Study on n-butane catalytic cracking for promoting propylene production over nMoOx·HZSM-5
-
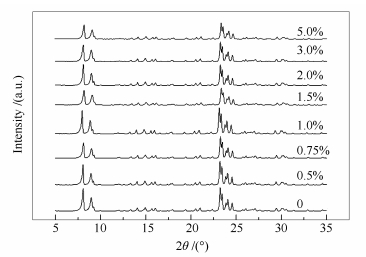
摘要: 采用等体积浸渍法制备了nMoOx·HZSM-5系列单相复合体,用XRD、NH3-TPD、Py-FTIR、BET、SEM等技术对其物相结构、表面酸性、比表面积进行了表征。在连续固定床微反装置中对nMoOx·HZSM-5单相复合体进行了催化正丁烷裂解性能的评价。结果表明,部分活性组分Mo以MoOx原子簇的形式定位于HZSM-5分子筛的Z形和直形孔道交叉孔处,与分子筛的骨架氧配位形成nMoOx·HZSM-5单相复合体,引起分子筛的骨架收缩,相应的晶胞参数及晶胞体积减小,比表面积下降;随Mo用量的增大,nMoOx·HZSM-5单相复合体的酸量呈先增加后减小趋势;在反应温度625℃,体积空速5600 h-1条件下,Mo用量为0.75%制备的nMoOx·HZSM-5-0.75%单相复合体催化正丁烷裂解反应的转化率为73.83%,略低于HZSM-5分子筛,但丙烯收率却达到了13.13%,较HZSM-5分子筛提高2个百分点以上,表现出较好的增产丙烯效果。Abstract: A series of nMoOx·HZSM-5 single-phase complexes were prepared by incipient wetness impregnation, and characterized by XRD, NH3-TPD, Py-FTIR, BET and SEM techniques. The n-butane catalytic cracking performance over nMoOx·HZSM-5 was investigated by using a continuous flowing micro reactor. The results indicate that active component Mo is located in the cross of Z form channel and straight channel of HZSM-5 in the form of MoOx clusters to generate a nMoOx·HZSM-5 single-phase complex, causing the contraction of HZSM-5 lattice cell and the reduction in the lattice parameters and cell volume of HZSM-5 as well as the decrease in specific surface area of HZSM-5. The acidity of nMoOx·HZSM-5 shows an increases firstly and then a decrease with the increasing dosage of active component Mo. The n-butane catalytic cracking conversion over nMoOx·HZSM-5-0.75% is 73.83% at reaction temperature of 625℃ and gas space velocity of 5600 h-1, slightly lower than that over HZSM-5. However, the propylene yield over nMoOx·HZSM-5-0.75% reaches 13.13%, 2 percent points higher than that over HZSM-5, exhibiting a better performance on the promotion of propylene yield.
-
Key words:
- nMoOx·HZSM-5 /
- single-phase complex /
- n-butane /
- propylene /
- conversion /
- yield
-
表 1 不同Mo用量的nMoOx·HZSM-5单相复合体的晶胞参数及晶胞体积
Table 1 Lattice parameters(a, b, c) and cell volume(V) of nMoOx·HZSM-5 single-phase complex with different dosages of Mo
Mo w/% a/nm b/nm c/nm V/nm3 0 2.0003 2.0181 1.3459 5.4333 0.5 1.9998 2.0189 1.3453 5.4318 0.75 2.0000 2.0187 1.3455 5.4327 1.0 1.9985 2.0187 1.3461 5.4311 1.5 1.9978 2.0196 1.3457 5.4296 2.0 1.9999 2.0188 1.3446 5.4291 3.0 1.9987 2.0178 1.3445 5.4225 5.0 1.9986 2.0175 1.3454 5.4241 表 2 nMoOx·HZSM-5单相复合体的比表面积
Table 2 Specific surface area of nMoOx·HZSM-5 single-phase complex
Mo w/% ABET/(m2·g-1) 0 399 0.5 395 0.75 388 1.0 372 1.5 365 2.0 356 3.0 354 5.0 340 表 3 nMoOx·HZSM-5单相复合体的酸量
Table 3 Acidic amount of nMoOx·HZSM-5 single-phase complex
Mo w/% Total /(mmol·g-1) Weak acid/% Strong acid/% 0 0.632 54.42 45.58 0.5 0.926 57.79 42.21 0.75 1.035 58.54 41.46 1.0 0.953 58.73 41.27 1.5 0.951 60.04 39.96 2.0 0.793 61.41 38.59 3.0 0.756 62.39 37.61 5.0 0.731 63.44 36.56 表 4 nMoOx·HZSM-5单相复合体的B酸和L酸酸量
Table 4 Amounts of B acid and L acid sites determined by Py-IR of nMoOx·HZSM-5 single-phase complex
Mo w/% Brønsted/ (mmol·g-1) Lewis/ (mmol·g-1) L/B 0 0.495 0.137 0.277 0.5 0.767 0.159 0.207 0.75 0.831 0.204 0.246 1.0 0.780 0.173 0.222 1.5 0.622 0.328 0.527 表 5 产物定性分析
Table 5 Identification result of product
Retention time t/min Peak substance Retention time t/min Peak substance 8.122 methane 14.187 propylene 8.948 ethane 16.613 isobutane 9.641 ethylene 17.128 n-butane 11.561 propane -
[1] 王梦瑶, 周嘉文, 任天华, 孟祥海, 张睿, 刘海燕.催化裂化多产丙烯[J].化工进展, 2015, 34(6):1619-1624. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=shihjs200403013WANG Meng-yao, ZHOU Jia-wen, REN Tian-hua, MENG Xiang-hai, ZHANG Rui, LIU Hai-yan. Catalytic cracking processes for maximizing propylene production[J]. Chem Ind Eng Prog, 2015, 34(6):1619-1624. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=shihjs200403013 [2] BOSWELL C, WEDDLE N, MEEHAN J, TERRY L. Propylene boosts prices downstream[J]. ICIS Chem Business, 2011, 279(4):20-21. http://connection.ebscohost.com/c/articles/58024109/propylene-boosts-prices-downstream [3] XIN L, AMIT K, YING H, HARSHUL V T, MARKTUS A A, FATEME R, DOUGLAS K L, ALI A R. Light olefins from renewable resources:Selective catalytic dehydration of bioethanol to propylene over zeolite and transition metal oxide catalysts[J]. Catal Today, 2016, 276(15):62-77. http://www.sciencedirect.com/science/article/pii/S0920586116300608 [4] SHINYA H, AZUSA M, SHUHEI W, RYUICHI K, FUYUKI Y. Catalytic conversion of light hydrocarbons to propylene over MFI-zeolite/metal-oxide composites[J]. Microporous Mesoporous Mater, 2016, 233(15):125-132. http://www.sciencedirect.com/science/article/pii/S138718111500709X [5] 陈硕, 王定博, 吉媛媛, 白杰.丙烯为目的产物的技术进展[J].石油化工, 2011, 40(2):217-224. http://epub.cqvip.com/articledetail.aspx?id=1000000437322CHEN-Shuo, WANG Ding-bo, JI Yuan-yuan, BAI Jie. Development in on-purpose propylene technology[J]. Petrochem Technol, 2011, 40(2):217-224. http://epub.cqvip.com/articledetail.aspx?id=1000000437322 [6] WANG H. Advances and prospect of low-carbon olefin production technology[J]. Sino Global Energy, 2010, 15(8):62-67. doi: 10.1007/s11244-012-9912-1 [7] 杨为民.碳四烃转化与利用技术研究进展及发展前景[J].化工进展, 2015, 34(1):1-9. http://www.oalib.com/paper/4206983WANG Wei-min. Progress and perspectives on conversion and utilization of C4 hydrocarbons[J]. Chem Ind Eng Prog, 2015, 34(1):1-9. http://www.oalib.com/paper/4206983 [8] GANG W, XU C, GAO J. Study of cracking FCC naphtha in a secondary riser of the FCC unit for maximum propylene production[J]. Fuel Process Technol, 2008, 89(9):864-873. doi: 10.1016/j.fuproc.2008.02.007 [9] LI X H, LI C Y, ZHANG J F, YANG C H, SHAN H H. Effects of temperature and catalyst to oil weight ratio on the catalytic conversion of heavy oil to propylene using ZSM-5 and USY catalysts[J]. J Nat Gas Chem, 2007, 16(1):92-99. doi: 10.1016/S1003-9953(07)60033-4 [10] ZHAO Z T, LIU Y, WANG F, LI X K, DENG S P, XU J, WEI W, WANG F. Life cycle assessment of primary energy demand and greenhouse gas (GHG) emissions of four propylene production pathways in China[J]. J Clean Prod, 2017, 163(9):285-292. https://www.researchgate.net/publication/290522544_Life_cycle_assessment_of_primary_energy_demand_and_GHG_emissions_of_four_propylene_production_pathways_in_China [11] RICCA A, PALMA V, LAQUANIELLO G, PALO E, SALLADINI A. Highly selective propylene production in a membrane assisted catalytic propane dehydrogenation[J]. Chem Eng J, 2017, 330(22):1119-1127. http://www.sciencedirect.com/science/article/pii/S1385894717314055 [12] EPELDE E, GAYUBO A G, OLAZAR M, BILBAO J, AGUAYO A T. Modified HZSM-5 zeolites for intensifying propylene production in the transformation of 1-butene[J]. Chem Eng J, 2014, 251(16):80-91. http://www.sciencedirect.com/science/article/pii/S1385894714004926 [13] 谢朝钢.催化裂解过程丙烯选择性的影响因数探究[J].石油学报(石油加工), 2018, 34(1):1-6. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=syxb-syjg201801001XIE Chao-gang. Study on inflencing factors of Propylene selectivity in a deep catalytic cracking process[J]. Acta Pet Sin(Pet Process Sect), 2018, 34(1):1-6. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=syxb-syjg201801001 [14] WANG P, TIAN X, YANG C, YANG C H, YUAN Z H. Economics-oriented nmpc of two-stage-riser catalytic pyrolysis processes for maximizing propylene yield[J]. IFAC-Papers Online, 2015, 48(8):32-37. doi: 10.1016/j.ifacol.2015.08.153 [15] KOTREL S, KNOZINGER H, GATES B C. The Haag-Dessau mechanism of protolytic cracking of alkanes[J]. Microporous Mesoporous Mater, 2000, 35(99):11-20. http://www.sciencedirect.com/science/article/pii/S1387181199002048 [16] XU X, LI C, SHAN H. Effect of phosphorus on novel bifunctional additives for enhancing the production of propylene and removal of SO2, in FCC process[J]. J Mol Catal A:Chem, 2011, 340(1/2):99-107. http://www.sciencedirect.com/science/article/pii/S1381116911001154 [17] VERSTRAETE J, COUPARD V, THOMAZEAU C, ETIENNE P. Study of direct and indirect naphtha recycling to a resid FCC unit for maximum propylene production[J]. Catal Today, 2005, 106(1/4):62-71. http://www.sciencedirect.com/science/article/pii/S0920586105005407 [18] MOHIUDDIN E, SA Y M, MDLELENI M M, SINCADU N, David Key, TSHABALALA T. Synthesis of ZSM-5 from impure and beneficiated Grahamstown kaolin:Effect of kaolinite content, crystallisation temperatures and time[J]. Appl Clay Sci, 2016, 119(2):213-221. http://www.sciencedirect.com/science/article/pii/S0169131715301368 [19] SHIMADA I, TAKIZAWA K, FUKUNAGA H, TAKAHASHI N, TAKATSUKA T. Catalytic cracking of polycyclic aromatic hydrocarbons with hydrogen transfer reaction[J]. Fuel, 2015, 161(28):207-214. http://www.sciencedirect.com/science/article/pii/S0016236115008601 [20] JIN H, ANSARI M B, PARK S E. Sulfonic acid functionalized mesoporous ZSM-5:Synthesis, characterization and catalytic activity in acidic catalysis[J]. Catal Today, 2015, 245(33):116-121. http://www.sciencedirect.com/science/article/pii/S0920586114003915 [21] CHEN X, DONG M, NIU X J, WANG K, CHEN G, FAN W B, WANG J G, QIN Z F. Influence of Zn species in HZSM-5 on ethylene aromatization[J]. Chin J Catal, 2015, 36(6):880-888. doi: 10.1016/S1872-2067(14)60289-8 [22] KONINGSVELD H V, BEKKUM H V, JANSEN J C. On the location and disorder of the tetrapropylammonium (TPA) ion in zeolite ZSM-5 with improved framework accuracy[J]. Acta Crystallogr, 1987, 43(2):127-132. doi: 10.1107/S0108768187098173 [23] ZHOU D, MA D, LIU X, BAO X. A simulation study on the absorption of molybdenum species in the channels of HZSM-5 zeolite[J]. J Mol Catal A:Chem, 2001, 168(1/2):225-232. http://www.sciencedirect.com/science/article/pii/S138111690000532X [24] QI C, WANG Y, DING X, SU H J. Catalytic cracking of light diesel over Au/ZSM-5 catalyst for increasing propylene production[J]. Chin J Catal, 2016, 37(10):1747-1754. doi: 10.1016/S1872-2067(16)62499-3 [25] PARK S, BILIGETU T, WANG Y, NISHITOBA T, KONDO J N, YOKOI T. Acidic and catalytic properties of ZSM-5 zeolites with different Al distributions[J]. Catal Today, 2017, 303(20):64-70. http://www.sciencedirect.com/science/article/pii/S0920586117305059 -





 下载:
下载: