Effect of aluminum source on the structure and performance of Ni/Al2O3 catalysts in CO2-CH4 reforming
-
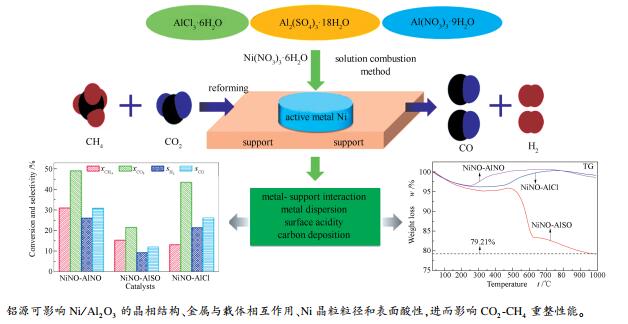
摘要: 以三种不同铝源采用溶液燃烧法制备了系列Ni/Al2O3催化剂,通过XRD、H2-TPR、NH3-TPD、N2吸附-脱附、TG-DTG和TPH等分析方法对反应前后催化剂进行了表征,研究了铝源对Ni/Al2O3催化剂结构、表面性质及其CO2-CH4重整性能的影响。结果表明,以Al(NO3)3·9H2O为铝源制备的NiNO-AlNO催化剂比表面积较大,达102 m2/g;高温还原峰面积大,峰型更为弥散;且载体Al2O3具有一定的结晶性。而以Al2(SO4)3·18H2O和AlCl3·6H2O为铝源制备的NiNO-AlSO和NiNO-AlCl催化剂,其载体以无定型Al2O3存在,活性组分Ni晶粒粒径大、分散性差,还原峰面积较小,与载体的相互作用较弱。其中,由于硫酸铝较为稳定,需要在更高温度下才能转化为Al2O3,且所制备NiNO-AlSO催化剂中残留有含硫物质,使得其表面酸性较强。评价结果显示,NiNO-AlNO催化剂活性较高,稳定性好,CH4转化率为31.21%,CO2转化率为48.97%。积炭分析结果发现,NiNO-AlNO催化剂表面积炭量最少,沉积炭主要以无定型态存在,具有良好的抗积炭性能。
-
关键词:
- Ni/Al2O3催化剂 /
- CO2-CH4重整 /
- 铝源 /
- 积炭
Abstract: Three Ni/Al2O3 catalysts were prepared with different aluminum sources by the solution combustion method and characterized by XRD, H2-TPR, NH3-TPD, N2 sorption, TG-DTG and TPH. The effect of aluminum source on the structure and performance of Ni/Al2O3 catalysts in CO2-CH4 reforming was then investigated. The results show that the NiNO-AlNO catalyst with Al(NO3)3·9H2O as aluminum source owns a large surface area of 102 m2/g and a wide and intense high-temperature reduction peak; besides, the Al2O3 support displays certain crystallinity. In contrast, the NiNO-AlSO and NiNO-AlCl catalysts, prepared with Al2(SO4)3·18H2O and AlCl3·6H2O sources, respectively, are composed of amorphous Al2O3 as support and crystal Ni as active component; the Ni species is poorly dispersed and present as large grains, with a small reduction peak and weak interaction with the support. In particular, because of the high stability of Al2(SO4)3 and difficulty in converting Al2(SO4)3 to active Al2O3 at high temperature, certain sulfur-containing substances are preserved and the resultant NiNO-AlSO catalyst shows strong surface acidity. The catalytic evaluation results indicate that the NiNO-AlNO catalyst exhibits high activity and stability in the CO2-CH4 reforming; the conversions of CH4 and CO2 are 31.21% and 48.97%, respectively. The carbon deposition analysis illustrates that the content of deposited carbon (present mainly in the amorphous form) on the NiNO-AlNO catalyst is rather low, suggesting a high resistance against carbon deposition.-
Key words:
- Ni/Al2O3 catalyst /
- CO2-CH4 reforming /
- aluminum source /
- carbon deposition
-
表 1 催化剂还原后和反应后的Ni晶粒粒径
Table 1 Size of Ni species on the reduced catalysts and the spent catalysts after reaction tests
Catalyst Ni particle size d/nm Increasing rate /% after reduction after reaction NiNO-AlNO 18.89 22.56 19 NiNO-AlSO 46.46 27.15 -42 NiNO-AlCl 36.69 39.51 8 表 2 催化剂比表面积、孔体积和平均孔径测试结果
Table 2 Surface area, pore volume, average pore diameter of three catalysts after reduction
Catalyst ABET/(m2·g-1) v/(m3·g-1) d/nm NiNO-AlNO 101.70 0.32 16.54 NiNO-AlSO 5.05 0.02 11.91 NiNO-AlCl 159.02 0.32 9.84 -
[1] ABDULRASHEE A, JALIL A A, GAMBO Y, IBRAHIM M, HAMBALI H U, SHAHUL HAMID M. A review on catalyst development for dry reforming of methane to syngas:Recent advances[J]. Renewable Sustainable Energy Rev, 2019, 108:175-193. doi: 10.1016/j.rser.2019.03.054 [2] ZHANG G J, LIU J W, XU Y, SUN Y H. A review of CH4-CO2 reforming to synthesis gas over Ni-based catalysts in recent years (2010-2017)[J]. Int J Hydrogen Energy, 2018, 43:15030-15054. doi: 10.1016/j.ijhydene.2018.06.091 [3] HORLYCK J, LEWIS S, AMAL R, SCOTT J. The impact of la doping on dry reforming ni-based catalysts loaded on fsp-alumina[J]. Top Catal, 2018, 61:1842-1855. doi: 10.1007/s11244-018-1015-1 [4] CHEN C J, WANG X G, HUANG H G, ZOU X J, GU F N, SU F B, LU X G. Synthesis of mesoporous Ni-La-Si mixed oxides for CO2 reforming of CH4 with a high H2 selectivity[J]. Fuel Process Technol, 2019, 185:56-57. doi: 10.1016/j.fuproc.2018.11.017 [5] JANG W J, SHIM J O, KIM H M, YOO S Y, ROH H S. A review on dry reforming of methane in aspect of catalytic properties[J]. Catal Today, 2019, 324:15-26. doi: 10.1016/j.cattod.2018.07.032 [6] NAVAS A Z, CRUZ P L, MARTIN G M, IRIBARREN D, DUFOUR J. Simulation and life cycle assessment of synthetic fuels produced via biogas dry reforming and Fischer-Tropsch synthesis[J]. Fuel, 2019, 235(1):1492-1500. https://www.sciencedirect.com/science/article/pii/S0016236118315199 [7] ARAMOUNIL N A K, TOUNMA J G, TARBOUSH B A, ZEAITER J, AHMAD M N. Catalyst design for dry reforming of methane:Analysis review[J]. Renewable Sustainable Energy Rev, 2018, 82:2570-2585. doi: 10.1016/j.rser.2017.09.076 [8] DAHDAH E, RACHED J A, AOUAD S, GENNEQUIN C, TIDAHY H L, ESTEPHANE J, ABOUKAIS A, AAD E A. CO2 reforming of methane over NixMg6-xAl2 catalysts:Effect of lanthanum doping on catalytic activity and stability[J]. Int J Hydrogen Energy, 2017, 42(17):12808-12817. doi: 10.1016/j.ijhydene.2017.01.197 [9] MO W L, MA F Y, MA Y Y, FAN X. The optimization of Ni-Al2O3 catalyst with the addition of La2O3 for CO2-CH4 reforming to produce syngas[J]. Int J Hydrogen Energy, 2019, 44(45):24510-24524. doi: 10.1016/j.ijhydene.2019.07.204 [10] SERRANO L A, DAZA L. Influence of the operating parameters over dry reforming of methane to syngas[J]. Int J Hydrogen Energy, 2014, 39(8):4089-4094. doi: 10.1016/j.ijhydene.2013.05.135 [11] TALKHONCHEH S K, HAGHIGHI M. Syngas production via dry reforming of methane over Ni-based nanocatalyst over various supports of clinoptilolite, ceria and alumina[J]. J Nat Gas Sci Eng, 2015, 23:16-25. doi: 10.1016/j.jngse.2015.01.020 [12] REZAEI M, ALAVI S M. Dry reforming over mesoporous nanocrystalline 5% Ni/M-MgAl2O4 (M:CeO2, ZrO2, La2O3) catalysts[J]. Int J Hydrogen Energy, 2019, 44(31):16516-16525. doi: 10.1016/j.ijhydene.2019.04.213 [13] LI B, XU Z X, JING F L, LUO S Z, WANG N, CHU W. Improvement of catalytic stability for CO2 reforming of methane by copper promoted Ni-based catalyst derived from layered-double hydroxides[J]. J Energy Chem, 2016, 25(6):1078-1085. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=trqhxzz-e201606023 [14] WANG Y, YAO L, WANG S H, MAO D H, HU C W. Low-temperature catalytic CO2 dry reforming of methane on Ni-based catalysts:A review[J]. Fuel Process Technol, 2018, 169:199-206. doi: 10.1016/j.fuproc.2017.10.007 [15] SEO H O. Recent scientific progress on developing supported Ni catalysts for dry (CO2) reforming of methane[J]. Catalysts, 2018, 8(3):110-118. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=MDPI000000203008 [16] AMIR E, REZAEI M, MESHKANI F. Investigation of the catalytic performance and coke formation of nanocrystalline Ni/SrO-Al2O3 catalyst in dry reforming of methane[J]. Iran J Hydrogen Fuel Cell, 2016, 3(4):315-322. https://www.researchgate.net/publication/222816867_Improvement_of_coke_resistance_of_NiAl2O3_catalyst_in_CH4CO2_reforming_by_ZrO2_addition [17] SHIRAZ M H A, REZAEI M, MESHKANI F. The effect of promoters on the CO2 reforming activity and coke formation of nanocrystalline Ni/Al2O3 catalysts prepared by microemulsion method[J]. Korean J Chem Eng, 2016, 33:3359-3366. doi: 10.1007/s11814-016-0203-6 [18] SHANG Z Y, LI S G, LI L, LIU G Z, LIANG X H. Highly active and stable alumina supported nickel nanoparticle catalysts for dry reforming of methane[J]. Appl Catal B:Environ, 2017, 201:302-309. doi: 10.1016/j.apcatb.2016.08.019 [19] CHEIN R Y, FUNG W Y. Syngas production via dry reforming of methane over CeO2 modified Ni/Al2O3 catalysts[J]. Int J Hydrogen Energy, 2019, 44:14303-14315. doi: 10.1016/j.ijhydene.2019.01.113 [20] RYOO H, MA B C, KIM Y C. Syngas production via combined steam and carbon dioxide reforming of methane over Ni-Mo-Sb/Al2O3 catalysts[J]. J Nanosci Nanotechnol, 2019, 19:988-990. doi: 10.1166/jnn.2019.15941 [21] 覃发玠, 刘雅杰, 庆绍军, 侯晓宁, 高志贤.甲醇制氢铜铝尖晶石缓释催化剂的研究-不同铜源合成的影响[J].燃料化学学报, 2017, 45(12):1481-1488. doi: 10.3969/j.issn.0253-2409.2017.12.010QIN Fa-jie, LIU Ya-jie, QING Shao-jun, HOU Xiao-ning, GAO Zhi-xian. Cu-Al spinel as a sustained release catalyst for H2 production from methanol steam reforming:Effects of different copper sources[J]. J Fuel Chem Technol, 2017, 45(12):1481-1488. doi: 10.3969/j.issn.0253-2409.2017.12.010 [22] 杜明仙, 翟效珍, 李源, 李林东, 朱华青, 谭长瑜.高比表面积窄孔分布氧化铝的制备Ⅰ.沉淀条件的影响[J].催化学报, 2002, 23(5):456-468. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=cuihuaxb200205019DU Ming-xian, ZHAI Xiao-zhen, LI Yuan, LI Lin-dong, ZHU Hua-qing, TAN Chang-yu. Preparation of alumina with high specific surface area and narrow pore size distributionⅠ. Effect of precipitation conditions[J]. Chin J Catal, 2002, 23(5):456-468. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=cuihuaxb200205019 [23] 谭亚南, 李枫, 伊晓东, 王跃敏, 方维平, 万惠霖.一种制备活性氧化铝的新方法[J].催化学报, 2008, 29(10):975-978. doi: 10.3321/j.issn:0253-9837.2008.10.006TAN Ya-nan, LI Feng, YI Xiao-dong, WANG Yue-min, FANG Wei-ping, WAN Hui-lin. A novel method for preparation of activated alumina[J]. Chin J Catal, 2008, 29(10):975-978. doi: 10.3321/j.issn:0253-9837.2008.10.006 [24] 莫文龙, 马凤云, 刘月娥, 刘景梅, 钟梅, 艾沙·努拉洪.制备方法对Ni-Al2O3催化剂在CO2-CH4重整反应中催化剂的影响[J].燃料化学学报, 2015, 43(9):1084-1091. http://www.ccspublishing.org.cn/article/id/100033402MO Wen-long, MA Feng-yun, LIU Yue-e, LIU Jing-mei, ZHONG Mei, AISHA Nu-la-hong. Effect of preparation methods on the catalytic performance of Ni-Al2O3 for CO2-CH4 reforming[J]. J Fuel Chem Technol, 2015, 43(9):1084-1091. http://www.ccspublishing.org.cn/article/id/100033402 [25] 郝志刚, 朱庆山, 雷泽, 李洪钟.流化床反应器中不同Ni/Al2O3催化剂上CH4-CO2重整反应性能的比较研究[J].燃料化学学报, 2007, 35(4):436-441. doi: 10.3969/j.issn.0253-2409.2007.04.010HAO Zhi-gang, ZHU Qing-shan, LEI Ze, LI Hong-zhong. Comparative study of CH4-CO2 reforming over different Ni/Al2O3 catalysts in a fluidized bed reactor[J]. J Fuel Chem Technol, 2007, 35(4):436-441. doi: 10.3969/j.issn.0253-2409.2007.04.010 [26] VETCHINKINA T N. Physicochemical properties of the alumina produced by alkaline and acidic methods[J]. Russ Metall, 2009, 2:120-128. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=3d2d379d4bd9f8055f2fb88feeea831b [27] HAO M M, ZENG Z Q, FAN G F, WANG X H, LÜ W Z, LIANG F. Influence of sulfate ion on phase and dispersion of Y3Al5O12 nanopowders with the co-crystallization method[J]. Solid State Phenom, 2018, 281:3-8. doi: 10.4028/www.scientific.net/SSP.281.3 [28] WANG J, SHI J, XU B. Effect of precursors on the morphology of hydroxyl aluminum prepared by hydrothermal treatment[J]. Adv Mater Res, 2011, 308/310:542-547. doi: 10.4028/www.scientific.net/AMR.308-310.542 [29] JABBOUR K, MASSIANI P, DAVIDSON A, CASALE S, HASSAN N E. Ordered mesoporous "one-pot" synthesized Ni-Mg (Ca)-Al2O3 as effective and remarkably stable catalysts for combined steam and dry reforming of methane (CSDRM)[J]. Appl Catal B:Environ, 2017, 201:527-542. doi: 10.1016/j.apcatb.2016.08.009 [30] 张荣俊, 夏国富, 李明丰, 吴玉, 聂红, 李大东.载体类型对Ni基催化剂甲烷干重整反应性能的影响[J].燃料化学学报, 2015, 43(11):1359-1365. doi: 10.3969/j.issn.0253-2409.2015.11.011ZHANG Rong-jun, XIA Guo-fu, LI Ming-feng, WU Yu, NIE Hong, LI Da-dong. Effect of support on the performance of Ni-based catalyst in methane dry reforming[J]. J Fuel Chem Technol, 2015, 43(11):1359-1365. doi: 10.3969/j.issn.0253-2409.2015.11.011 [31] 周建良, 朱健, 陈肇雄, 邱丽玲, 何雪琴, 霍艳.不同原料对镁铝水滑石晶体结构及形貌影响的研究[J].华中师范大学学报(自然科学版), 2010, 44(2):247-250. http://d.old.wanfangdata.com.cn/Periodical/hzsfdxxb201002018ZHOU Jian-liang, ZHU Jian, CHEN Zhao-xiong, QIU Li-ling, HE Xue-qin, HUO Yan. Influence of different raw materials on the crystal structure and morphology of Mg-Al-CO3 hydrotalcite[J]. J Huazhong Norm Univ (Nat Sci), 2010, 44(2):247-250. http://d.old.wanfangdata.com.cn/Periodical/hzsfdxxb201002018 [32] 王晶, 徐冰.铝盐前驱体对水热法制备薄水铝石微观结构的影响[J].中国有色金属学报, 2012, 22(6):1821-1825. http://d.old.wanfangdata.com.cn/Periodical/zgysjsxb201206037WANG Jing, XU Bing. Effect of aluminum salt precursors on microstructure of boehmite prepared by hydrothermal treatment[J]. Chin J Nonferrous Met, 2012, 22(6):1821-1825. http://d.old.wanfangdata.com.cn/Periodical/zgysjsxb201206037 [33] 莫文龙, 马凤云, 刘月娥, 刘景梅, 钟梅, 艾沙·努拉洪.溶液燃烧法制备Ni-Al2O3催化剂用于CO2-CH4重整研究[J].无机材料学报, 2016, 31(5):485-491. http://d.old.wanfangdata.com.cn/Periodical/wjclxb201605007MO Wen-long, MA Feng-yun, LIU Yue-e, LIU Jing-mei, ZHONG Mei, AISHA Nu-la-hong. Preparation of Ni-Al2O3 Catalysts by Solution Combustion Method for CO2 Reforming of CH4[J]. J Inorg Mater, 2016, 31(5):485-491. http://d.old.wanfangdata.com.cn/Periodical/wjclxb201605007 [34] AL-FATESH A S, NAEEM M A, FAKEEHA A H, ABASAEED A E. Role of La2O3 as promoter and support in Ni/γ-Al2O3 catalysts for dry reforming of methane[J]. Chin J Chem Eng, 2014, 22(1):28-37. doi: 10.1016/S1004-9541(14)60029-X [35] 张鹏, 张晴, 刘静, 高濂.甲烷干气重整镍基复合结构催化剂的研究进展[J].无机材料学报, 2018, 33(9):931-941. http://d.old.wanfangdata.com.cn/Periodical/wjclxb201809002ZHANG Peng, ZHANG Qing, LIU Jing, GAO Lian. Research progress of ni-based composite catalysts for methane dry reforming[J]. J Inorg Mater, 2018, 33(9):931-941. http://d.old.wanfangdata.com.cn/Periodical/wjclxb201809002 [36] 莫文龙, 马风云, 刘景梅, 钟梅, 艾沙·努拉洪.基于程序升温氢化表征的Ni-Al2O3催化剂上CO2-CH4重整反应积碳研究[J].燃料化学学报, 2019, 47(5):549-557. doi: 10.3969/j.issn.0253-2409.2019.05.005MO Wen-long, MA Feng-yun, LIU Jing-mei, ZHONG Mei, AISHA Nu-la-hong. A study on the carbonaceous deposition on Ni-Al2O3 catalyst in CO2-CH4 reforming on the basis of temperature programmed hydrogenation characterization[J]. J Fuel Chem Technol, 2019, 47(5):549-557. doi: 10.3969/j.issn.0253-2409.2019.05.005 -





 下载:
下载: