Effect of the promoters on oxidation behavior of Fe-based Fischer-Tropsch catalyst: Deciphering the role of H2O
-
摘要: 以纯Fe催化剂为研究对象,采用XRD、Raman和TPH等手段考察了催化剂的碳化程度、还原程度对H2O氧化过程的影响,获得了H2O氧化过程与催化剂中碳物种转变之间的相互影响规律;系统考察了典型的费托合成助剂K和SiO2存在时对催化剂物化性质以及H2O氧化行为的影响,发现催化剂的碳化程度越高,碳化铁的抗H2O氧化能力越强,氧化过程使得碳物种的石墨化程度增加。适量K助剂可促进碳化铁和催化剂表面石墨碳的形成,提高了碳化铁在H2O氧化过程中的稳定性;SiO2助剂的加入显著抑制了催化剂的碳化,但可有效提高碳化铁以及碳物种的稳定性。Abstract: The effect of carburization and reduction degree on H2O oxidation behaviour for the iron carbides in Fe-based FTS catalyst were firstly investigated using a combination method including X-ray diffraction (XRD), Raman and temperature-programmed-hydrogenation (TPH). The relationship between carbon species transformation and H2O oxidation behaviour of iron carbides was investigated simultaneously. Based on these observations, the influence of typical promoters like K and SiO2 on the structure and H2O oxidation behaviour of Fe-based FTS catalysts was further studied. The results indicated that, for the iron catalyst, the stability of iron carbides against H2O oxidation was increased with the increase of iron carbides content, and the H2O oxidation process led to the formation of more graphitic carbon. The carburization ability was effectively enhanced when certain amount of K promoter was incorporated. Addition of K into Fe-based FTS catalyst increased the number of graphitic carbons, which increased the stability of iron carbides toward H2O oxidation ultimately. It was also found that promotion of SiO2 greatly degraded the carburization degree of the catalyst, while their tendency to be oxidized to form Fe3O4 in the H2O atmosphere was obviously hindered.
-
Key words:
- Fischer-Tropsch synthesis /
- iron-based catalysts /
- H2O oxidation /
- stability of iron carbide /
- promoter
-
表 1 纯Fe催化剂碳化及氧化后的XRD & Raman表征
Table 1 XRD and Raman results of Fe catalysts after carbonization and oxidization
Sample Content
wmol/%FeCx
phase shift
wmol/%Oxidation
degreea
D/%Particle
size d/nmID/IG Peak area of
carbon deposition
via Raman
/(×104 a.u.)Increase ratio
of carbon deposition
after oxidation
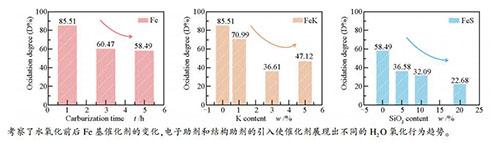
by H2Ob /%Fe5C2 Fe3O4 Fe5C2 Fe3O4 Fe-C1 36.16 63.85 30.92 85.51 7.65 21.23 1.66 4.42 130.77 Fe-C1W2 5.24 94.75 2.75 25.83 0.87 10.18 Fe-C3 68.02 31.98 41.13 60.47 9.04 20.82 1.37 5.23 57.17 Fe-C3W2 26.89 73.11 4.26 26.97 0.96 8.22 Fe-C5 84.30 15.70 49.31 58.49 10.48 17.74 1.41 5.52 7.25 Fe-C5W2 34.99 65.02 7.40 23.31 1.08 5.92 a: calculated as: (FeCx content before oxidation -FeCx content after oxidation)/ FeCx content before oxidation ×100%;
b: calculated from (area of deposited carbon after oxidation-area of deposited carbon before oxidation)/ area of deposited carbon before oxidation ×100%表 2 纯Fe催化剂H2还原和氧化后的XRD拟合
Table 2 XRD results of the Fe catalysts after reduction and oxidization
Sample Content w/% Particle size d/nm Fe3O4 Fe Fe3O4 Fe Fe-H1 90.18 9.82 26.02 16.82 Fe-H1W2 100.00 0.00 29.41 - Fe-H3 68.60 31.40 26.12 20.29 Fe-H3W2 100.00 0.00 35.70 - Fe-H5 50.35 49.65 29.81 22.58 Fe-H5W2 100.00 0.00 43.48 - 表 3 纯Fe催化剂的TPH拟合
Table 3 TPH results of the Fe catalysts
Sample α atomic
carbon
/(×10-6 a.u.)β polymeric,
amorphous
aggregates
/(×10-5 a.u.)γ iron
carbides
/(×10-5 a.u.)δ graphitic
(crystalline)
films
/(×10-6 a.u.)Total δ
graphitic
/(×10-6 a.u.)aTotal
carbon
/(×10-4 a.u.)bFe-C5 2.24 16.42 11.85 9.09 0.08 0.84 - 0.84 3.77 Fe-C5W0.5 - - 8.35 3.12 2.77 2.80 0.63 3.43 1.46 Fe-C5W1 - - 2.80 9.05 3.35 6.69 1.82 8.51 1.60 Fe-C5W2 - 4.21 3.93 5.91 1.13 2.94 3.48 6.42 1.58 a: sum of two different kind of δ-carbon; b: sum of all carbon species 表 4 FeK系列催化剂的XRD & Raman表征
Table 4 XRD and Raman results of FeK catalysts after carbonization and oxidization
Sample Content wmol/% FeCx
phase shift
wmol/%Oxidation
degreea D/%Particle size d/nm ID/IG Peak area of
carbon deposition via
Raman/(×104 a.u.)FeCx Fe3O4 Fe5C2 Fe3O4 Fe-C1 36.16 63.85 30.92 85.51 7.65 21.23 1.66 4.19 Fe-C1W2 5.24 94.75 2.75 25.83 0.87 9.65 Fe1K-C1 64.11 35.89 45.51 70.99 5.85 20.66 1.47 4.11 Fe1K-C1W2 18.59 81.41 5.34 22.16 0.9 9.34 Fe3K-C1 61.38 38.62 22.47 36.61 6.14 18.52 1.41 3.93 Fe3K-C1W2 38.91 61.09 3.30 22.06 1.10 5.13 Fe5K-C1 33.85 66.15 15.95 47.12 5.32 21.31 1.45 3.63 Fe5K-C1W2 17.90 82.10 3.97 25.88 0.92 3.71 a: calculated as: (FeCx content before oxidation -FeCx content after oxidation)/ FeCx content before oxidation ×100% 表 5 FeK催化剂的TPH拟合
Table 5 TPH results of FeK catalysts
Sample
no.α atomic
carbon
/(×10-8 a.u.)β polymeric,
amorphous
aggregates
/(×10-7 a.u.)γ iron
carbides
/(×10-6 a.u.)δ graphitic
(crystalline) films
/(×10-7 a.u.)Total δ
graphitic
/(×10-7 a.u.)aTotal
carbon
/(×10-6 a.u.)bFe-C1 4.05 - 33.04 0.93 0.31 0.13 - 0.13 4.59 Fe-C1W2 - 6.83 10.96 0.44 0.19 0.22 - 0.22 2.44 Fe1K-C1 9.75 - 24.42 0.42 0.87 - 2.74 2.74 4.11 Fe1K-C1W2 - 2.37 - 1.48 0.59 2.05 2.31 4.36 2.74 Fe3K-C1 5.37 - 2.50 1.78 1.94 2.36 0.79 3.15 4.34 Fe3K-C1W2 - - 5.13 2.27 0.94 3.40 - 3.40 4.06 Fe5K-C1 3.80 - 1.19 1.67 2.02 - 2.84 2.84 4.13 Fe5K-C1W2 - - 0.37 2.22 1.53 - 2.97 2.97 4.08 a: sum of two different kind of δ-carbon; b: sum of all carbon species 表 6 FeSi系列催化剂的XRD & Rama表征
Table 6 XRD and Raman results of FeSi catalysts after carbonization and oxidization
Sample Content
wmol/%FeCx
phase
shift wmol/%Oxidation
degreea
D/%Particle size d/nm ID/IG Peak area of
carbon deposition via
Raman/(×104 a.u.)FeCx Fe3O4 Fe5C2 Fe3O4 Fe-C5 84.30 15.70 49.31 58.49 10.48 17.74 1.28 4.92 Fe-C5W2 34.99 65.02 7.40 23.31 0.94 7.87 Fe5Si-C5 66.29 33.71 24.25 36.58 6.47 12.99 1.67 2.68 Fe5Si-C5W2 42.04 57.96 5.38 18.92 1.11 2.13 Fe10Si-C5 58.31 41.69 18.71 32.09 6.72 4.44 1.43 1.44 Fe10Si-C5W2 39.60 60.40 5.58 16.24 1.48 1.29 Fe20Si-C5 42.50 57.50 9.64 22.68 5.53 2.02 1.07 1.02 Fe20Si-C5W2 32.86 67.14 4.66 8.77 1.04 0.54 a: calculated as: (FeCx content before oxidation -FeCx content after oxidation)/ FeCx content before oxidation ×100% 表 7 FeSi催化剂的TPH拟合
Table 7 TPH results of FeSi catalysts
Sample α atomic
carbon
/(×10-7 a.u.)β polymeric,
amorphous
aggregates
/(×10-6 a.u.)γ iron
carbides
/(×10-6 a.u.)δ graphitic
(crystalline)
films
/(×10-7 a.u.)Total δ
graphitic
/(×10-7 a.u.)aTotal
carbon
/(×10-5 a.u.)bFe-C5 22.36 164.20 118.52 90.93 0.77 8.43 - 8.43 37.75 Fe-C5W2 - 42.12 39.27 59.07 11.27 29.39 34.60 63.99 15.81 Fe5Si-C5 - - 2.72 3.40 7.91 51.70 - 51.70 1.92 Fe5Si-C5W2 - - 1.08 1.63 2.56 0.39 - 0.39 0.53 Fe10Si-C5 6.38 1.19 2.46 1.17 3.44 - 30.78 30.78 1.20 Fe10Si-C5W2 3.28 1.83 1.99 1.09 1.38 - 27.39 27.39 0.94 Fe20Si-C5 0.97 0.25 0.68 0.54 2.27 - - 0 0.38 Fe20Si-C5W2 1.08 0.19 1.38 1.26 1.53 - - 0 0.45 a: sum of two different kind of δ-carbon; b: sum of all carbon species -
[1] 温晓东, 杨勇, 相宏伟, 焦海军, 李永旺.费托合成铁基催化剂的设计基础:从理论走向实践[J].中国科学:化学, 2017, 47(11):1298-1311. http://www.cnki.com.cn/Article/CJFDTotal-JBXK201711007.htmWEN Xiao-dong, YANG Yong, XIANG Hong-wei, JIAO Hai-jun, LI Yong-wang. The design principle of iron-based catalysts for fischer-tropsch synthesis:from theory to practice[J]. Sci Sin Chim, 2017, 47(11):1298-1311. http://www.cnki.com.cn/Article/CJFDTotal-JBXK201711007.htm [2] ZHANG Q H, KANG J C, WANG Y. Development of novel catalysts for Fischer-Tropsch synthesis:Tuning the product selectivity[J]. ChemCatChem, 2010, 2(9):1030-1058. doi: 10.1002/cctc.201000071 [3] 张成华, 杨勇, 陶智超, 李廷真, 万海军, 相宏伟, 李永旺. Cu、K助剂对FeMn/SiO2催化费托合成的影响[J].物理化学学报, 2006, 22(11):1310-1316. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=wlhxxb200611002ZHANG Cheng-hua, YANG Yong, TAO Zhi-chao, LI Ting-zhen, WAN Hai-jun, XIANG Hong-wei, LI Yong-wang. Effects of Cu and K on Co-precepitated FeMn/SiO2 catalysts for Fischer-Tropsch synthesis[J]. Acta Phys-Chim Sin, 2006, 22(11):1310-1316. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=wlhxxb200611002 [4] YANG Y, XIANG H W, TIAN L, WANG H, ZHANG C H, TAO Z C, XU Y Y, ZHONG B, LI Y W. Structure and Fischer-Tropsch performance of iron-manganese catalyst incorporated with SiO2[J]. Appl Catal A:Gen, 2005, 284(1):105-122. http://www.sciencedirect.com/science/article/pii/S0926860X05000323 [5] ARGYLE M D, BARTHOLOMEW C H. Heterogeneous catalyst deactivation and regeneration:A review[J]. Catalysts, 2015, 5:145-269. doi: 10.3390/catal5010145 [6] DRY M E, SHINGLES T, BOSHOFF L J, BOTHA C S H. Factors influencing the formation of carbon on iron Fischer-Tropsch catalysts:Ⅱ. The effect of temperature and of gases and vapors present during Fischer-Tropsch synthesis[J]. J Catal, 1970, 17(3):347-354. doi: 10.1016/0021-9517(70)90110-7 [7] SARKAR A, SETH D, DOZIER A K, NEATHERY J K, HAMDEH H H, DAVIS B H. Fischer-Tropsch synthesis:Morphology, phase transformation and particle size growth of nano-scale particles[J]. Catal Lett, 2007, 117(1/2):1-17. doi: 10.1007/s10562-007-9194-6 [8] MANSKER L D, JIN Y, BUKUR D B, DATYE A K. Characterization of slurry phase iron catalysts for Fischer-Tropsch synthesis[J]. Appl Catal A:Gen, 1999, 186(1/2):277-296. http://www.sciencedirect.com/science/article/pii/S0926860X99001490 [9] DRY M E, HOOGENDOORN J C. Technology of the Fischer-Tropsch process[J]. Catal Rev, 1981, 23(1/2):265-278. http://d.old.wanfangdata.com.cn/Periodical/ddhg201905032 [10] NING W, KOIZUMI N, CHANG H, MOCHIZUKU T, ITOH T, YAMADA M. Phase transformation of unpromoted and promoted Fe catalysts and the formation of carbonaceous compounds during Fischer-Tropsch synthesis reaction[J]. Appl Catal A:Gen, 2006, 312(9):35-44. http://www.sciencedirect.com/science/article/pii/S0926860X06004790 [11] PENDYALA V R R, JACOBS G, MOHANDAS J C, LUO M S, HAMDEH H H, JI Y Y, RIBEIRO M C, DAVIS B H. Fischer-Tropsch Synthesis:Effect of water over iron-based catalysts[J]. Catal Lett, 2010, 140(3/4):98-105. doi: 10.1007/s10562-010-0452-7 [12] THÜNE P, MOODLEY P, SCHEIJEN F, FREDRIKSSON H, LANCEE R, KROPF J, MILLER J, NIEMANTSVERDRIET J W. The effect of water on the stability of iron oxide and iron carbide nanoparticles in hydrogen and syngas followed by in situ X-ray absorption spectroscopy[J]. J Phys Chem C, 2012, 116(13):7367-7373. doi: 10.1021/jp210754k [13] SATTERFIELD C N, HANLON R T, TUNG S E, ZOU Z M, PAPAEFTHYMIOU G C. Effect of water on the iron-catalyzed Fischer-Tropsch synthesis[J]. Ind Eng Chem Pro Res Dev, 1986, 25(3):407-414. doi: 10.1021/i300023a007 [14] QING M, YANG Y, WU B S, XU J, ZHANG C H, GAO P, LI Y W. Modification of Fe-SiO2 interaction with zirconia for iron-based Fischer-Tropsch catalysts[J]. J Catal, 2011, 279(1):111-122. doi: 10.1016/j.jcat.2011.01.005 [15] BUTT J B. Carbide phases on iron-based Fischer-Tropsch synthesis catalysts part Ⅰ:Characterization studies[J]. Catal Lett, 1990, 7(1/4):61-81. doi: 10.1007/BF00764492 [16] TUINSTRA F, KOENIG J L. Raman spectrum of graphite[J]. J Chem Phys, 1970, 53(3):1126-1130. doi: 10.1063/1.1674108 [17] NEMANICH R J, SOLIN S A. First- and second-order Raman scattering from finite-size crystals of graphite[J]. Phys Rev B, 2015, 20(2):392-401. http://www.researchgate.net/publication/235593141_First-_and_second-order_Raman_scattering_from_finite-size_crystals_of_graphite [18] DE FARIA D L A, VENÂNCIO SILVA S, DE OLIVEIRA M T. Raman microspectroscopy of some iron oxides and oxyhydroxides[J]. J Raman Spectrosc, 1997, 28(11):873-878. doi: 10.1002/(SICI)1097-4555(199711)28:11<873::AID-JRS177>3.0.CO;2-B [19] TAN P H, ZHANG S L, KWOK T Y, HUANG F M, SHI Z J, ZHOU X H, GU Z N. Comparative Raman study of carbon nanotubes prepared by D.C. arc discharge and catalytic methods[J]. J Raman Spectrosc, 1997, 28(5):369-372. doi: 10.1002/(SICI)1097-4555(199705)28:5<369::AID-JRS107>3.0.CO;2-X [20] SHULTZ J F, HALL W K, SELIGMAN B, ANDERSON R B. Studies of the Fischer-Tropsch synthesis. XIV. Hägg iron carbide as catalysts[J]. J Am Chem Soc, 1955, 77(1):213-221. doi: 10.1021/ja01606a079 [21] XU J, BARTHOLOMEW C H. Temperature-programmed hydrogenation (TPH) and in situ Mössbauer spectroscopy studies of carbonaceous species on silica-supported iron Fischer-Tropsch catalysts[J]. J Phys Chem B, 2005, 109(6):2392-2403. doi: 10.1021/jp048808j [22] MILLER D G, MOSKOVITS M. A study of the effects of potassium addition to supported iron catalysts in the Fischer-Tropsch reaction[J]. J Phys Chem, 1988, 92(21):6081-6085. doi: 10.1021/j100332a047 [23] BONZEL H P, KREBS H J. Enhanced rate of carbon deposition during Fischer-Tropsch synthesis on K promoted Fe[J]. Surf Sci, 1981, 109(2):L527-L531. doi: 10.1016/0039-6028(81)90486-6 [24] FERDI S, SING K S W, WEITKAMP J. Handbook of Porous Solids(vol.3)[M]. Germany:Wiley-VCH, 2002:1543-1591. -





 下载:
下载: