Morphologic effect of CeO2 on the catalytic performance of Ni/CeO2 in CO methanation
-
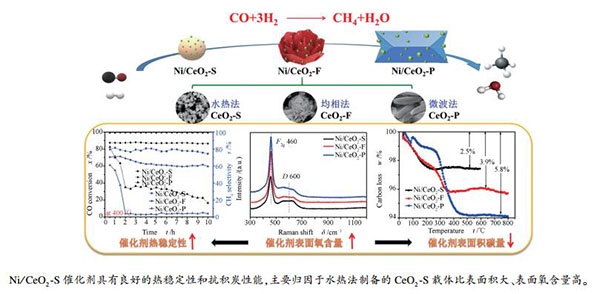
摘要: 通过改变制备方法合成了不同形貌的CeO2载体(包括球状CeO2-S、花苞状CeO2-F和多面体状CeO2-P),并用氨水配位浸渍法制备了Ni/CeO2催化剂。研究了CeO2载体结构与Ni/CeO2催化剂上CO甲烷化反应性能的关系。结果表明,CeO2-S、CeO2-F和CeO2-P载体暴露的晶面和氧空位不同,对Ni/CeO2催化剂催化活性影响也不相同。CeO2-S氧空位最多,Ni/CeO2-S在350 ℃下CO转化率和CH4选择性分别达到99.19%和88.88%。10 h热稳定性测试结果表明,Ni/CeO2-S催化剂上的积炭量最少(2.5%),CH4选择性一直保持在80%左右,分别是Ni/CeO2-F的1.3倍和Ni/CeO2-P的17.6倍。这主要归因于CeO2-S载体比表面积较大,主要暴露[111]晶面,且表面氧空位含量较多,使Ni/CeO2-S催化剂的载体与活性中心的相互作用增强,从而呈现出优异的抗积炭性能。Abstract: CeO2 supports with different morphologies (including spherical CeO2-S, bud-shaped CeO2-F, and polyhedral CeO2-P) were synthesized and the supported Ni/CeO2 catalysts were prepared by ammonia-water coordination impregnation method; the effect of CeO2 morphology on the catalytic performance of Ni/CeO2 in CO methanation was then investigated. The results indicate that CeO2-S, CeO2-F, and CeO2-P supports are rather different in the exposed crystal planes and oxygen vacancies, which have a significant effect on the catalytic performance of Ni/CeO2 in CO methanation. In particular, CeO2-S has the most oxygen vacancies; for CO methanation over the Ni/CeO2-S catalyst, the conversion of CO and selectivity to CH4 at 350 ℃ reach 99.19% and 88.88%, respectively. After 10 h thermal stability test, the Ni/CeO2-S catalyst displays lowest carbon deposit (2.5%); the selectivity to CH4 over the Ni/CeO2-S catalyst remains above 80%, which is 1.3 times of that over Ni/CeO2-F and 17.6 times of that over Ni/CeO2-P. The excellent catalytic performance of Ni/CeO2-S may be ascribed to that CeO2-S support has large surface area and mainly exposes the [111] crystal plane with a large amount of oxygen vacancies, which can enhance the interaction between the support and the active center and alleviate the carbon deposition.
-
Key words:
- CO methanation /
- Ni/CeO2 /
- support structure /
- morphology /
- preparation method
-
表 1 CeO2载体和Ni/CeO2催化剂的结构参数
Table 1 Structural properties of CeO2 supports and Ni/CeO2catalysts
Sample Ni loadinga w/% ABETb /(m2·g-1) vporeb /(m3·g-1) dporeb /nm d(Ni)c /nm Ni dispersiond/% CeO2-S - 79.89 0.042 44.34 - - CeO2-F - 59.65 0.041 50.03 - - CeO2-P - 39.71 0.017 52.39 - - Ni/CeO2-S 9.50 55.42 0.056 56.90 22.13 17.23 Ni/CeO2-F 9.25 52.21 0.076 57.50 46.24 14.65 Ni/CeO2-P 10.02 40.36 0.030 48.80 52.65 10.98 a: determined by ICP-AES measurement; b: measured using N2 adsorption-desorption, the surface area was calculated by the BET method, and the pore volume and pore size were calculated by the Barrett-Joyner-Halenda method; c: calculated with the Scherrer equation applied to the Ni (111) peak; d: H2 consumption was determined based on the H2-TPD, Ni dispersion by calculation 表 2 催化剂表面物种的定量分析
Table 2 Quantitative XPS analysis results of the Ni/CeO2 catalysts
Sample Ce3+/% Oα/% Ni0:Ni2+:Ni3+ Ni/CeO2-S 16.16 45.17 0.39:0.36:0.25 Ni/CeO2-F 14.59 40.49 0.27:0.31:0.42 Ni/CeO2-P 11.34 28.29 0.32:0.31:0.37 -
[1] YANG X Z, WANG X, GAO G J, WENDURIMA, LIU E M, SHI Q Q, ZHANG J N, HAN C H, WANG J, LU H L, LIU J, TONG M. Nickel on a macro-mesoporous Al2O3@ZrO2 core/shell nanocomposite as a novel catalyst for CO methanation[J]. Int J Hydrogen Energy, 2013, 38(32):13926-13937. doi: 10.1016/j.ijhydene.2013.08.083 [2] BOESCH F T. Synthesis of reliable networks:A survey[J]. IEEE Trans Reliab, 2007, 35(3):240-246. http://d.old.wanfangdata.com.cn/OAPaper/oai_arXiv.org_1002.2013 [3] GALLETTI C, SPECCHIA S, SARACCO G, SPECCHIA V. Co-selective methanation over Ru-γAl2O3 catalysts in H2-rich gas for PEM-FC applications[J]. Chem Eng Sci, 2008, 65(1):590-596. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=2c9442e40e0ee7572019fbff9a33a2c7 [4] TRIMM D, ILSENÖNSAN Z. Onboard fuel conversion for hydrogen-fuel-cell-driven vehicles[J]. Catal Rev, 2000, 43(1/2):31-84. doi: 10.1081/CR-100104386 [5] 李茂华, 杨博, 鹿毅, 刘玉梅.煤制天然气甲烷化催化剂及机理的研究进展[J].工业催化, 2014, 22(1):10-24. doi: 10.3969/j.issn.1008-1143.2014.01.002LI Mao-hua, YANG Bo, LU Yi, LIU Yu-mei. Research advance in methanation catalysts for synthetic natural gas and their catalytic mechanisms[J]. Ind Catal, 2014, 22(1):10-24. doi: 10.3969/j.issn.1008-1143.2014.01.002 [6] AHMAD ZAMANI AB HALIM, RUSMIDAH ALI, WAN AZELEE WAN ABU BAKAR. CO2/H2 methanation over M*/Mn/Fe-Al2O3 (M*:Pd, Rh, and Ru) catalysts in natural gas; optimization by response surface methodology-central composite design[J]. Clean Technol Environ, 2015, 17(3):627-636. doi: 10.1007/s10098-014-0814-8 [7] 李春启.新型合成气甲烷化催化剂La2O3-ZrO2-Ni/Al2O3的制备与性能[J].化工进展, 2019, 38(6):2776-2783. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=hgjz201906026LI Chun-qi. Preparation of a novel catalyst of La2O3-ZrO2-Ni/Al2O3 and its performance in syngas methanation[J]. Chem Ind Eng Prog, 2019, 38(6):2776-2783. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=hgjz201906026 [8] 王辉, 张俊峰, 白云星, 王文峰, 谭猗生, 韩怡卓. NiO@SiO2核壳催化剂在浆态床中低温甲烷化研究[J].燃料化学学报, 2016, 44(5):548-556. doi: 10.3969/j.issn.0253-2409.2016.05.006WANG Hui, ZHANG Jun-feng, BAI Yun-xing, WANG Wen-feng, TAN Yi-sheng, HAN Yi-zhuo. NiO@SiO2 core-shell catalyst for low-temperature methanation of syngas in slurry reactor[J]. J Fuel Chem Technol, 2016, 44(5):548-556. doi: 10.3969/j.issn.0253-2409.2016.05.006 [9] LE T A, KANG J K, PARK E D. CO and CO2 methanation over Ni/SiC and Ni/SiO2 catalysts[J]. Top Catal, 2018, 61(15/17):1537-1544. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=448e36651c547decd3e6763146fe871a [10] HAN Y, WEN B, ZHU M Y, DAI B. Lanthanum incorporated in MCM-41 and its application as a support for a stable Ni-based methanation catalyst[J]. J Rare Earth, 2018, 36(4):367-373. http://d.old.wanfangdata.com.cn/Periodical/zgxtxb-e201804007 [11] 周亭, 郭芳, 许俊强, 陈志, 李军, 刘奇. CO2甲烷化Ni基分子筛催化剂的研究进展[J].功能材料, 2017, 48(6):6029-6033. http://d.old.wanfangdata.com.cn/Periodical/gncl201706006ZHOU Ting, GUO Fang, XU Jun-qiang, CHEN Zhi, LI Jun, LIU Qi. Research progress in the Ni-based molecular sieve catalysts for the methanation of carbon dioxide[J]. J Funct Mater, 2017, 48(6):6029-6033. http://d.old.wanfangdata.com.cn/Periodical/gncl201706006 [12] LIN Y, ZHU Y, PAN X, BAO X. Modulating the methanation activity of Ni by the crystal phase of TiO2[J]. Catal Sci Technol, 2017, 7. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=01f9c567324f062670b403b06a3d1043 [13] LIU Q, LIAO L, LIU Z, DONG X. Effect of ZrO2 crystalline phase on the performance of Ni-B/ZrO2 catalyst for the CO selective methanation[J]. Chin J Chem Eng, 2011, 19(3):434-438. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=cjce201103011 [14] ZYRYANOVA M M, SNYTNIKOV P V, GULYAEV R V, AMOSOV Y I, BORONIN A I, SOBYANIN V A. Performance of Ni/CeO2 catalysts for selective CO methanation in hydrogen-rich gas[J]. Chem Eng J, 2014, 238:189-197. doi: 10.1016/j.cej.2013.07.034 [15] ZHANG X, RUI N, JIA X, HU X, LIU C. Effect of decomposition of catalyst precursor on Ni/CeO2 activity for CO methanation[J]. Chin J Catal, 2019, 40(4):495-503. doi: 10.1016/S1872-2067(19)63289-4 [16] WANG J B, TAI Y L, DOW W P, HUANG T J. Study of ceria-supported nickel catalyst and effect of yttria doping on carbon dioxide reforming of methane[J]. Appl Catal A:Gen, 2001, 218(1/2):69-79. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=9cbe5f19c4dc3746099ce0e47711aadb [17] ODEDAIRO T, CHEN J, ZHU Z. Metal-support interface of a novel Ni-CeO2 catalyst for dry reforming of methane[J]. Catal Commun, 2013, 31(Complete):25-31. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=c25a75f8185cb44c951655318e412db9 [18] 张涛, 张亚文.金属-氧化物界面中的金属-载体强相互作用及其对贵金属-氧化铈负载型催化材料性质调控的研究进展[J].中国稀土学报, 2014, 32(2):129-142. http://d.old.wanfangdata.com.cn/Periodical/zgxtxb201402001ZHANG Tao, ZHANG Ya-wen. Research advances on strong metal-support interactions at metal-oxide interfaces and their roles in regulating catalytic properties of noble metal-ceria supported catalysts[J]. J Rare Earth, 2014, 32(2):129-142. http://d.old.wanfangdata.com.cn/Periodical/zgxtxb201402001 [19] MAI H X, SUN L D, ZHANG Y W, SI R, FENG W, ZHANG H P, LIU H C, YAN C H. Shape-selective synthesis and oxygen storage behavior of ceria nanopolyhedra, nanorods, and nanocubes[J]. J Phys Chem B, 2005, 109(51):24380-24385. doi: 10.1021/jp055584b [20] 刘玉娟, 王东哲, 张磊, 白金, 陈琳, 刘道胜. CeO2形貌对甲醇水蒸汽重整CuO/CeO2催化剂的影响[J].精细化工, 2018, 35(12):71-77+112. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=jxhg201812011LIU Yu-juan, WANG Dong-zhe, ZHANG Lei, BAI Jin, CHEN Lin, LIU Dao sheng. Effect of CeO2 morphology on the performance of CuO/CeO2 catalysts for methanol steam reforming[J]. Fine Chem, 2018, 35(12):71-77+112. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=jxhg201812011 [21] 刘浩文, 刘荷芬, 乐琦, 韩晓彦.微波法合成蝴蝶型超细CeO2及其性能研究[J].中南民族大学学报(自然科学版), 2016, 35(3):17-20. http://d.old.wanfangdata.com.cn/Periodical/znmzxyxb-zrkx201603004LIU Hao-wen, LIU He-fen, YUE Qi, HAN Xiao-yan. Microwave synthesis of butterfly-type superfine CeO2 and its properties[J]. J South-Cent Univ Natl (Nat Sci Ed), 2016, 35(3):17-20. http://d.old.wanfangdata.com.cn/Periodical/znmzxyxb-zrkx201603004 [22] LE T A, KIM M S, LEE S H, KIM T W, PARK E D. CO and CO2 methanation over supported Ni catalysts[J]. Catal Today, 2016, 293(4):1-7. http://d.old.wanfangdata.com.cn/NSTLQK/NSTL_QKJJ0228845933/ [23] SHAN W, LUO M, YING P, SHEN W, LI C. Reduction property and catalytic activity of Ce1-xNixO2 mixed oxide catalysts for CH4 oxidation[J]. Appl Catal A:Gen, 2003, 246(1):1-9. doi: 10.1016/S0926-860X(02)00659-2 [24] WANG L H, LIU H, LIU Y, CHEN Y, YANG S Q. Effect of precipitants on Ni-CeO2 catalysts prepared by a co-precipitation method for the reverse water-gas shift reaction[J]. J Rare Earth, 2013, 31:969. doi: 10.1016/S1002-0721(13)60014-9 [25] TAN H Y, WANG J, YU S Z, ZHOU K B. Support morphology-dependent catalytic activity of Pd/CeO2 for formaldehyde oxidation[J]. Environ Sci Technol, 2015, 49(14):8675-82. doi: 10.1021/acs.est.5b01264 [26] GROSVENOR A P, BIESINGER M C, SMART R S C, MCINTYRE N S. New interpretations of XPS spectra of nickel metal and oxides[J]. Surf Sci, 2006, 600(9):1771-1779. doi: 10.1016/j.susc.2006.01.041 [27] ZHANG S, HUANG Z Q, MA Y, GAO W, LI J, CAO F. Solid frustrated-Lewis-pair catalysts constructed by regulations on surface defects of porous nanorods of CeO2[J]. Nat Commun, 2017, 8:15266. doi: 10.1038/ncomms15266 [28] HENDERSON M A, PERKINS C L, ENGELHARD M H, THEVUTHASN S, PEDEN C H F. Redox properties of water on the oxidized and reduced surfaces of CeO2(111)[J]. Surf Sci, 2003, 526(1):1-18. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=812de0e890f65cf3272f63360e545f15 [29] MCCARTY J G, WISE H. Hydrogenation of surface carbon on alumina-supported nickel[J]. J Catal, 1979, 57(3):406-416. doi: 10.1016/0021-9517(79)90007-1 [30] 杨霞, 田大勇, 孙守理, 孙琦. CeO2助剂对Ni基催化剂甲烷化性能的影响[J].工业催化, 2014, 22(2):137-143. doi: 10.3969/j.issn.1008-1143.2014.02.012YANG Xia, TIAN Da-yong, SUN Shou-li, SUN Qi. Effect of CeO2 on the performance of nickel-based catalysts for methanation[J]. Ind Catal, 2014, 22(2):137-143. doi: 10.3969/j.issn.1008-1143.2014.02.012 -





 下载:
下载: