-
摘要: 用溶胶-凝胶法制备了不同组成的Mg-Co和Mg-Mn-Co复合氧化物,用于催化分解N2O。在较高活性的Mg-Mn-Co表面浸渍K2CO3溶液,制备K改性催化剂。用X射线衍射(XRD)、N2物理吸附(BET)、扫描电镜(SEM)、H2程序升温还原(H2-TPR)、O2程序升温脱附(O2-TPD)等技术表征催化剂结构,考察了复合氧化物的组成、K负载量等制备参数对催化剂活性的影响。结果表明,加入助剂K显著提高了催化剂活性,其中,0.02 K/MgMn0.2Co1.8O4活性较高,有氧无水、有氧有水气氛400℃连续反应50 h,N2O转化率分别保持97%和60%。有水-无水气氛交替实验表明,有水反应后再进行无水实验,K改性催化剂的稳定性较好。
-
关键词:
- N2O催化分解 /
- Mg-Co复合氧化物 /
- Mg-Mn-Co复合氧化物 /
- K改性催化剂 /
- 催化性能
Abstract: Mg-Co and Mg-Mn-Co composite oxides with different compositions were prepared by sol-gel method for N2O catalytic decomposition in the presence of oxygen. Of Mg-Mn-Co catalysts, the one with higher activity was impregnated by K2CO3 solution to make K-modified catalyst. These catalysts were characterized by X-ray diffraction(XRD), nitrogen physisorption (BET), scanning electron microscopy(SEM), temperature-programmed reduction of hydrogen(H2-TPR), and temperature-programmed desorption of oxygen(O2-TPD). The effect of preparation parameters such as compositions and potassium loadings on their catalytic activity has been investigated. The results show that K-modified catalysts exhibit better activity and higher resistance towards water in contrast to un-modified catalyst due to the weakness of surface metal-oxygen bonds. Among these catalysts, 0.02K/MgMn0.2Co1.8O4 is the most active, over which 97% and 60% conversions of N2O can be reached at 400℃ after continuous running for 50 h under the atmosphere of oxygen-alone and oxygen-steam together, respectively. When the steam is switched off, the catalytic activity of 0.02K/MgMn0.2Co1.8O4 can be restored to large extent, indicating the good water-resistance of K-modified catalyst. -
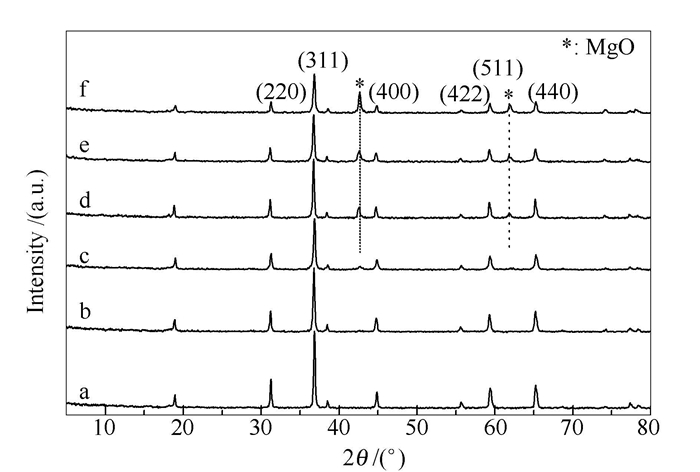
表 1 Mg-Co复合氧化物的晶粒粒径和比表面积
Table 1 Crystallite size and BET surface area of Mg-Co composite oxides
Catalyst Crystallite size
d/nmaBET surface area
A/(m2·g-1)Co3O4 113.4 4.0 Mg0.2Co2.8O4 85.0 8.4 Mg0.4Co2.6O4 66.1 13.0 Mg0.6Co2.4O4 104.4 18.8 Mg0.8Co2.2O4 74.1 16.8 MgCo2O4 58.8 20.1 acalculated by Scherrer equation on the basis of (311) crystallographic plane data in XRD patterns 表 2 Mg-Mn-Co复合氧化物的晶粒粒径和比表面积
Table 2 Crystallite size and BET Surface area of Mg-Mn-Co composite oxides
Catalyst Crystallite size
d/nmaBET surface area
A/(m2·g-1)MgCo2O4 58.8 20.1 MgMn0.2Co1.8O4 17.6 75.0 MgMn0.4Co1.6O4 7.8 77.7 MgMn0.6Co1.4O4 8.6 81.0 MgMn0.8Co1.2O4 16.0 72.4 MgMnCoO4 11.9 73.3 a calculated by Scherrer equation on the basis of (311) crystallographic plane data in XRD patterns 表 3 K/MgMn0.2Co1.8O4催化剂的晶粒粒径和比表面积
Table 3 Crystallite size and BET surface area of K/MgMn0.2Co1.8O4 catalysts
Catalyst Crystallite size
d/nmaBET surface area
A/(m2·g-1)MgMn0.2Co1.8O4 17.6 75.0 0.01K/MgMn0.2Co1.8O4 21.2 60.8 0.02K/MgMn0.2Co1.8O4 33.6 54.6 0.03K/MgMn0.2Co1.8O4 25.4 45.7 0.04K/MgMn0.2Co1.8O4 24.7 56.8 0.05K/MgMn0.2Co1.8O4 27.0 44.9 a calculated by Scherrer equation on the basis of (311) crystallographic plane data in XRD patterns -
[1] PIETROGIACOMI D,CAMPA M C,CARBONE L R,TUTI S,OCCHIUZZI M.N2O decomposition on CoOx,CuOx,FeOx or MnOx supported on ZrO2:The effect of zirconia doping with sulfates or K+ on catalytic activity[J].Appl Catal B:Environ,2016,187:218-227. doi: 10.1016/j.apcatb.2016.01.018 [2] ASANO K,OHNISHI C,IWAMOTO S,SHIOYA Y,INOUE M.Potassium-doped Co3O4 catalyst for direct decomposition of N2O[J].Appl Catal B:Environ,2008,78(3/4):242-249. [3] XUE L,ZHANG C B,HE H,TERAOKA Y.Promotion effect of residual K on the decomposition of N2O over cobalt-cerium mixed oxide catalyst[J].Catal Today,2007,126(3/4):449-455. [4] HUSSAIN M,FINO D,RUSSO N.N2O decomposition by mesoporous silica supported Rh catalysts[J].J Hazard Mater,2012,211-212:255-265. doi: 10.1016/j.jhazmat.2011.08.024 [5] KLYUSHINA A,PACULTOVÁK,KREJČOVÁS,SLOWIK G,JIRÁTOVÁK,KOVANDA F,RYCZKOWSKI J,OBALOVÁL.Advantages of stainless steel sieves as support for catalytic N2O decomposition over K-doped Co3O4[J].Catal Today,2015,257(1):2-10. [6] ZABILSKIY M,DJINOVĆ P,ERJAVEC B,DRAŽĆG,PINTAR A.Small CuO clusters on CeO2 nanospheres as active species for catalytic N2O decomposition[J].Appl Catal B:Environ,2015,163:113-122. doi: 10.1016/j.apcatb.2014.07.057 [7] AMROUSSE R,KATSUMI T.Substituted ferrite MxFe1-xFe2O4(M=Mn,Zn) catalysts for N2O catalytic decomposition processes[J].Catal Commun,2012,26:194-198. doi: 10.1016/j.catcom.2012.05.024 [8] KUMAR S,VINU A,SUBBRT J,BAKARDJIEVA S,RAYALU S,TERAOKA Y,LABHSETWAR N.Catalytic N2O decomposition on Pr0.8Ba0.2MnO3 type perovskite catalyst for industrial emission control[J].Catal Today,2012,198(1):125-132. doi: 10.1016/j.cattod.2012.06.015 [9] XUE Z W,SHEN Y S,SHEN S B,LI C L,ZHU S M.Promotional effects of Ce4+,La3+ and Nd3+ incorporations on catalytic performance of Cu-Fe-Ox for decomposition of N2O[J].J Ind Eng Chem,2015,30:98-105. doi: 10.1016/j.jiec.2015.05.008 [10] LIN Y,MENG T,MA Z.Catalytic decomposition of N2O over RhOx supported on metal phosphates[J].J Ind Eng Chem,2015,28:138-146. doi: 10.1016/j.jiec.2015.02.009 [11] DACQUIN J P,DUJARDIN C,GRANGER P.Surface reconstruction of supported Pd on LaCoO3:Consequences on the catalytic properties in the decomposition of N2O[J].J Catal,2008,253(1):37-49. doi: 10.1016/j.jcat.2007.10.023 [12] BEYER H,EMMERICH J,CHATZIAPOSTOLOU K,KÖHLER K.Decomposition of nitrous oxide by rhodium catalysts:Effect of rhodium particle size and metal oxide support[J].Appl Catal A:Gen,2011,391(1/2):411-416. https://www.researchgate.net/publication/244108871_Decomposition_of_nitrous_oxide_by_rhodium_catalysts_Effect_of_rhodium_particle_size_and_metal_oxide_support?_sg=Rfvd1ymI19OYOXGNk-833LB0G7yeMc9b4Pax8SHTMg6hfSirheDn3UcS9w6w5-GQwyDaXPzjjJ2bRfzdZ3tidA [13] PACHATOURIDOU E,PAPISTA E,DELIMITIS A,VASILIADES M A,EFSTATHIOU A M,AMIRIDIS M D,ALEXEEV O S,BLOOM D,MAMELLOS G E,KONSOLAKIS M,ILIOPOULOU E.N2O decomposition over ceria-promoted Ir/Al2O3 catalysts:The role of ceria[J].Appl Catal B:Environ,2016,187:259-268. doi: 10.1016/j.apcatb.2016.01.049 [14] BERRIER E,OVSITSER O,KONDRATENKO E V,SCHWIDDER M,GRÜNERT W,BRÜCKNER A.Temperature-dependent N2O decomposition over Fe-ZSM-5:Identification of sites with different activity[J].J Catal,2007,249(1):67-78. doi: 10.1016/j.jcat.2007.03.027 [15] CÜRDANELI P E,ÖZKAR S.Ruthenium (Ⅲ) ion-exchanged zeolite Y as highly active and reusable catalyst in decomposition of nitrous oxide to sole nitrogen and oxygen[J].Microporous Mesoporous Mater,2014,196:51-58. doi: 10.1016/j.micromeso.2014.04.052 [16] MENG T,REN N,MA Z.Silicalite-1@Cu-ZSM-5 core-shell catalyst for N2O decomposition[J].J Mol Catal A:Chem,2015,404-405:233-239. doi: 10.1016/j.molcata.2015.05.006 [17] YAN L,REN T,WANG X L,GAO Q,JI D,SUO J S.Excellent catalytic performance of ZnxCo1-xCo2O4 spinel catalysts for the decomposition of nitrous oxide[J].Catal Commun,2003,4(10):505-509. doi: 10.1016/S1566-7367(03)00131-6 [18] YAN L,REN T,WANG X L,JI D,SUO J S.Catalytic decomposition of N2O over MxCo1-xCo2O4(M=Ni,Mg) spinel oxides[J].Appl Catal B:Environ,2003,45(2):85-90. doi: 10.1016/S0926-3373(03)00174-7 [19] ABU-ZIED B M,SOLIMAN S A,ABDELLAH S E.Pure and Ni-substituted Co3O4 spinel catalysts for direct N2O decomposition[J].Chin J Catal,2014,35(7):1105-1112. doi: 10.1016/S1872-2067(14)60058-9 [20] MANIAK G,STELMACHOWSKI P,STANEK J J,KOTARBA A,SOJKA Z.Catalytic properties in N2O decomposition of mixed cobalt-iron spinels[J].Catal Commun,2011,15(1):127-131. doi: 10.1016/j.catcom.2011.08.027 [21] 窦喆,张海杰,潘燕飞,徐秀峰.N2O在钾改性Cu-Co尖晶石型复合氧化物上的催化分解[J].燃料化学学报,2014,42(2):238-245. doi: 10.1016/S1872-5813(14)60016-5DOU Zhe,ZHANG Hai-jie,PAN Yan-fei,XU Xiu-feng.Catalytic decomposition of N2O over potassium-modified Cu-Co spinel oxides[J].J Fuel Chem Technol,2014,42(2):238-245. doi: 10.1016/S1872-5813(14)60016-5 [22] 王建,窦喆,潘燕飞,徐秀峰.MnxCo3-xO4复合氧化物及改性催化剂催化分解N2O[J].分子催化,2015,29(3):246-255.WANG Jian,DOU Zhe,PAN Yan-fei,XU Xiu-feng.MnxCo3-xO4 composite oxide and modified catalyst catalytic decomposition of N2O[J].J Mol Catal (China),2015,29(3):246-255. [23] CHELLAM U,XU Z P,ZENG H C.Low-temperature synthesis of MgxCo1-xCo2O4 spinel catalysts for N2O decomposition[J].Chem Mater,2000,12:650-658. doi: 10.1021/cm990355l [24] KUBOŇOVÁL,FRIDRICHOVÁD,WACH A,KUŚTROWSKR P,OBALOVÁL,COOL P.Catalytic activity of rhodium grafted on ordered mesoporous silicamaterials modified with aluminum in N2O decomposition[J].Catal Today,2015,257:51-58. doi: 10.1016/j.cattod.2015.03.019 [25] PRRUTKO L V,CHERNYAVSKY V S,STAROKON E V,IVANOV A A,KHARITONOV A S,PANOV G.The role of a-sites in N2O decomposition over FeZSM-5.Comparison with the oxidation of benzene to phenol[J].Appl Catal B:Environ,2009,91(1/2):174-179. [26] 吴藏藏,张海杰,王建,郑丽,徐秀峰.N2O分解催化剂Co-Al尖晶石型复合氧化物制备参数的优化[J].分子催化,2016,30(1):62-71.WU Cang-cang,ZHANG Hai-jie,WANG Jian,ZHENG Li,XU Xiu-feng.The preparation parameters screening of Co-Al spinel oxides for N2O catalytic decomposition[J].J Mol Catal (China),2016,30(1):62-71. [27] AMROUSSE R,TSUTSUMI A,BACHAR A,LAHCENE D.N2O catalytic decomposition over nano-sized particles of Co-substituted Fe3O4 substrates[J].Appl Catal A:Gen,2013,450:253-260. doi: 10.1016/j.apcata.2012.10.036 [28] FRANKEN T,PALKOVITS R.Investigation of potassium doped mixed spinels CuxCo3-xO4 as catalysts for an efficient N2O decomposition in real reaction conditions[J].Appl Catal B:Environ,2015,176-177:298-305. doi: 10.1016/j.apcatb.2015.04.002 -





 下载:
下载: