.12 0.15 25 0.46 0.62 34.78 20 0.15 0.16 6.67 0.53 0.64 20.75 25 0.16 0.17 6.25 0.53 0.64 20.75 表 4 两煤样萃取物中各官能团的相对含量及其变化
Table 4 Contents and change of groups in the two coals' extracts
Band position σ/cm-1 Functional groups Area percentage/% RC-E2 AC-E2 3600-3500 OH- π 19.28 27.98 3500-3350 self-associated OH 31.72 30.36 3350-3260 OH-ether O 29.73 27.40 3260-3170 cyclic OH 19.26 14.25 2950 aliphatic -CH3 16.72 23.34 2920 asymmetric aliphatic -CH2 32.02 25.87 2890 aliphatic -CH 29.39 25.87 2850 symmetric aliphatic -CH2 21.88 24.91 1742 aryl esters 6.07 5.09 1711 carboxyl acids 14.38 15.82 1610 conjugated 9.73 12.57 1543 aromatic C=C 3.42 0.94 1460 CH3-, CH2- 18.94 19.30 1372 CH3-Ar, R 13.20 13.37 1263 carboxyl C-O 13.72 13.66 1156 grease C-O 9.16 10.61 1051 alkyl eters 11.38 8.64 900-860 five adjacent H deformation 16.68 20.74 860-810 four adjacent H deformations 21.96 21.55 810-750 three adjacent H deformations 42.66 23.13 750-720 two adjacent H deformations 18.70 34.58 表 5 两种煤样石油醚萃取物的GC-MS分析
Table 5 GC-MS analysis of two coals' extracts
RC-E1 AC-E1 No. RT/min name content/% No. RT/min name content/% 1 4.03 2, 4-dimethylhexane 21.20 1 4.03 2, 4-dimethylhexane 13.50 2 4.39 hexyl acetate 1.22 2 4.24 1, 3-dimethyl-cyclohexane 1.78 3 4.99 pentanol 1.42 3 5.05 ethylcyclohexane 2.41 4 5.35 octanol 3.48 4 12.30 isobutylbenzene 1.32 5 7.62 β-hydroxy-isovaleric acid 34.74 5 13.14 1-methylindane 2.10 6 8.20 3-methyl-3-pentanethiol 19.60 6 14.29 N-formylmorpholine 7.49 7 8.86 3-hexyl hydroperoxide 1.43 7 15.29 tetrahydronaphthalene 50.65 8 9.19 2-[2-(hexyloxy)ethoxy]-ethanol 1.49 8 15.94 naphthalene 16.84 9 9.59 4-methyl-5-decanol 5.81 9 19.12 2-methylnaphthalene 1.56 10 10.77 3, 3-dimethylbutyric acid 1.91 10 23.24 tetradecane 1.27 11 42.25 hexacosane 1.22 11 25.27 pentadecane 1.07 12 43.46 heptacosane 1.28 13 44.76 octacosane 1.46 14 46.30 nonacosane 1.41 15 48.16 triacontane 1.22 16 50.43 hentriacontane 1.12 表 6 两种煤样CS2萃取物GC-MS分析
Table 6 GC-MS analysis of two coals' extracts
RC-E2 AC-E2 No. RT/min name content/% No. RT/min name content/% 1 26.76 1, 4-dimethyl-7-isopropylazulene 2.42 1 15.28 tetrahydronaphthalene 2.37 2 27.19 tetradecane 1.70 2 15.98 naphthalene 3.15 3 27.24 bute hydrocarbon 1.88 3 26.76 1, 4-dimethyl-7-isopropylazulene 3.18 4 29.01 heptadecane 1.76 4 27.25 bute hydrocarbon 1.52 5 30.75 hexadecane 1.57 5 29.01 heptadecane 2.09 6 33.34 estrone 2.84 6 30.75 hexadecane 1.47 7 33.99 nonadecane 4.12 7 33.34 estrone 1.82 8 34.50 diacetylbenzene 4.74 8 33.99 nonadecane 3.33 9 35.51 heneicosane 3.78 9 34.35 tetradecanal 4.02 10 35.78 phenanthrene 12.87 10 34.50 diacetylbenzene 1.39 11 36.26 N, N-dimethyldecanamide 1.76 11 35.51 heneicosane 5.66 12 36.96 docosane 6.42 12 35.78 phenanthrene 2.84 13 38.06 ethanone 1.56 13 36.96 docosane 13.54 14 38.36 tetracosane 5.59 14 37.36 13-docosen 6.18 15 39.71 pentacosane 6.98 15 38.07 ethanone 2.07 16 40.13 cyclohexadecane 1.76 16 38.36 tetracosane 1.88 17 40.45 hexacosane 1.66 17 38.77 2-hexadecyloxirane 4.33 18 41.00 octacosane 3.79 18 39.71 pentacosane 1.56 19 42.25 nonacosane 5.99 19 40.13 cyclohexadecane 5.65 20 42.70 elaidyl alcohol 2.01 20 41.00 octacosane 2.93 21 43.01 triacontane 2.08 21 41.43 tetracosanal 2.49 22 43.19 erucylamide 8.83 22 42.25 nonacosane 1.64 23 43.25 hentriacontane 1.57 23 42.69 elaidyl alcohol 4.57 24 43.46 octadecenoic 4.06 24 43.01 triacontane 2.30 25 44.77 dotriacontane 6.16 25 43.19 erucylamide 1.63 26 45.72 tritriacontane 2.10 26 43.46 octadecenoic 8.07 27 44.77 dotriacontane 1.92 28 45.73 tritriacontane 4.74 29 48.17 tetratriacontane 1.67 -
[1] MARZEC A. Towards an understanding of the coal structure:A review[J]. Fuel Process Technol, 2002, 77:25-32. doi: 10.1016-S0378-3820(02)00045-0/ [2] ASHIDA R, MORIMOTO M, MAKINO Y, UMEMOTO S, NAKAGAWA H, MIURA K. Fractionation of brown coal by sequential high temperature solvent extraction[J]. Fuel, 2009, 88(8):1485-1490. doi: 10.1016/j.fuel.2008.12.003 [3] TAHMASEBI A, YU J, HAN Y, YIN F, BHATTACHARYA S, STOKIE D. Study of chemical structure changes of Chinese lignite upon drying in superheated steam, microwave, and hot air[J]. Energy Fuels, 2012, 26(6):3651-3660. doi: 10.1021/ef300559b [4] ZHENG A L, FAN X, WANG S Z, LIU F J, WEI X Y, ZHAO Y P. Analysis of the products from the oxidation of Geting bituminous coal by atmospheric pressure photoionization-mass spectrometry[J]. Anal Lett, 2014, 47(6):958-969. doi: 10.1080/00032719.2013.860541 [5] IINO M. Network structure of coals and association behavior of coal-derived materials[J]. Fuel Process Technol, 2000, 62(2):89-101. http://www.sciencedirect.com/science/article/pii/S0378382099001204 [6] WANG J, TAKARADA T. Characterization of high-temperature coal tar and supercritical-water extracts of coal by laser desorption ionization-mass spectrometry[J]. Fuel Process Technol, 2003, 81(3):247-258. doi: 10.1016/S0378-3820(03)00025-0 [7] WANG S Q, TANG Y G, SCHOBERT H H, GUO Y N, GAO W C, LU X K. FTIR and simultaneous TG/MS/FTIR study of Late Permian coals from Southern China[J]. J Anal Appl Pyrolysis, 2013, 100:75-80. doi: 10.1016/j.jaap.2012.11.021 [8] MAO J D, SCHIMMELMANN A, MASTALERZ M, HATCHER P, LI Y. Structural features of a bituminous coal and their changes during low-temperature oxidation and loss of volatiles investigated by advanced solid-state NMR spectroscopy[J]. Energy Fuels, 2010, 24(4):2536-2544. doi: 10.1021/ef9015069 [9] FAN X, JIANG J, CHEN L, ZHOU C C, ZHU J L, ZHU T G, WEI X Y. Identification of organic fluorides and distribution of organic species in an anthracite with high content of fluorine[J]. Fuel Process Technol, 2016, 142:54-58. doi: 10.1016/j.fuproc.2015.09.024 [10] GRYGLEWICZ G, RUTKOWSKI P, YPERMAN J. Characterization of sulfur compounds in supercritical coal extracts by gas chromatography-mass spectrometry[J]. Fuel Process Technol, 2002, 77:167-172. https://www.sciencedirect.com/science/article/pii/S0378382002000462 [11] YU X Y, WEI X Y, LI Z K, CHEN Y, ZONG Z M, MA F Y. Comparison of three methods for extracting Liuhuanggou bituminous coal[J]. Fuel, 2017, 210:290-297. doi: 10.1016/j.fuel.2017.08.071 [12] IINO M, TAKANOHASHI T, OHSUGA H, TODA K. Extraction of coals with CS2-N-methyl-2-pyrrolidinone mixed solvent at room temperature[J]. Fuel, 1988, 67(12):1639-1647. doi: 10.1016/0016-2361(88)90208-6 [13] WEI X Y, SHEN J L, TAKANOHASHI T, IINO M. Effect of extractable substances on coal dissolution. Use of CS2-N-methyl-2-pyrrolidinone mixed solvent for dissolution re-action products[J]. Energy Fuels, 1989, 3(5):575-579. doi: 10.1021/ef00017a008 [14] TAKANOHASHI T, XIAO F, TAKAHIRO YOSHIDA A, SAITO I. Difference in extraction yields between CS2/NMP and NMP for upper freeport coal[J]. Energy Fuels, 2003, 17(1):255-256. doi: 10.1021/ef020141h [15] TAKANOHASHI T, TAKAYUKI YANAGIDA A, IINO M, MAINWARING D E. Extraction and swelling of low-rank coals with various solvents at room temperature[J]. Energy Fuels, 1996, 10(5):1128-1132. doi: 10.1021/ef960033t [16] LU H Y, WEI X Y, YU R, PENG Y L, QI X Z, QIE L M. Sequential thermal dissolution of Huolinguole lignite in methanol and in ethanol[J]. Energy Fuels, 2011, 25(6):2741-2745. doi: 10.1021/ef101734f [17] ISHIZUKA T, TAKANOHASHI T, ITO O, LINO M. Effects of additives and oxygen on ex-traction yield with CS2-NMP mixed solvent for argonne premium coal samples[J]. Fuel, 1993, 72(4):579-580. doi: 10.1016/0016-2361(93)90120-Q [18] NISHIOKA M. Multistep extraction of coal[J]. Fuel, 1991, 70(12):1413-1419. doi: 10.1016/0016-2361(91)90007-W [19] LI Q C, TAKANOHASHI T, YOSHIDA T, SAITO I, AOKI H, MASHIMO K. Effect of acid treatment on thermal extraction yield in ashless coal production[J]. Fuel, 2004, 83(6):727-732. doi: 10.1016/j.fuel.2003.06.002 [20] LIU L L, YUAN Y, KUMAR S, WANG Z H, HE Y, LV Y, LIU J Z, GUL-E-RANA J, CEN K F. Catalytic effect of metal chlorides on coal pyrolysis and gasification part Ⅱ. Effects of acid washing on coal characteristics[J]. Thermochim Acta, 2018, 666:41-50. doi: 10.1016/j.tca.2018.06.001 [21] GENG W, NAKAJIMA T, TAKANASHI H, OHKI A. Analysis of carboxyl group in coal and coal aromaticity by Fourier transform infrared (FT-IR) spectrometry[J]. Fuel, 2009, 88(1):139-144. doi: 10.1016/j.fuel.2008.07.027 [22] WIJAYA N, ZHANG L. A critical review of coal demineralization and its implication on understanding the speciation of organically bound metals and submicrometer mineral grains in coal[J]. Energy Fuels, 2011, 25(1):1-16. https://www.researchgate.net/publication/231272542_A_Critical_Review_of_Coal_Demineralization_and_Its_Implication_on_Understanding_the_Speciation_of_Organically_Bound_Metals_and_Submicrometer_Mineral_Grains_in_Coal [23] LIU X P, WU X T, WANG J. Substantial upgrading of a high-ash lignite by hydrothermal treatment followed by Ca(OH)2 digestion/acid leaching[J]. Fuel, 2018, 222:269-277. doi: 10.1016/j.fuel.2018.02.034 [24] TANG L F, CHEN S J, WANG S W, TAO X X, HE H, FENG L, ZHENG L, MA C Y, ZHAO Y D. Exploration on the action mechanism of microwave with peroxyacetic acid in the process of coal desulfurization[J]. Fuel, 2018, 214:554-560. doi: 10.1016/j.fuel.2017.10.087 -





 下载:
下载: