Influence of different supports on the physicochemical properties and denitration performance of the supported MnCe-based catalysts for NH3-SCR
-
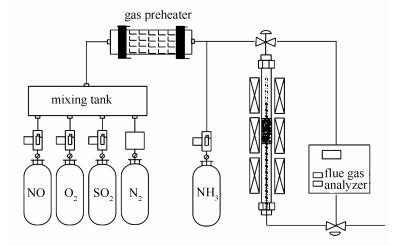
摘要: 选取TiO2、SAPO-34、Al2O3三种常用载体,通过浸渍法以Mn-Ce-O为活性组分制备了负载型MnCeOx脱硝催化剂。采用XRD、BET、H2-TPR、XPS、Py-FTIR等手段对催化剂的固相结构、比表面积、还原性能、表面元素及酸量进行表征分析。结果表明,MnCeOx/SAPO-34催化剂具有最大的比表面积(439.87 m2/g),酸量适中,还原性能最差;MnCeOx/Al2O3催化剂中Mn4+、Ce3+所占比例较高,但酸性最弱;MnCeOx/TiO2催化剂还原性能最优,表面Mn、Ce元素浓度最高,并具有大量Lewis酸性位。通过气固相催化反应装置对催化剂性能进行了NH3-SCR脱硝评价,结果表明,MnCeOx/TiO2催化剂具有较好的脱硝性能,反应温度为280 ℃时,NO转化率达100%(空速为42000 h-1);与催化剂物化性质对比分析,催化剂的氧化还原能力和Lewis酸性位对其脱硝性能至关重要。Abstract: A series of supported Mn-Ce-based catalysts were prepared using TiO2, SAPO-34, and Al2O3 as supports. The physicochemical properties of the obtained catalysts, such as structure, specific surface area, reduction properties, surface elements and acidity were characterized with XRD, BET, H2-TPR, XPS and Py-FTIR. The results showed that MnCeOx/SAPO-34 catalyst exhibited a larger specific surface area (439.87 m2/g), medium amount of Lewis acid sites and the weakest reduction property. In the MnCeOx/Al2O3 catalyst, the concentration of Mn4+ and Ce3+ was relatively high, and the amount of acid sites is the lowest. However, TiO2 as the catalyst support could enhance the reduction property, and increase the amount of Lewis acid sites and the concentration of Mn and Ce. NH3-SCR performances of the catalysts were evaluated using a flow type fixed bed reactor. The results showed that MnCeOx/TiO2 catalyst presented the best catalytic performance, over which near 100% NO conversion was reached at 280 ℃ under a gas hourly space velocity of 42000 h-1. The combination of characterization and reaction results indicated that the good reduction behavior and large amount of Lewis acid sites were beneficial to the enhancement of the catalytic performance for low-temperature NH3-SCR reaction.
-
Key words:
- supports /
- Mn-Ce oxides /
- reducibility /
- surface acidity /
- low-temperature NH3-SCR
-
表 1 催化剂的结构特征
Table 1 Structural parameters of the catalysts
Sample Specific surface area A/ (m2·g-1) Mean pore diameter d/nm Total pore volume v/(cm3·g-1) TiO2 108.72 20.68 0.59 MnCeOx/TiO2 101.21 21.39 0.54 SAPO-34 479.32 1.59 0.49 MnCeOx/SAPO-34 439.87 2.13 0.23 Al2O3 230.15 14.56 0.93 MnCeOx/Al2O3 223.57 15.21 0.85 表 2 催化剂表面原子浓度及比例
Table 2 Atomic concentration and ratio of the catalysts
Catalyst Atomic concentration w/% Atomic ratio /% Mn Ce Mn2+/Mnn+ Mn3+/Mnn+ Mn4+/Mnn+ Ce3+/Cen+ MnCeOx/TiO2 1.96 2.32 10.91 50.97 38.11 18.69 MnCeOx/SAPO-34 1.02 1.11 32.23 36.51 31.25 15.12 MnCeOx/Al2O3 0.53 0.78 16.41 35.07 48.51 20.97 表 3 催化剂表面B酸和L酸分布及浓度
Table 3 Concentration of pyridine on Brønsted (B) and Lewis acid (L) sites
Sample 100 ℃ /(mmol·g-1) 200 ℃ /(mmol·g-1) 300 ℃ /(mmol·g-1) B L B L B L MnCeOx/TiO2 0.009 0.184 0.006 0.134 0.002 0.087 MnCeOx/SAPO-34 0.018 0.069 0.009 0.025 0.003 0.005 MnCeOx/Al2O3 0 0.058 0 0.039 0 0.013 -
[1] BUSCA G, LIETT L, RAMIS G, BERTI F. Chemical and mechanistic aspects of the selective catalytic reduction of NOx by ammonia over oxide catalysts[J]. Appl Catal B:Environ, 1998, 18(1):1-36. https://www.researchgate.net/publication/298897586_Cation_synergies_affect_ammonia_adsorption_over_VOX_and_VWOX_dispersed_on_a-Al2O3_0001_and_a-Fe2O3_0001 [2] QI G S, YANG R T, CHANG R. MnOx-CeO2 mixed oxides prepared by co-precipitation for selective catalytic reduction of NO with NH3 at low temperatures[J]. Appl Catal B:Environ, 2004, 51(2):93-106. doi: 10.1016/j.apcatb.2004.01.023 [3] DJERAD S, TIFOUTI L, CROCOLL M, WEISWEILER W. Effect of vanadia and tungsten loadings on the physical and chemical characteristics of V2O5-WO3/TiO2 catalysts[J]. J Mol Catal A:Chem, 2004, 208(1):257-265. https://www.sciencedirect.com/science/article/pii/S1381116903005545 [4] XUE J J, WANG X Q, QI G S, WANG J, SHENG M Q, LI W. Characterization of copper species over Cu/SAPO-34 in selective catalytic reduction of NOx with ammonia:Relationships between active Cu sites and de-NOx performance at low temperature[J]. J Catal, 2013, 297(1):56-64 https://www.sciencedirect.com/science/article/pii/S0021951712002990 [5] FANG D, XIE J L, MEI D, ZHANG Y M, HE F, LIU X Q, LI Y M. Effect of CuMn2O4 spinel in Cu-Mn oxide catalysts on selective catalytic reduction of NOx with NH3 at low temperature[J]. RSC Adv, 2014, 4(49):25540-25551. doi: 10.1039/c4ra02824d [6] CAO F, SU S, XIANG J, WANG P Y, HU S, SUN L S, ZHANG A C. The activity and mechanism study of Fe-Mn-Ce/gamma-Al2O3 catalyst for low temperature selective catalytic reduction of NO with NH3[J]. Fuel, 2015, 139(1):232-239. [7] TIAN W, YANG H S, FAN X Y, ZHANG X B. Catalytic reduction of NOx with NH3 over different-shaped MnO2 at low temperature[J]. J Hazard Mater, 2011, 188(1/3):105-109 http://downloads.hindawi.com/journals/jnm/2016/6201546.xml [8] SJOERD KIJLSTRA W, BIERVLIET M, POELS E D, BLIEK A. Deactivation by SO2 of MnOx/Al2O3 catalysts used for the selective catalytic reduction of NO with NH3 at low temperatures[J]. Appl Catal B:Environ, 1998, 16(1):327-337. http://downloads.hindawi.com/journals/jnp/2015/601941.xml [9] WANG L S, HUANG B C, SU Y X, ZHOU G Y, WANG K L, LUO H C. Manganese oxides supported on multi-walled carbon nanotubes for selective catalytic reduction of NO with NH3:Catalytic activity and characterization[J]. Chem Eng J, 2012, 192(1):232-241. [10] JIANG B Q, LIU Y, WU Z B. Low-temperature selective catalytic reduction of NO on MnOx/TiO2 prepared by different methods[J]. J Hazard Mater, 2009, 162(2/3):1249-1254. http://downloads.hindawi.com/journals/jchem/2017/2937108.xml [11] FANG D, HE F, MEI D, ZHANG Z, XIE J L, HU H. Thermodynamic calculation for the activity and mechanism of Mn/TiO2 catalyst doped transition metals for SCR at low temperature[J]. Catal Commun, 2014, 52(5):45-48. [12] THIRUPATHI B, SMIRNIOTIS P G. Co-doping a metal (Cr, Fe, Ni, Cu, Zn, Ce and Zr) on Mn/TiO2 catalyst and its effect on the selective reduction of NO with NH3 at low-temperature[J]. Appl Catal B:Environ, 2011, 110(2):195-206. [13] WU Z B. JIN R B, WANG H Q, LIU Y. Effect of ceria doping on SO2 resistance of Mn/TiO2 for selective catalytic reduction of NO with NH3 at low temperature[J]. Catal Commun, 2007, 10(6):935-939. http://www.cjche.com.cn/EN/abstract/abstract14652.shtml [14] LIU C, SHI J W, GAO C, NIU C M. Manganese oxide-based catalysts for low-temperature selective catalytic reduction of NOx with NH3:A review[J]. Appl Catal A:Gen, 2016, 522(25):54-69. https://www.sciencedirect.com/science/article/pii/S0926860X16302071 [15] CAO F, XIANG J, SU S, WANG P Y, HU S, SUN S L. Ag modified Mn-Ce/gamma-Al2O3 catalyst for selective catalytic reduction of NO with NH3 at low-temperature[J]. Fuel Process Technol, 2015, 135(1):66-72. [16] ZHANG L, ZHANG D S, ZHANG J P, CAI S X, FANG C, HUANG L, LI H R, GAO R H, SHI L Y. Design of meso-TiO2@MnOx-CeOx/CNTs with a core-shell structure as DeNOx catalysts:promotion of activity, stability and SO2-tolerance[J]. Nanoscale, 2013, 5(20):9821-9829. doi: 10.1039/c3nr03150k [17] 闫东杰, 玉亚, 徐颖, 黄学敏. Mn、Ce负载顺序对催化剂Mn-Ce/TiO2低温脱硝活性的影响[J].化工进展, 2015, 34(6):1652-1655. http://www.hgjz.com.cn/CN/abstract/abstract17332.shtmlYANG Dong-jie, YU Ya, XU Ying, HUANG Xue-ming. Effect of loading sequence of Mn and Ce on the activity of Mn-Ce/TiO2catalysts at low-temperature[J]. Chem Ind Eng Prog (China), 2015, 34(6):1652-1655. http://www.hgjz.com.cn/CN/abstract/abstract17332.shtml [18] YAO X J, MA K L, ZOU W X, HE S G, AN J B, YANG F M, DONG L. Influence of preparation methods on the physicochemical properties and catalytic performance of MnOx-CeO2 catalysts for NH3-SCR at low temperature[J]. Chin J Catal, 2017, 38(1):146-159. doi: 10.1016/S1872-2067(16)62572-X [19] YOU X C, SHENG Z Y, YU D Q, YANG L, XIAO X, WNAG S. Influence of Mn/Ce ratio on the physicochemical properties and catalytic performance of graphene supported MnOx-CeO2 oxides for NH3-SCR at low temperature[J]. Appl Surf Sci, 2017, 423(30):845-854. [20] LI L L, SUN B W, SUN J F, YU S H, GE C Y, TANG C J, DONG L. Novel MnOx-CeO2 nanosphere catalyst for low-temperature NH3-SCR[J]. Catal Commun, 2017, 100(1):98-102. [21] LI S H, HUANG B C, YU C L. A CeO2-MnOx core-shell catalyst for low-temperature NH3-SCR of NO[J]. Catal Commun, 2017, 98(10):47-51. [22] SHEN Q, ZHANG L Y, SUN N N, WANG H, ZHONG L S, HE C, WEI W, SUN Y H. Hollow MnOx-CeO2 mixed oxides as highly efficient catalysts in NO oxidation[J]. Chem Eng J, 2017, 322(15):46-55. [23] GAO F Y, TANG X L, YI H H, LI J Y, ZHAO S Z, WANG J G, CHU C, LI C L. Promotional mechanisms of activity and SO2 tolerance of Co- or Ni-doped MnOx-CeO2 catalysts for SCR of NOx with NH3 at low temperature[J]. Chem Eng J, 2017, 98(1):47-51. [24] QIU L, PANG D D, ZHANG C L, MENG J J, ZHU R S, OUYANG F. In situ IR studies of Co and Ce doped Mn/TiO2 catalyst for low-temperature selective catalytic reduction of NO with NH3[J]. Appl Surf Sci, 2015, 357(1):189-196. [25] CAO F, XIANG J, SU S, WNAG P Y, SUN L S, HU S, LEI S. The activity and characterization of MnOx-CeO2-ZrO2/γ-Al2O3 catalysts for low temperature selective catalytic reduction of NO with NH3[J]. Chem Eng J, 2014, 243(1):347-354. http://downloads.hindawi.com/journals/jnm/2017/8707289.xml [26] KWON D W, NAM K B, HONG S C. Influence of tungsten on the activity of a Mn/Ce/W/Ti catalyst for the selective catalytic reduction of NO with NH3 at low temperatures[J]. Appl Catal A:Gen, 2015, 497(1):160-166. [27] XIONG Y, TANG C J, YAO X J, ZHANG L, LI L L, WANG X B, DENG Y, GAO F, DONG L. Effect of metal ions doping (M=Ti4+, Sn4+) on the catalytic performance of MnOx/CeO2 catalyst for low temperature selective catalytic reduction of NO with NH3[J]. Appl Catal A:Gen, 2015, 492(1):206-216. http://priede.bf.lu.lv/ftp/pub/RakstuDarbi/OpenOffice/Vardnicas/3/dict-en_20130731.oxt [28] ZHNAG L J, CUI S P, GUO H X, MA X Y, LUO X G. The influence of K+ cation on the MnOx-CeO2/TiO2 catalysts for selective catalytic reduction of NOx with NH3 at low temperature[J]. J Mol Catal A:Chem, 2014, 390(1):14-21. https://www.researchgate.net/publication/298897586_Cation_synergies_affect_ammonia_adsorption_over_VOX_and_VWOX_dispersed_on_a-Al2O3_0001_and_a-Fe2O3_0001 [29] YAO X, ZHAO R D, CHEN L, DU J, TAO C Y, YANG F M, DONG L. Selective catalytic reduction of NOx by NH3 over CeO2 supported on TiO2:Comparison of anatase, brookite, and rutile[J]. Appl Catal B:Environ, 2017, 208(5):82-93. doi: 10.1186/1743-8977-10-15 [30] YU C L, HUANG B C, DONG L F, CHEN F, YANG Y, FAN Y M, YANG Y X, LIU X Q, WANG X N. Effect of Pr/Ce addition on the catalytic performance and SO2 resistance of highly dispersed MnOx/SAPO-34 catalyst for NH3-SCR at low temperature[J]. Chem Eng J, 2017, 98(15):47-51. [31] YAO X J, KONG T T, YU S H, LI L I, YANG F M, DONG. Influence of different supports on the physicochemical properties and denitration performance of the supported Mn-based catalysts for NH3-SCR at low temperature[J]. Appl Surf Sci, 2017, 402(30):208-217. [32] LI J H, CHEN J, KE R, LUO C K, HAO J M. Effects of precursors on the surface Mn species and the activities for NO reduction over MnOx/TiO2 catalysts[J]. Catal Commun, 2007, 8(12):1896-1900. doi: 10.1016/j.catcom.2007.03.007 [33] HE C, LI P, CHENG J, WANG H L, LI J J, LI Q, HAO Z P. Synthesis and characterization of Pd/ZSM-5/MCM-48 biporous catalysts with superior activity for benzene oxidation[J]. Appl Catal A:Gen, 2010, 382(2):167-175. doi: 10.1016/j.apcata.2010.04.033 [34] 甄开吉.催化剂作用基础[M].北京:科学出版社, 2005.ZHEN Kai-ji. Catalyst Basis[M]. Beijing:The Science Publishing Company, 2005. [35] KAPTEIJN F, SINGOREDJO L, ANDREINI A, NOULIJN J A. Activity and selectivity of pure manganese oxides in the selective catalytic reduction of nitric oxide with ammonia[J]. Appl Catal B:Environ, 1994, 3(2):173-189. [36] CARNO J, FERRANDON M, BJORNBOM E, JARAS S. Mixed manganese oxide/platinum catalysts for total oxidation of model gas from wood boilers[J]. Appl Catal A:Gen, 1997, 155(2):265-281. doi: 10.1016/S0926-860X(97)80129-9 [37] GAO G, SHI J W, LIU C, GAO C, FAN Z Y, NIU C M. Mn/CeO2 catalysts for SCR of NOx with NH3:comparative study on the effect of supports on low-temperature catalytic activity[J]. Appl Surf Sci, 2017, 411(31):338-346. http://downloads.hindawi.com/journals/amse/2017/3958319.xml [38] PARRY E P. An infrared study of pyridine adsorbed on acidic solids. Characterization of surface acidity[J]. J Catal, 1963, 2(5):371-379. doi: 10.1016/0021-9517(63)90102-7 [39] WU Z B, JIANG B Q, LIU Y, WNAG H Q, JIN R B. DRIFT study of manganese/titania-Based catalysts for low-temperature selective catalytic reduction of NO with NH3[J]. Environ Sci Technol, 2007, 41(16):5812-5817. doi: 10.1021/es0700350 -





 下载:
下载: