Effect of synthesis conditions on the catalytic performance of phosphotungstic acid encapsulated metal-organic framework in the oxidative desulfurization
-
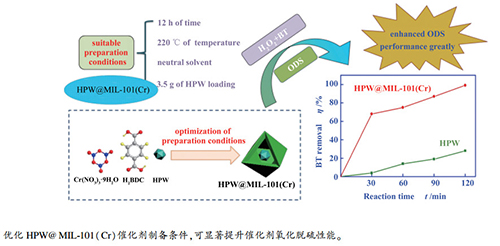
摘要: 通过一步水热合成法制备了大比表面积、高脱硫活性的磷钨酸(HPW)负载的金属有机框架HPW@MIL-101(Cr)催化剂,对其进行了FT-IR、XRD和氮吸附等表征,并研究了合成时间、合成温度、酸碱度及HPW负载量等参数对催化剂脱硫性能的影响。结果表明,随着合成时间的延长、合成温度的提高,HPW@MIL-101(Cr)孔道有序度提高;合成温度低于等于140℃时,不能形成MIL-101(Cr)晶体结构;酸性合成条件合成的HPW@MIL-101(Cr)的孔道有序度降低;随着HPW负载量的增加,HPW@MIL-101(Cr)的催化性能呈现先升高后降低的趋势。在12 h、220℃和中性条件下制备得到的负载量为3.5 g的HPW@MIL-101(Cr)催化剂具有最佳脱硫活性;在模拟油20 mL、催化剂用量0.24 g、氧硫比为8和50℃条件下反应120 min,对苯并噻吩、二苯并噻吩和4,6-二甲基二苯并噻吩脱硫率分别为99%、100%和99%;与HPW相比,苯并噻吩脱硫率提高了2.4倍。Abstract: A series of phosphotungstic acid (HPW) encapsulated metal-organic HPW@MIL-101(Cr) catalysts, with high surface area and high activity in the oxidative desulfurization (ODS), were synthesized by one-step hydrothermal method and characterized by FT-IR, XRD and nitrogen physisorption. The influences of synthesis time, temperature, HPW loading, and acidity/alkalinity on the catalytic performance of HPW@MIL-101(Cr) in ODS were then investigated. The results indicated that the order degree of channels in HPW@MIL-101(Cr) is improved with the increase of synthesis time and temperature. The crystal structure of MIL-101(Cr) cannot be formed at a synthetic temperature below 140℃ and the channel order of HPW@MIL-101(Cr) decreases under an acidic synthetic environment. The catalytic activity of HPW@MIL-101(Cr) displays a trend of first increasing and then decreasing with the increase of HPW loading. The HPW@MIL-101(Cr) catalyst with a HPW loading of 3.5 g, synthesized at 220℃ under neutral environment for 12 h, exhibits the highest activity in ODS. At 50℃, with a catalyst dosage of 0.24 g, an model oil of 20 mL, and an O/S molar ratio of 8, the desulfurization rates over the HPW@MIL-101(Cr) catalyst towards benzothiophene, dibenzothiophene, and 4, 6-dimethyl dibenzothiophene after reaction for 120 min reach 99%, 100% and 99%, respectively; in particular, the desulfurization rate for benzothiophene is 2.4 times higher than that obtained over HPW.
-
表 1 催化剂的N2吸附数据
Table 1 N2 adsorption data of various catalysts
Sample ABET /(m2·g-1) Pore volume v/(cm3·g-1) Pore size d/nm HPW 13 10.46 17.9 MIL-101(Cr) 2465 0.93 3.1 HPW(3.5)@MIL-101(Cr)-2-220-zh 798 0.49 5.3 HPW(3.5)@MIL-101(Cr)-4-220-zh 871 0.43 4.7 HPW(3.5)@MIL-101(Cr)-8-220-zh 984 0.32 2.9 HPW(3.5)@MIL-101(Cr)-12-220-zh 1054 0.31 2.7 HPW(3.5)@MIL-101(Cr)-12-100-zh 156 7.48 9.1 HPW(3.5)@MIL-101(Cr)-12-140-zh 214 7.56 8.6 HPW(3.5)@MIL-101(Cr)-12-180-zh 899 0.39 3.5 HPW(3.5)@MIL-101(Cr)-12-220-s 813 0.37 3.3 HPW(3.5)@MIL-101(Cr)-12-220-j 1182 0.31 2.8 HPW(0.5)@MIL-101(Cr)-12-220-zh 1475 0.51 3.0 HPW(2.0)@MIL-101(Cr)-12-220-zh 1213 0.39 2.8 HPW(5.0)@MIL-101(Cr)-12-220-zh 916 0.29 2.6 表 2 HPW(3.5)@MIL-101(Cr)-12-220-z催化剂BT催化氧化脱硫性能的影响
Table 2 Effect of synthesis environment on the ODS performance of HPW(3.5)@MIL-101(Cr)-12-220-z catalysts
Catalyst HPW(3.5)@MIL-101
(Cr)-12-220-s (acidic)HPW(3.5)@MIL-101
(Cr)-12-220-zh (neutral)HPW(3.5)@MIL-101
(Cr)-12-220-j (alkalic)BT removal η/% 68 99 85 表 3 其他油品的氧化脱硫性能
Table 3 Oxidative desulfurization results
Sulfur removal η/% TP BT DBT 4, 6-DMDBT 58 99 100 99 -
[1] ZHU W S, LI H M, JIANG X, YAN Y S, LU J D, XIA J X. Oxidative desulfurization of fuels catalyzed by peroxotungsten and peroxomolybdenum complexes in ionic liquids[J]. Energy Fuels, 2007, 21(5):2514-2516. doi: 10.1021/ef700310r [2] HUANG D, WANG Y J, YANG L M, LUO G S. Chemical oxidation of dibenzothiophene with a directly combined amphiphilic catalyst for deep desulfurization[J]. Ind Eng Chem Res, 2006, 45:1880-1885. doi: 10.1021/ie0513346 [3] CAMPOS-MARTIN J M, CAPEL-SANCHEZ M C, PEREZ-PRESAS P, FIERRO J L G. Oxidative processes of desulfurization of liquid fuels[J]. J Chem Technol Biotechnol, 2010, 85:879-890. doi: 10.1002/jctb.v85:7 [4] KOMINTARACHAT C, TRAKARNPRUK W. Oxidative desulfurization using polyoxometalates[J]. Ind Eng Chem Res, 2006, 45:1853-1856. doi: 10.1021/ie051199x [5] YUAN D, SONG H, SONG H L, YOU M Y, WANG B H, LI F, HAO Y L, YU Q. Heterogeneous oxidative desulfurization for model fuels using novel PW-coupled polyionic liquids with carbon chains of different lengths[J]. J Taiwan Inst Chem E, 2017, 76:83-88. doi: 10.1016/j.jtice.2017.04.013 [6] LI H M, HE L N, LU J D, ZHU W S, JIANG X, WANG Y, YOU Y S. Deep oxidative desulfurization of fuels catalyzed by phosphotungstic acid in ionic liquids at room temperature[J]. Energy Fuels, 2009, 23:1354-1357. doi: 10.1021/ef800797n [7] HU X F, LU Y K, DAI F N, LIU C G, LIU Y Q. Host-guest synthesis and encapsulation of phosphotungstic acid in MIL-101 via "bottle around ship":An effective catalyst for oxidative desulfurization[J]. Microporous Mesoporous Mater, 2013, 170:36-44. doi: 10.1016/j.micromeso.2012.11.021 [8] ZHENG J J, JIAO Z B. Modified Bi2WO6 with metal-organic frameworks for enhanced photocatalytic activity under visible light[J]. J Colloid Interf Sci, 2017, 488:234-239. doi: 10.1016/j.jcis.2016.11.007 [9] DING J W, WANG R. A new green system of HPW@MOFs catalyzed desulfurization using O2 as oxidant[J]. Chin Chem Lett, 2016, 27(5):655-658. doi: 10.1016/j.cclet.2016.03.005 [10] CHEN M, YAN J Q, TAN Y, LI Y F, WU Z M, PAN L S, LIU Y J. Hydroxyalkylation of phenol with formaldehyde to bisphenol F catalyzed by Keggin phosphotungstic acid encapsulated in metal-organic frameworks MIL-100(Fe or Cr) and MIL-101(Fe or Cr)[J]. Ind Eng Chem Res, 2015, 54(47):11804-11813. doi: 10.1021/acs.iecr.5b02746 [11] ZHOU Z Y, CHENG B H, MA C, XU F, XIAO J, XIA Q B, LI Z. Flexible and mechanically-stable MIL-101(Cr)@PFs for efficient benzene vapor and CO2 adsorption[J]. RSC Adv, 2015, 5(114):94276-94282. doi: 10.1039/C5RA17270E [12] YANG X, LIU J, FAN K, RONG L. Hydrocracking of jatropha oil over non-sulfided PTA-NiMo/ZSM-5 catalyst[J]. Sci Rep, 2017, 7:41654. doi: 10.1038/srep41654 [13] WAN H, CHEN C, WU Z W, QUE Y G, FENG Y, WANG W, WANG L, GUAN G F, LIU X Q. Encapsulation of heteropolyanion-based ionic liquid within the metal-organic framework MIL-100(Fe) for biodiesel production[J]. ChemCatChem, 2015, 7(3):441-449. doi: 10.1002/cctc.v7.3 [14] ZHU Y F, ZHU M Y, KANG L H, YU F, DAI B. Phosphotungstic acid supported on mesoporous graphitic carbon nitride as catalyst for oxidative desulfurization of fuel[J]. Ind Eng Chem Res, 2015, 54(7):2040-2047. doi: 10.1021/ie504372p -





 下载:
下载: