Wet ball-milling method to prepare nanocrystalline Li4SiO4 materials for CO2 capture at high temperatures
-
摘要: 采用不同硅源、锂源以湿磨法结合高温焙烧制备了纳米Li4SiO4材料,利用X射线衍射(XRD)、扫描电子显微镜(SEM)和透射电子显微镜(TEM)表征了合成材料的结构和表面形貌,利用热重分析仪(TG)研究了Li4SiO4材料高温下的CO2吸收性能和循环使用稳定性。结果表明,湿磨法制备的Li4SiO4材料在550℃、2.5×104 Pa下,10 min可达吸收平衡,平衡吸收量为27.9%(质量分数),经五次吸收-解吸后仍保持初始吸收性能,显示了良好的循环稳定性。将25%CO2-25%N2-50%He混合气通过Li4SiO4材料床层,发现在550℃下,CO2能被高效捕集,在相对湿度为10%的水汽存在下,Li4SiO4捕集CO2的性能没有明显下降。Abstract: A wet ball-milling method followed by calcination was adopted to prepare nanocrystalline Li4SiO4 materials by using different silicon and lithium sources. X-ray diffraction (XRD), scanning electron microscope (SEM) and transmission electron microscope (TEM) were applied to characterize the structure and morphology of the as-prepared Li4SiO4 materials. CO2 uptakes and recycle stability of the prepared Li4SiO4 materials were investigated on a thermogravity (TG) analyzer. Absorption equilibrium of 27.9% was achieved within 10 min at 550℃ and CO2 partial pressure of 2.5×104 Pa. The prepared nanocrystalline Li4SiO4 material kept the original absorption properties after 5 capture-regeneration cycles, indicating the good cycle stability. A mixture of 25% CO2-25% N2-50% He was introduced through the Li4SiO4 absorption bed, showing that CO2 can be efficiently captured at 550℃. The adsorption capacity showed no significant decrease in the presence of 10% humidity.
-
Key words:
- Li4SiO4 /
- wet ball-milling /
- CO2 capture /
- high temperature /
- moisture
-
表 1 不同硅源、锂源制备的Li4SiO4材料及其CO2吸收性能
Table 1 Li4SiO4 materials prepared by various silicon and lithium sources and their CO2 absorption properties
Sample Lithium source Silicon source Absorption
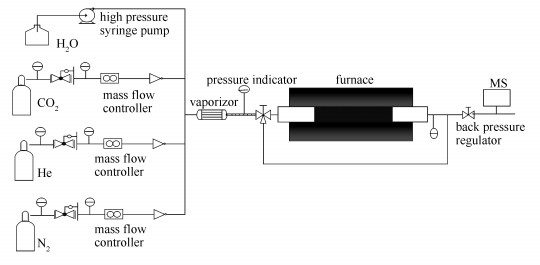
equilibrium w/%1 LiOH·H2O silica sol 19.9 2 LiOH·H2O fumed silica 2.3 3 LiOH·H2O silicic acid 25.6 4 LiOH·H2O TEOS 27.9 5 Li2CO3 TEOS no absorption 6 LiNO3 TEOS - -: the precursor is in paste form and cannot produce the solid precursor 表 2 湿磨法制备的Li4SiO4材料与文献中报道的锂基类吸收剂的CO2吸收性能比较
Table 2 Comparison of the CO2 capture properties of the Li4SiO4 sorbent prepared using the wet ball-milling method and lithium based sorbents reported in references
Sorbent CO2 capture conditions Maximum of
CO2 capture rate
/(%·min-1)aEquilibrium
sorption amount
w/%Time required
to equilibrium
t/minReference temp
t/ ℃CO2
pressure
p/Patotal feed
flow
q/(mL·min-1)Li4SiO4 550 2.5×104 40 6.4 27.8 ~10 this work Li2ZrO3 550 2.5×104 40 0.5 5.20 ~20 [14] K-Li2ZrO3 550 2.5×104 40 1.9 22.0 < 20 [16] Li4SiO4 550 2.5×104 40 3.1 23.9 >60 [19] Li4SiO4 550 2.5×104 40 3.2 24.5 >60 [20] Fe0.15Li3.45SiO4 550 2.5×104 40 3.4 26.0 ~25 [20] Li4SiO4 550 2.5×104 40 5.5 29.8 ~7.5 [21] Li4SiO4 680 1.0×105 - 2.0 27.5 ~40 [18] Li4SiO4 620 5×104 100 2.7 28.62 80 [17] a: differential values of the uptake curves -
[1] 《联合国气候变化框架公约》京都议定书[Z].联合国:日本京都, 1998.《United Nations Framework Convention on Climate Change》 Kyoto Protocol[Z]. UN: Kyoto, Japan, 1998. [2] 陈长虹, 鲍仙华.全球能源消费与CO2排放量[J].上海环境科学, 1999, 18(2): 62-64.CHEN Chang-hong, BAO Xian-hua. Global energy consumption and CO2 emission[J]. Shanghai Environ Sci, 1999, 18(2): 62-64. [3] LI H, HELENE B, DE C. Approaching sustainable H2 production: Sorption enhanced steam reforming of ethanol[J]. J Phy Chem A, 2010, 114(11): 3834-3844. doi: 10.1021/jp906146y [4] 张元卓, 于兹瀛, 张富民, 肖强, 钟依均, 朱伟东.纳米Li2ZrO3吸收剂原位移除CO2强化水煤气变换反应制氢[J].催化学报, 2012, 33(9): 1572-1577.ZHANG Yun-zhuo, YU Zi-ying, ZHANG Fu-ming, XIAO Qiang, ZHONG Yi-jun, ZHU Wei-dong. Li2ZrO3 nanoparticles as absorbent for in-situ removal of CO2 in water-gas shift reaction to enhance H2 production[J]. Chin J Catal, 2012, 33(9): 1572-1577. [5] LI H, PAARA J M S, BLEKKAN E A, DE C. Towards efficient hydrogen production from glycerol by sorption enhanced steam reforming[J]. Energy Environ Sci, 2010, 3(8): 1046-1056. doi: 10.1039/b922355j [6] 高峰, 李存梅, 王媛, 孙国华, 李开喜.树脂基球状活性炭的制备及对二氧化碳吸附性能的研究[J].燃料化学学报, 2014, 42(1): 116-120. http://www.wenkuxiazai.com/doc/dfc8895816fc700aba68fc48.htmlGAO Feng, LI Cun-mei, WANG Yuan, SUN Guo-hua, LI Kai-xi. Preparation of resin-base spherical activated carbon and study on adsorption properties towards CO2[J]. J Fuel Chem Technol, 2014, 42(1): 116-120. http://www.wenkuxiazai.com/doc/dfc8895816fc700aba68fc48.html [7] HU J X, SHANG H, WANG J G, LUO L, XIAO Q, ZHONG Y J, ZHU W D. Highly enhanced selectivity and easy regeneration for the separation of CO2 over N2 on melamine-based microporous organic polymers[J]. Ind Eng Chem Res, 2014, 53(29): 11828-11837. doi: 10.1021/ie501736t [8] ZHAO J, SIMEON F, WANG Y, LUO G, HATTON T A. Polyethylenimine-impregnated siliceous mesocellular foam particles as high capacity CO2 adsorbents[J]. RSC Adv, 2012, 2(16): 6509-6519. doi: 10.1039/c2ra20149f [9] 李勇, 李磊, 闻霞, 王峰, 赵宁, 肖福魁, 魏伟, 孙予罕.二次嫁接法制备氨基修饰的硅基二氧化碳吸附剂[J].燃料化学学报, 2013, 41(9): 1122-1128. http://rlhxxb.sxicc.ac.cn/CN/abstract/abstract18261.shtmlLI Yong, LI Lei, WEN Xia, WANG Feng, ZHAO Ning, XIAO Fu-kui, WEI Wei, SUN Yu-han. Synthesis of amine modified silica for the capture of carbon dioxide by a twice grafting method[J] J Fuel Chem Technol, 2013, 41(9): 1122-1128. http://rlhxxb.sxicc.ac.cn/CN/abstract/abstract18261.shtml [10] 杨刚胜, 曾淦宁, 赵强, 陈徐, 陈盛积, 艾宁.负载型氨基酸离子液体的制备及其对二氧化碳的吸附性能[J].燃料化学学报, 2016, 44(1): 106-112.YANG Gang-sheng, ZENG Gan-ning, ZHAO Qiang, CHEN Xu, CHEN Sheng-ji, AI Ning. Preparation of silica gel supported amino acid ionic liquids and their performance capacity towards carbon dioxide[J]. J Fuel Chem Technol, 2016, 44(1): 106-112. [11] LI J R, SCULLEY J, ZHOU H C. Metal-organic frameworks for separations[J]. Chem Rev, 2011, 112(2): 869-932. https://www.researchgate.net/publication/51704912_Metal-Organic_Frameworks_for_Separations [12] 左臣盛, 周思宇, 孙成志, 王兴之, 刘道胜, 徐煇旼, 朴容起, 桂建舟, 刘丹.变温镁基CO2吸附剂的制备及应用I. Na/Mg物质的量比[J].燃料化学学报, 2014, 42(7): 884-889. http://rlhxxb.sxicc.ac.cn/CN/abstract/abstract18461.shtmlZUO Cheng-sheng, ZHOU Si-yu, SUN Cheng-zhi, WANG Xing-zhi, LIU Dao-sheng, XU Hui-min, PIAO Rong-qi, GUI Jian-zhou, LIU Dan. Preparation and application of magnesium-based CO2 sorbent for temperature swing absorption I. Na/Mg mol ratio[J]. J Fuel Chem Technol, 2014, 42(7): 884-889. http://rlhxxb.sxicc.ac.cn/CN/abstract/abstract18461.shtml [13] FLORIN N H, HAARRIS A T. Reactivity of CaO derived from nano-sized CaCO3 particles through multiple CO2capture-and-release cycles[J]. Chem Eng Sci, 2009, 64(2): 187-191. doi: 10.1016/j.ces.2008.10.021 [14] XIAO Q, LIU Y F, ZHONG Y J, ZHU W D. A citrate sol-gel method to synthesize Li2ZrO3 nanocrystals with improved CO2 capture properties[J]. J Mater Chem, 2011, 21(11): 3838-3842. doi: 10.1039/c0jm03243c [15] XIAO Q, TANG X D, LIU Y F, ZHONG Y J, ZHU W D. Comparison study on strategies to prepare nanocrystalline Li2ZrO3-based absorbents for CO2 capture at high temperatures[J]. Front Chem Sci Eng, 2013, 7(3): 297-302. doi: 10.1007/s11705-013-1346-1 [16] XIAO Q, TANG X D, ZHONG Y J, ZHU W D. A facile starch-assisted sol-gel method to synthesize K-doped Li2ZrO3 sorbents with excellent CO2 capture properties[J]. J Am Ceram Soc, 2012, 95(5): 1544-1548. doi: 10.1111/jace.2012.95.issue-5 [17] SHAN S, JIA Q, JIANG L, LI Q, WANG Y, PENG J. Preparation and kinetic analysis of Li4SiO4 sorbents with different silicon sources for high temperature CO2 capture[J]. Chin Sci Bull, 2012, 57(19): 2475-2479. doi: 10.1007/s11434-012-5188-x [18] YIN Z, WANG K, ZHAO P, TANG X. Enhanced CO2 chemisorption properties of Li4SO4, using a water hydration-calcination technique[J]. Ind Eng Chem Res, 2016, 55(4): 1142-1146. doi: 10.1021/acs.iecr.5b03746 [19] 唐晓丹.纳米Li2ZrO3和Li4SiO4二氧化碳吸收材料制备与表征[D].金华:浙江师范大学, 2012.TANG Xiao-dan. Synthesis and characterization of nanosized Li2ZrO3 and Li4SiO4 materials as carbon dioxide sorbents[D]. Jinhua: Zhejiang Normal University, 2012. [20] 悦灵丽, 肖强, 钟依均, 朱伟东.金属元素掺杂对硅酸锂材料二氧化碳吸收性能的影响[J].现代化工, 2014, (34): 70-73. http://www.cnki.com.cn/Article/CJFDTOTAL-XDHG201408020.htmYUE Ling-li, XIAO Qiao, ZHONG Yi-jun, ZHU Wei-dong. Influence of metallic element dopant on CO2, absorption properties of Li4SiO4 materials[J] Modern Chem Ind, 2014, (34): 70-73. http://www.cnki.com.cn/Article/CJFDTOTAL-XDHG201408020.htm [21] 童沂, 黄雪芹, 许春慧, 肖强, 钟依均, 朱伟东.液相法结合冷冻干燥技术制备Li4SiO4材料及其高温二氧化碳吸收性能[J].物理化学进展, 2015, 04(2): 77-83. doi: 10.12677/JAPC.2015.42010TONG Yi, HUANG Xue-qin, XU Chun-hui, XIAO Qiang, ZHONG Yi-jun, ZHU Wei-dong. A liquid phase method combined with freeze-drying technique to lithium silicate materials and their carbon dioxide absorption properties at high temperatures[J]. J Adv Phys Chem, 2015, 04(2): 77-83. doi: 10.12677/JAPC.2015.42010 [22] VENEGAS M J, FREGOSO-ISRAEL E, ESCAMILLA R, PFEIFFER H. Kinetic and reaction mechanism of CO2 sorption on Li4SiO4: Study of the particle size effect[J]. Ind Eng Chem Res, 2007, 46(8): 2407-2412. doi: 10.1021/ie061259e [23] CHEN D L, SHANG H, ZHU W D, KRISHNA R. Transient breakthroughs of CO2/CH4 and C3H6/C3H8 mixtures in fixed beds packed with Ni-MOF-74[J]. Chem Eng Sci, 2014, 117: 407-415. doi: 10.1016/j.ces.2014.07.008 -





 下载:
下载: